Back to Journals » Journal of Inflammation Research » Volume 14
Increasing Age Affected Polymorphonuclear Neutrophils in Prognosis of Mycoplasma pneumoniae Pneumonia
Authors Zhao Q, Zhang T, Zhu B , Bi Y, Jiang SW, Zhu Y, Zhao D, Liu F
Received 31 May 2021
Accepted for publication 30 July 2021
Published 14 August 2021 Volume 2021:14 Pages 3933—3943
DOI https://doi.org/10.2147/JIR.S321656
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Qianye Zhao,1,2,* Teng Zhang,2,* Beibei Zhu,1 Ying Bi,3 Shi-Wen Jiang,4 Yifan Zhu,1 Deyu Zhao,1 Feng Liu1
1Department of Respiratory Medicine, Children’s Hospital of Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China; 2Department of Pediatrics, Lianyungang Maternal and Child Health Care Hospital, Lianyungang, Jiangsu, People’s Republic of China; 3Department of Respiratory Medicine, Xuzhou children’s Hospital, Xuzhou, Jiangsu, People’s Republic of China; 4Research Institute for Reproductive Health and Genetic Diseases, Center of Reproductive Medicine, The Affiliated Wuxi Maternity and Child Health Care Hospital of Nanjing Medical University, Wuxi, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Deyu Zhao; Feng Liu
Department of Respiratory Medicine, Children’s Hospital of Nanjing Medical University, No. 72, Guangzhou Road, Gulou District, Nanjing, Jiangsu, 210008, People’s Republic of China
Tel +86 189 5176 9559
; +86 189 5176 8373
Fax +86 025 83117376
Email [email protected]; [email protected]
Purpose: It is well known that age is related to the incidence of Mycoplasma pneumoniae pneumonia (MPP), and how age and other factors contribute to MPP remains unclear. In this study, we investigate how age affects the prognosis of MPP.
Patients and Methods: A total number of 1875 hospitalized children with pneumonia were enrolled in this study, including 52 children with refractory M. pneumoniae pneumonia (RMPP) and 298 children with non-RMPP. We used multiple logistic regression analysis to further identify the risk factors of RMPP, and found that age and polymorphonuclear neutrophils (PMNs) count were the key independent risk factors for the occurrence of RMPP. In order to improve specificity, 4.5 years old was taken as the cut-off value. Then, according to the cut-off value of age, 76 participants were recruited and divided into four groups: < 4.5y MPP group, ≥ 4.5y MPP group, < 4.5y health control (< 4.5yHC) and ≥ 4.5y HC group. We explored the diverse functions of primary PMNs from children of different ages with MPP at cellular level. Besides, we studied the relationship between lung injury and PMNs in mice model with MPP of different ages.
Results: We found that the age and PMNs count of RMPP group were significantly higher than those of the non-RMPP group. Importantly, there is a linear correlation between the age of patients with RMPP and the percentage of PMNs. Further analysis showed that elderly patients infected with M. pneumoniae had more active PMNs function. Meanwhile, proteomics showed that children with M. pneumoniae infection in different age groups have differences in PMNs apoptosis, nicotinamide adenine dinucleotide phosphate, mitochondrial function and oxidative stress. Finally, we found that age is also involved in the pathogenesis of mouse model with MPP.
Conclusion: We speculate that age may contribute to the development of RMPP.
Keywords: refractory Mycoplasma pneumoniae pneumonia, Mycoplasma pneumoniae, polymorphonuclear neutrophils, reactive oxygen species, neutrophil extracellular traps
Introduction
Mycoplasma pneumoniae pneumonia (MPP) accounts for 10–40% of community-acquired pneumonia cases in hospitalized children, most of which occur over 5 years old.1 In recent years, more and more cases of M. pneumoniae (MP) infection are manifested as refractory Mycoplasma pneumoniae pneumonia (RMPP), followed by pulmonary embolism, plastic bronchitis, necrotizing pneumonia, lung abscess and other extrapulmonary complications.2,3 According to reports, the main population of fulminant MP infection is young people without serious underlying diseases, and they required early administration of steroids as cellular immunosuppressants.4 These studies indicate that MP infection can trigger excessive lung and systemic inflammation in children with RMPP or fulminant Mycoplasma pneumoniae pneumonia.
