Back to Journals » International Journal of General Medicine » Volume 14
Increased Platelet-to-Lymphocyte Ratio is an Independent Predictor of Hemorrhagic Transformation and In-Hospital Mortality Among Acute Ischemic Stroke with Large-Artery Atherosclerosis Patients
Authors Yang Y, Xie D, Zhang Y
Received 22 July 2021
Accepted for publication 1 October 2021
Published 1 November 2021 Volume 2021:14 Pages 7545—7555
DOI https://doi.org/10.2147/IJGM.S329398
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Yi Yang, Dan Xie, Yongbo Zhang
Department of Neurology, Beijing Friendship Hospital, Capital Medical University, Beijing, People’s Republic of China
Correspondence: Yongbo Zhang
Department of Neurology, Beijing Friendship Hospital, Capital Medical University, Beijing, People’s Republic of China
Email [email protected]
Background: The platelet-to-lymphocyte ratio (PLR) is an inflammation marker of acute ischemic stroke, but its significance in patients with hemorrhage transformation (HT) after acute ischemic stroke with large-artery atherosclerosis (AIS-LAA) is unclear, and we also identified the relationship between PLR and in-hospital mortality of HT after AIS-LAA.
Methods: This was a retrospective analysis of patients with AIS-LAA. The PLR was calculated according to platelet and lymphocyte counts on admission. HT was defined on follow-up magnetic resonance imaging or computed tomography when neurologic deterioration worsened during hospitalization. The univariate analysis and multivariate logistic regression were performed to assess the association of PLR, HT and in-hospital mortality of HT after AIS-LAA.
Results: We included 328 Chinese AIS-LAA patients (mean age 67.2± 11.1 years; 70.4% male). HT occurred in 38 patients (11.6%). After multivariate regression analyses, NRL (odds ratio [OR] 1.354, 95% confidence interval [CI] 1.176– 1.559, P< 0.001) and PLR (odds ratio [OR] 3.869, 95% confidence interval [CI] 2.233– 5.702, P< 0.001) were independently associated with HT after AIS-LAA. The area under the ROC curve (AUC) value of PLR (0.72, 95% CI (0.64– 0.80), P< 0.001) tested a greater discriminatory ability compared with neutrophil-lymphocyte ratio (NLR) (0.67, 95% CI (0.58– 0.76), P< 0.001). Meanwhile, PLR was found to be significantly related to HT after AIS-LAA, including in subtypes of artery-to-artery embolization (aOR 1.699, 95% CI 1.298– 3.215, P< 0.001), in-situ thrombosis (aOR4.499, 95% CI 1.344– 9.054, P< 0.001) and branch atheromatous disease (aOR3.239, 95% CI 1.098– 8.354, P< 0.001). Increased PLR predicts high in-hospital mortality of HT after AIS-LAA (OR 1.041, 95% CI (1.006– 1.077), P=0.020; aOR 1.053, 95% CI (1.004– 1.104), P=0.034).
Conclusion: High PLR is associated with greater risk of HT in AIS-LAA patients, including in artery-to-artery embolization, in-situ thrombosis and branch atheromatous disease. Meanwhile, increased PLR predicts high in-hospital mortality of HT after AIS-LAA.
Keywords: acute ischemic stroke, large-artery atherosclerosis, hemorrhagic transformation, platelet-to-lymphocyte ratio, in-hospital mortality
Acute ischemic stroke (AIS) is one of the most common reasons for high mortality and morbidity in Chinese adults. AIS is classified by large-artery atherosclerosis (LAA), small-artery occlusion, cardio-embolism, undetermined etiology and other etiology according to The Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification system.1,2 Although intravenous thrombolysis demonstrates efficacy, there are still several patients whose symptoms would worsen because of hemorrhagic transformation (HT). Meanwhile, HT is a part of AIS natural progression; it occurs in 10–40% of AIS, and contributes to early mortality and adversely affects functional recovery of AIS.3–5 Previous researches indicate that patients with AIS-LAA are prone to early neurological deterioration, especially HT.6 Therefore, it is significant to explore an easy measurable diagnostic biomarker of HT after AIS-LAA which may further improve the recovery of patients.
The pathogenesis of AIS with large-artery atherosclerosis is much more complex, with a key role for inflammation.7,8 Several studies investigating inflammatory response have regarded as valuable HT after AIS, such as neutrophil-lymphocyte ratio (NLR), lymphocyte-monocyte ratio (LMR) and neutrophil-platelet ratio (NPR).9–16 Recently, platelet-to-lymphocyte ratio (PLR) has been reported as a potential biomarker in various diseases, including AIS, carotid artery stenosis, and post-stroke depression.17–20 To our interest, it has been mentioned that high PLR may be associated with poor prognosis after intravenous thrombolysis in AIS.17 However, the relationship between PLR and HT after AIS-LAA remains unclear; we are even unclear about the relationship between PLR and in-hospital mortality of HT after AIS-LAA. Therefore, in our single-center cross-sectional study, we aim to analyse the association between PLR, HT after AIS-LAA and in-hospital mortality of HT after AIS-LAA.