MP infection is more common in children aged 5 years or older than children under 5 years old in the United States.1 In northern China, the peak age of MPP is between 6 and 10 years.5 Zhang et al reported that the peak age of patients is much older than that of Non-RMPP patients in East China.6 Bi et al regarded age as one of the predictors of RMPP.7 These studies indicate that age may be the main factor associated with MP infection, but the underlying mechanism remains unclear. Previous studies have shown that age may have an impact on the function of polymorphonuclear neutrophils (PMNs). It has been observed that premature infants migrate fewer PMNs than full-term newborns, and the migration rate is slower than that of young adults.8 In addition, Peniche et al demonstrated that middle-aged mice exhibit significant defects in the recruitment of PMNs to the colon.9 There are also data supporting that PMNs from the elderly group have significantly reduced targeting accuracy10 and Fc-mediated phagocytosis.11 Further studies have shown that epigenetic changes related to aging in PMNs, such as changes in DNA methylation, may affect cell function through modification of gene expression.12 However, whether age affects the function of PMNs after MP infection remains unclear.
In order to investigate how age affects the prognosis of MPP, in this study, we firstly looked for the differences in PMNs and age between RMPP and non-RMPP group, and then we explored the diverse functions of PMNs from children of different age with MPP at cellular level; besides, we studied the lung injury in mice model with MPP of different ages.
Materials and Methods
Ethics Statements
All animals’ experiments were approved by the Institutional Animal Care and Use Committee of the Nanjing Medical University at Nanjing (Approval number IACUC-1905057) and followed the Guidelines for the Ethical Review of Laboratory Animal Welfare People’s Republic of China National Standard (GB/T 35892–2018).13 The BALB/c mice were kept in a pathogen-free environment and fed ad lib. The clinical study (Protocol number 201703058) was consented by the Ethics Committee of the Children’s Hospital of Nanjing Medical University in compliance with the Declaration of Helsinki. Informed consents were obtained from the parents of all patients included in this study.
Population
This study included patients with MPP who were hospitalized at the Department of Respiratory Medicine, Children’s Hospital of Nanjing Medical University from September 1, 2016 to March 31, 2017. The criteria for diagnosis of MPP are provided in Supplement 1. RMPP was defined as MPP cases whose chest X-ray or low-dose multi-slice spiral CT scan showed clinical and radiological deteriorations, such as malabsorption, atelectasis, pleural effusion, lung abscess, and lung necrosis, despite the appropriate macrolide antibiotic therapy for 7 days or longer.14 Patients whose chest imaging showed clear absorption after 7 days of treatment were assigned into non-RMPP groups. Patients with immune dysfunction, congenital ciliary dysplasia, cystic fibrosis, bronchiectasis, diffuse interstitial lung disease, and bronchopulmonary dysplasia were excluded from the study.
Isolation and Culture of PMNs from Peripheral Blood in vitro
Seventy-six participants were recruited and we divided them into 4 groups: <4.5y healthy control (<4.5yHC), ≥4.5y HC, <4.5y MPP group (MPP patients under 4.5 years old) and ≥4.5y MPP group (MPP patients greater than or equal to 4.5 years old). All subjects were recruited from March 2018 to November 2019. Peripheral blood samples of 76 participants at admission were collected in centrifuge tubes containing ethylenediaminetetraacetic acid (EDTA) solution to prevent blood clotting. The detailed medical information about 76 participants was provided in Supplement 2 (Tables S1 and S2). Among them, 46 children of the MPP groups met the diagnostic and exclusive criteria for MPP. The sample was then centrifuged at 3000 rpm for 10 minutes at 4°C. The plasma was collected and stored at −80°C. The cell components were used to separate PMNs by Ficoll2 Hypaque density gradient centrifugation. PMNs were divided into control group (MP-) and MP stimulation group (MP+), and they were cultured in the medium of PMNs containing 10% fetal calf serum in a humidified atmosphere containing 5% CO2 at 37°C. MP stimulation group was treated with 108 CFU of M129 (Supplement 3). This standard MP strain was provided by Professor Zhimin Chen from Zhejiang Children’s Hospital.
Plasma Concentration of Inflammatory Factors
The levels of plasma interleukin-8 (IL-8) and granulocyte colony stimulating factor (G-CSF) were measured by using Enzyme-Linked Immunosorbent Assay (ELISA) (RayBio, America) according to the manufacturer’s instructions.
PMNs Oxidative Stress and Generation of NETs
The intracellular reactive oxygen molecules (ROS) levels were determined by flow cytometry (DCFH-DA, Beyotime). In brief, the cell culture supernatants were collected and subjected to centrifugation under 3000 rev/min for 10 min at 4°C after 30 minutes of incubation. The level of myeloperoxidase (MPO) in the supernatant was detected by colorimetry (Sigma-Aldrich). Quantification of neutrophil extracellular traps (NETs) in the culture supernatants of PMN was determined by PicoGreen staining (Invitrogen) after 4 hours of incubation with cells.
Proteomic Analysis of PMNs
Eleven samples of PMNs were analyzed using label-free proteomics, including 7 PMNs specimens of different ages with MPP and 4 healthy children. Raw data files were acquired from triple TOF 5600. Protein identification was performed using the Andromeda search engine integrated with maxquant. Data are filtered to a 1% false-discovery rate (FDR). Each protein contains at least one unique peptide. While P value <0.05 and fold-changes >1.5 were considered significant using Welch’s t-test. In total, 2985 proteins and 19,016 peptides were identified.
Model of BALB/c Mice with MPP
The mice aged 3–4 and 7–8 weeks old were provided by the Animal Experimental Center of Nanjing Medical University. Two independent trials of the experiments were performed. Six BALB/c mice were used for per group and randomly allocated to the different groups. Mice were anaesthetized with 1% pentobarbital Sodium, endotracheally infected with 1×108 CFU of M129 MP strain in 10 μL PMNs culture medium (Supplement 4).
Histopathological Staining
Mice were infected with MP for 72 hours before euthanasia and their lungs were harvested. The right inferior lobe was fixed in 4% paraformaldehyde, followed by dehydration with ethanol and embedding in paraffin. Paraffin-embedded lung tissues were sectioned at 8 μm and processed for H&E and immunohistochemical staining. Immunohistochemistry was performed to detect PMNs infiltration using anti-Ly6G antibody (Abcam, America).
Preparation and Cytological Examination of Bronchoalveolar Lavage Fluid
Mice were infected with MP for 72 hours before euthanasia. Bronchoalveolar lavage fluid (BALF) was obtained by irrigation with 1 mL of PBS using a blunted needle inserted into the trachea after a tracheotomy. The BALF was centrifuged at 12000 rpm and 4°C for 10 minutes and stored at −80°C for further analysis. Cell components were used for measuring the total number of cytology and percentage of PMNs using Diff-Quik Stain (Solarbio, China).
Detection of Pulmonary Vascular Permeability of the Lung Injury
The residual left lung in the above experiment was weighed, and then dried in a 65°C incubator for 24 hours. The wet-to-dry weight ratio was calculated after 24 hours. Pulmonary vascular permeability was assessed by the ratio of protein quantity of alveolar lavage fluid to serum. Proteins in serum samples from mice were isolated. Protein concentration in the supernatant of 10% bronchoalveolar lavage fluid (BALF) and mice serum were determined using bicinchoninic acid (BCA) Protein Assay kit (Beyotime, China) according to the manufacturer’s instructions.
Preparation of Lung Homogenate
Following euthanasia, the right lungs were dissociated from the pulmonary hilum. The inferior lobes of the right lung were separated and placed in a pre-frozen PBS balance salt. The 10% lung tissue homogenate was prepared by the fully automatic freeze-mixed ball mill and was centrifuged at 4°C 12000 rpm for 15 minutes, and the supernatant was aliquoted and stored at −80 °C until use.
Measurement of Surrogate Markers
Malondialdehyde (MDA) and MPO in 5% lung tissue homogenate were determined by thiobarbituric acid (TBA) colorimetric method (JIAN CHENG TECHNOLOGY, China) and ELISA (Fcmacs, America), respectively. NETs of BALF were examined with the PicoGreen fluorescent dye (Invitrogen, America).
Data Analysis
Statistical analyses were performed by using SPSS software, version 17.0 (IBM, Armonk, NY, USA). Data with normal distribution were expressed as mean±standard deviation, and compared using with the independent sample t-test and paired-samples t-test. The Levene homogeneity variance was analyzed for quantitative data of multiple groups. One-way ANOVA was performed on data with multiple groups before application of t-test for one-to-one comparison. The qualitative data were compared using Chi-square test. Linear correlation of quantitative data was analyzed using with the Pearson correlation analysis, and nonlinear correlation was analyzed using with the restricted cubic spline method. Statistical significance was defined as P < 0.05.
Results
Age and PMNs Counts Were Critical Independent Risk Factors for the Occurrence of RMPP
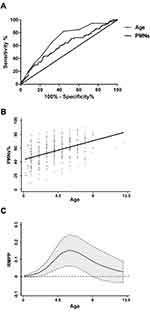
Age is reported to be associated with the incidence and adverse outcomes of MPP. To determine the optimal cut-off value of age for assessing the incidence of RMPP, a total number of 1875 hospitalized children with pneumonia were enrolled in this study, including 298 children with Non-RMPP and 52 children with RMPP. The demographic and clinical data of these patients are presented in Table 1. There were significant differences between non-RMPP group and RMPP group in age distribution and duration of fever. As for laboratory findings, there was no significant difference in gender, peripheral WBC counts, HB, PLT, ALT, AST and LDH between the two groups as well. However, we found that peripheral PMNs count and CRP in the RMPP group were significantly higher than those in the non-RMPP group. We then use multiple logistic regression analysis to further identify the risk factors of RMPP, and find that age and PMNs count are the key independent risk factors for the occurrence of RMPP. In order to improve specificity, 4.5 years old was taken as the cut-off value. The diagnostic sensitivity and specificity at the cut-off of 4.5 years old were 61.54% and 65.77%, respectively. The area under the ROC curve (AUC) was 0.68 (Figure 1A). The correlation coefficient between age and the percentage of PMNs in cases with RMPP was found to be 0.45 (Figure 1B). The age distribution of RMPP group is presented in Figure 1C. As presented in Table 2, the odds ratio (OR) values of each variable were calculated and evaluated. The incidence of RMPP in children aged 4.5~9 years was 4.22 times higher than that in children under 4.5 years old, and OR value was 1.90 to 9.38 (95% CI) by adjusted WBC, the count and percentage of PMNs, duration days of fever, and levels of HB, PLT, AST, ALT, LDH and CRP. Collectively, these data suggested that age affected the percentage of PMNs in peripheral blood, and that age ≥4.5 years could partially predict the development of RMPP.
 |
Table 1 Demographic Data and Clinical and Laboratory Characteristics of Non-RMPP and RMPP Patients |
 |
Table 2 OR for RMPP by Various Factors |
Age is Correlated with the Counts and Function of PMNs in Children with MPP
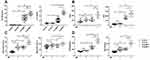
G-CSF can stimulate the proliferation and differentiation of granulocyte precursor cells in the bone marrow. To determine the influence of age on PMNs counts in peripheral blood, serum inflammatory factors were detected in children with MPP. The MPP patients were classified into <4.5y MPP group and ≥4.5y MPP group at a cut-off of 4.5 years of age. As documented in Table 3, it was found that PMNs counts were significantly higher in ≥4.5y MPP group than in <4.5y MPP group. As shown in Figure 2A, the level of G-CSF was significantly increased in ≥4.5y MPP group compared with <4.5y MPP or HC group. Proinflammatory cytokine IL-8 act as recruiter of PMNs infiltrating into the infection sites. A similar pattern was observed for both G-CSF and IL-8 levels in our study (Figure 2A).
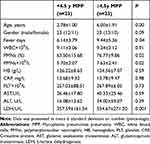 |
Table 3 Comparison of Clinical Characteristics of Non-RMPP in Different Age Groups |
To characterize the effect of age on activated PMNs function after MP infection, we separated and cultured primary PMNs in vitro. Compared with HC or MPP group, intracellular ROS fluorescence intensity (Figure 2B) in PMNs stimulated by MP, MPO (Figure 2C) and NETs (Figure 2D) in the culture supernatants of PMN at different post-infection time were significantly increased. The levels of ROS, MPO and NETs of PMNs in ≥4.5y MPP group were significantly higher than those of < 4.5y age group with or without MP stimulation. These data suggest that age acts as a factor in inducing different activations of PMNs in response to MP.
PMNs Responded Diversely to MP Infection in Children of Different Ages
In order to further understand the functional differences of PMNs in children of different ages after MP stimulation, eleven PMNs specimens, including 4 cases of ≥4.5y MPP (A9, A21, A30 and A31), 3 cases of <4.5y MPP (A13, A28 and A37) and 4 cases of HC group were analyzed by label free proteomics approach. A total of 2684 proteins were identified, of which 46 proteins were classified as differentially accumulated proteins (DAPs), including 8 up-regulated DAPs (G3V126, Q6N093, MOQXF7, AOAO24QZCO, E5KNY5, J3QSX6, Q8ND71 and Q9NZ08) and 38 down-regulate DAPs (W8QEY1, Q8BFZ3, V9HWO4, Q53Y51, P20292, AOA14OVJY7, C9J8E1, Q9NXV6 and so on) (Figure 3A and B). DAPs have been found to be associated with apoptosis, phagosome, nitrogen metabolism and leukocyte transendothelial migration, etc. (Figure 3C). In addition to chemotaxis and phagocytosis, PMNs function scores, such as apoptosis, nicotinamide adenine dinucleotide phosphate (NADPH), mitochondrion and oxidative stress, statistically increased in older MPP cases (Figure 3D), which indicate that there are multiple functional differences of PMNs after MP infection in children of different age.
Age Affected the Consequence of Mice with Experimental MPP
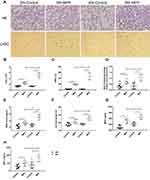
To further clarify the correlation between the infiltration and activation of PMNs and lung injury in mice of different ages, 3-week-old weaned and 8-week-old puberty male BALB/c mice were selected according to age conversion of human and the lifespan of mice.15 The model of BALB/c mice with MPP is established by intratracheal instillation with MP at 72 h post infection. Histopathological sections of the lung tissues stained with HE and showed that the alveolar septums were thickened obviously, and a large number of inflammatory cells were infiltrated in peribronchial, perivascular and vascular lumens (Figure 4A), which proved that the MPP mice model was established successfully.
Compared to 3-week-old mice group with MPP, the total number of cells (Figure 4B) and percentage of PMNs (Figure 4C) in BALF were significantly increased, and increasing numbers of pulmonary infiltrative PMNs were observed in histopathological sections in 8-week-old mice group with MPP by immunohistochemical staining using Anti-Ly6g antibody (Figure 4A). Meanwhile, the level of MDA (Figure 4E), MPO (Figure 4G) and production of NETs (Figure 4H) in lung homogenate were significantly higher in MP infected group of 8-week-old mice. Finally, the indicators related to lung injury, including the ratio of protein quantity of BALF to serum (Figure 4D), wet-to-dry weight ratio of lung tissue (Figure 4F) of 8-week-old mice MPP model, were significantly higher than those of 3-week-old mice group infected with MP. These data suggest that age-related aberrant infiltration and activation of PMNs in mice lung tissue lead to different degrees of pulmonary damage after MP infection.
Discussion
MP is the most common pathogenic microorganism in children over 5 years old in the America,1,16 it can also affect infants and young children during epidemic years in Denmark.17 In recent years, the onset age of MPP has shown a downtrend in Eastern China. However, RMPP mainly occurs in school-age children.18 Our findings indicate that age is a key factor in the prognosis of MPP, which is consistent with previous reports.
This study presents novel data on the effect of age on the number of circulating PMNs after pulmonary MP infection. Lymphocytes are the most abundant leukocyte type in the circulation of children aged from 4–6 days to 4–6 years, the percentage of PMNs continue to increase with age and gradually reach adult level after 4–6 years of age. Since G-CSF drives myeloid progenitors to bias differentiation towards the granulocytic lineage and regulates production of neutrophils by controlling their release into blood,19 so we may speculate that age affects the amounts of PMNs in acute inflammation after MP infection by increasing G-CSF.
In this study, we found that age can also affect the activation function of PMNs stimulated by MP. Human PMNs were capable of triggering superoxide and MPO releasing.20 PMNs separated from the elder cases showed enhanced respiratory burst and NETs production. Therefore, it is suggested that age has an impact on the activation of PMNs through promoting the secretion of IL-8, and may be related to the adverse consequences of MPP.
It is well known that NETs formation occurs via NADPH oxidase (NOX)-dependent and mitochondria NOX-independent pathways. The suppression of mitochondrial oxidative stress reduced NETs release from PMNs of aged mice.21 Functional analysis of PMNs implies that the enhancement of mitochondrial function in elder MPP patients could be related to the increase in injury NETs and oxidative stress injury.
Recently, a large amount of studies focused on abnormal cellular immunity in children with MPP. Macrophages are implicated in the clearance of MP.22 A decreased percentage of macrophages and an increased percentage of PMNs in bronchoalveolar lavage fluid were observed in children with MPP,23 as well as RMPP.24 Recruitment of PMNs into the lung tissue is more associated with the severity and progression in mice with MPP.25 In mice model with MPP, increased PMNs in airspaces and interstitium mediate microvascular damage, pulmonary edema, leading to lung injury. Meanwhile, reduced pulmonary infiltration of PMNs decreased lung injury in immunosuppressed mice with MPP. Therefore, PMNs are deemed to play an important role in the development of MPP. Inhibition of pulmonary PMNs infiltration could reduce lung injury. In our study, we focused on the effects of age on the mobilizing function of PMNs stimulated by MP. In pulmonary infection, activated PMNs eliminate invasive pathogens by producing abundant ROS/nitrogen species, concomitant delivery of MPO and other antimicrobial enzymes into the phagosome.26 MPO represents one of the most abundant protein enzymes that participates in immune defense through the formation of microbicidal reactive oxidants and diffusible radical species.27 However, overproduction of MPO will lead to oxidative stress and tissue damage when they exceed the limits of local antioxidant defense protection. Long-term exposure to a high concentration of ROS can lead to inflammatory injury, which may be associated with cardiovascular diseases,28 neurological diseases,29 cancer and aging.30 Our study confirmed that age affects the “oxidative burst” of PMNs after MP infection, and eventually contributes to different degrees of lung injury.
Recent studies have shown that NETs are another mechanism for PMNs to eliminate pathogens by forming physical barriers and its chemical bactericidal effects, which depends on the transport of MPO from granules to nucleus31 and the release of mitochondrial DNA triggered by ROS.32 Meanwhile, NETs play a critical role against fungal infection,33 and respiratory syncytial virus34 in vitro. Furthermore, NETs are thought to be associated with the pathological damage of severe influenza virus infection. Excessive NETs can also damage normal tissues, leading to thrombosis,35 acute respiratory distress syndrome36 and so on. In this study, age was found to be related to PMNs’ production of NETs stimulated by MP in human and mice. Excessive NETs release results in MP-induced pulmonary tissue damage. Therefore, NETs clearance might be helpful in attenuating pathological injury of MPP.
Conclusion
This study identified that age is associated with the prognosis of MPP by increasing PMNs’ counts and activation in children under 9 years old, which could explain the difference in age sensitivity to MP infection. New therapeutic strategies that inhibit neutrophil recruitment and target neutrophil function can improve the adverse outcome of RMPP.
Abbreviations
RMPP, refractory Mycoplasma pneumoniae pneumonia; MPP, Mycoplasma pneumoniae pneumonia; PMNs, polymorphonuclear neutrophils; HC, healthy control; IL-8, interleukin-8; G-CSF, granulocyte colony stimulating factor; AUC, areas under curves; OR, odds ratio; ROS, reactive oxygen species; MPO, Myeloperoxidase; NETs, neutrophil extracellular traps; MDA, malondialdehyde; MP-, without Mycoplasma pneumoniae stimulation; MP+, Mycoplasma pneumoniae stimulation.
Acknowledgment
The work is supported by Jiangsu Provincial Social Development Project Foundation (BE 2019607), and Key Projects of Nanjing Health and Planning Commission (ZKX 18041). We thank Yun Guo for her technical assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835–845. doi:10.1056/NEJMoa1405870
2. Miyashita N, Akaike H, Teranishi H, et al. Chest computed tomography for the diagnosis of Mycoplasma pneumoniae infection. Respirology. 2014;19(1):144–145. doi:10.1111/resp.12218
3. Nambu A, Ozawa K, Kobayashi N, et al. Imaging of community-acquired pneumoniae: roles of imaging examinations, imaging diagnosis of specific pathogens and discrimination from noninfectious disease. World J Radiol. 2014;6(10):779–793. doi:10.4329/wjr.v6.i10.779
4. Izumikawa K, Izumikawa K, Takazono T, et al. Clinical features, risk factors and treatment of fulminant Mycoplasma pneumoniae pneumonia: a review of the Japanese literature. J Infect Chemother. 2014;20(3):181–185. doi:10.1016/j.jiac.2013.09.009
5. Gao LW, Yin J, Hu YH, et al. The epidemiology of paediatric Mycoplasma pneumoniae pneumonia in North China: 2006 to 2016. Epidemiol Infect. 2019;147:e192. doi:10.1017/S0950268819000839
6. Zhang Y, Zhou Y, Li S, et al. The clinical characteristics and predictors of refractory mycoplasma pneumoniae pneumonia in children. PLoS One. 2016;11(5):e0156465. doi:10.1371/journal.pone.0156465
7. Bi Y, Zhu Y, Ma X, et al. Development of a scale for early prediction of refractory Mycoplasma pneumoniae pneumonia in hospitalized children. Sci Rep. 2021;11(1):6595. doi:10.1038/s41598-021-86086-5
8. Raymond SL, Mathias BJ, Murphy TJ, et al. Neutrophil chemotaxis and transcriptomics in term and preterm neonates. Transl Res. 2017;190(190):4–15. doi:10.1016/j.trsl.2017.08.003
9. Peniche AG, Spinler JK, Boonmer P, Savidge TC, Dann SM. Aging impairs protective host defenses against Clostridioides (Clostridium) difficile infection in mice by suppressing neutrophil and IL-22 mediated immunity. Anaerobe. 2018;54(54):83–91. doi:10.1016/j.anaerobe.2018.07.011
10. Sapey E, Greenwood H, Walton G, et al. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood. 2014;123(2):239–248. doi:10.1182/blood-2013-08-519520
11. Butcher SK, Chahal H, Nayak L, et al. Senescence in innate immune responses: reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J Leukoc Biol. 2001;70(6):881–886.
12. Chatterjee A, Stockwell PA, Rodger EJ, et al. Genome-wide DNA methylation map of human neutrophils reveals widespread inter-individual epigenetic variation. Sci Rep. 2015;5(5):17328. doi:10.1038/srep17328
13. MacArthur Clark JA, Sun D. Guidelines for the ethical review of laboratory animal welfare People’s Republic of China national standard GB/T 35892-2018 [issued 6 February 2018 effective from 1 September 2018]. Anim Model Exp Med. 2020;3(1):103–113. doi:10.1002/ame2.12111
14. Tamura A, Matsubara K, Tanaka T, et al. Methylprednisolone pulse therapy for refractory Mycoplasma pneumoniae pneumonia in children. J Infect. 2008;57(3):223–228. doi:10.1016/j.jinf.2008.06.012
15. Dutta S, Sengupta P. Men and mice: relating their ages. Life Sci. 2016;152(152):244–248. doi:10.1016/j.lfs.2015.10.025
16. Kutty PK, Jain S, Taylor TH, et al. Mycoplasma pneumoniae among children hospitalized with community-acquired pneumonia. Clin Infect Dis. 2019;68(1):5–12. doi:10.1093/cid/ciy419.
17. Søndergaard MJ, Friis MB, Hansen DS, et al. Clinical manifestations in infants and children with Mycoplasma pneumoniae infection. PLoS One. 2018;13(4):e0195288. doi:10.1371/journal.pone.0195288
18. Wang Z, Li YC, Chen L. Early identification of refractory Mycoplasma pneumoniae pneumonia in children. Zhongguo Dang Dai Er Ke Za Zhi. 2015;17(11):1189–1192.
19. Semerad CL, Liu F, Gregory AD, et al. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17(2):413–423. doi:10.1016/S1074-7613(02)00424-7
20. Krupa A, Kato H, Matthay MA, et al. Proinflammatory activity of anti-IL-8 autoantibody: IL-8 complexes in alveolar edema fluid from patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2004;286(6):L1105–13. doi:10.1152/ajplung.00277.2003
21. Wang Y, Wang W, Wang N, Tall AR, Tabas I. Mitochondrial oxidative stress promotes atherosclerosis and neutrophil extracellular traps in aged mice. Arterioscler Thromb Vasc Biol. 2017;37(8):e99–e107. doi:10.1161/ATVBAHA.117.309580
22. Lai JF, Zindl CL, Duffy LB, et al. Critical role of macrophages and their activation via MyD88-NFκB signaling in lung innate immunity to Mycoplasma pneumoniae. PLoS One. 2010;5(12):e14417. doi:10.1371/journal.pone.0014417
23. Liu Y, Zhang X, Wang Y, et al. The role of granulocyte macrophage colony stimulating factor in hospitalized children with Mycoplasma pneumoniae pneumonia. J Infect Chemother. 2018;24(10):789–794. doi:10.1016/j.jiac.2018.06.003
24. Zhao QY, Shi SJ, Sun DQ, et al. Correlation between galectin-3 level in bronchoalveolar lavage fluid and cellular immunity in children with refractory Mycoplasma pneumoniae pneumonia. Zhongguo Dang Dai Er Ke Za Zhi. 2019;21(2):150–154. doi:10.7499/j.issn.1008-8830.2019.02.008.
25. Shi S, Zhang X, Zhou Y, et al. Immunosuppression reduces lung injury caused by Mycoplasma pneumoniae infection. Sci Rep. 2019;9(1):7147. doi:10.1038/s41598-019-43451-9
26. Malle E, Furtmüller PG, Sattler W, et al. Myeloperoxidase: a target for new drug development? Br J Pharmacol. 2007;152(6):838–854. doi:10.1038/sj.bjp.0707358
27. Santos SS, Brunialti MK, Rigato O, et al. Generation of nitric oxide and reactive oxygen species by neutrophils and monocytes from septic patients and association with outcomes. Shock. 2012;38(1):18–23. doi:10.1097/SHK.0b013e318257114e
28. Senoner T, Dichtl W. Oxidative stress in cardiovascular diseases: still a therapeutic target? Nutrients. 2019;11(9):2090. doi:10.3390/nu11092090
29. Tönnies E, Trushina E. Oxidative stress, synaptic dysfunction, and alzheimer’s disease. Alzheimers Dis. 2017;57(4):1105–1121. doi:10.3233/JAD-161088
30. Grimm A, Eckert A. Brain aging and neurodegeneration: from a mitochondrial point of view. J Neurochem. 2017;143(4):418–431. doi:10.1111/jnc.14037
31. Papayannopoulos V, Metzler KD, Hakkim A, et al. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191(3):677–691. doi:10.1083/jcb.201006052
32. Yousefi S, Mihalache C, Kozlowski E, et al. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16(11):1438-1444. doi:10.1038/cdd.2009.96.
33. Abi Abdallah DS, Denkers EY. Neutrophils cast extracellular traps in response to protozoan parasites. Front Immunol. 2012;3:382. doi:10.3389/fimmu.2012.00382.
34. Funchal GA, Jaeger N, Czepielewski RS, et al. Respiratory syncytial virus fusion protein promotes TLR-4-dependent neutrophil extracellular trap formation by human neutrophils. PLoS One. 2015;10(4):e0124082. doi:10.1371/journal.pone.0124082
35. Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107(36):15880–15885. doi:10.1073/pnas.1005743107
36. Narasaraju T, Yang E, Samy RP, et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179(1):199–210. doi:10.1016/j.ajpath.2011.03.013
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.