Materials and Methods
Study Population
This retrospective study was based on consecutive AIS-LAA patients at Department of Neurology in Beijing Friendship Hospital, Capital Medical University from February 2018 to March 2019. Patients were included if they met the following criteria: (1) they were diagnosed with AIS within 3 days of onset; (2) they underwent brain CT on admission; and (3) magnetic resonance imaging (MRI), magnetic resonance angiography (MRA) or CT angiography (CTA) within 3 days after admission immediately revealed worsening or abrupt neurological changes within 24 hours. This diagnosis of AIS was made based on World Health Organization criteria.21 Patients with AIS-LAA were classified into four groups: artery-to-artery embolization, in-situ thrombosis, hypoperfusion, and branch athermanous disease39 (Figure 1).
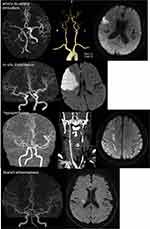 |
Figure 1 Representative cases of the four types of LAA mechanism (diffusion-weighted image and CTA images of the four types of LAA mechanism). |
Patients were excluded if they (1) had hemorrhage detected on initial CT; (2) had acute or chronic infection, systemic immune diseases, liver and kidney diseases, hematological diseases, or cancer; or (3) had received end-vascular thrombectomy (Figure 2).
 |
Figure 2 Study flow diagrams. |
This study was approved by the Ethics Committee of Beijing Friendship Hospital (2021-P2-167-01). This is a retrospective study, and all participants’ information is protected. The study does not involve personal privacy or commercial interests. It is difficult to trace the recruitment and sign informed consent, so ethics committee of Beijing Friendship Hospital agreed to waive informed consent in this clinical trial. All procedures were carried out in accordance with the code of ethics of the 1975 Declaration of Helsinki.
Data Collection
Data were collected on the admission day, containing demographic characteristics (age and sex), cerebrovascular accident risk factors (hypertension, diabetes mellitus, dyslipidemia, coronary artery disease, prior stroke or TIA, smoking, alcohol drinking), admission NIHSS, systolic blood pressure (SBP), diastolic blood pressure (DBP), and treatment in hospital (antiplatelet, anticoagulation, thrombolysis). According to MRI, MRA or CTA within 3 days after admission, we identified AIS-LAA patients who had stenosis greater than 50% in the relevant vessels. Mechanisms of AIS-LAA were classified into four subtypes: artery-to-artery embolization, in-situ thrombosis, hypoperfusion, and branch atheromatous disease. According to the area of the DWI infarction, the infarct volume was calculated using the Pullicino formula: t (mL) = π/6 × L (maximum long axis) × L (short axis) × slice (layer thickness) (cm).40 All laboratory examinations were collected next morning (5:00am) after admission, so we collected laboratory data including white blood cell counts (WBC), neutrophil counts (N), lymphocyte counts (L), platelet counts (P), NLR (calculated as neutrophil-to-lymphocyte ratio), and PLR (calculated as platelet-to-lymphocyte ratio).
HT which was not detected in initial CT or MRI on admission but later presented on the following CT and MRI because of worsened neurologic deterioration emergence in hospitalization was classified into hemorrhagic infarction (HI) and parenchymal hematoma (PH).22
Statistical Analysis
All patients were divided into the HT and non-HT groups. Continuous variables were expressed as means (standard deviation, SD) or medians (interquartile range, IQR). Categorical variables were expressed as percentages. Univariate analysis using the student’s t-test or Mann–Whitney U-test evaluated possible predictors of HT events, while the chi-square test or Fisher’s exact test were applied for proportions. We used the violin plots in Python to show the distribution of PLR among groups in mechanism subtypes of LAA. Receiver operating characteristic (ROC) curves were used to test predictive indicators for HT and to determine the optimal cutoff value at which the total sensitivity and specificity was highest. The area under the ROC curve (AUC) was considered a critical diagnostic index because the prognosis is more accurate when the AUC is larger. Furthermore, multiple logistic regression analysis was adjusted for all potential confounders and assessed whether PLR was associated with incidence of HT after AIS-LAA and in-hospital mortality of HT after AIS-LAA. In the study, variables with P<0.05 were considered statistically significant and all statistical analyses were performed using SPSS version 23 (IBM SPSS, Chicago, II, USA).
Results
Patient Characteristics
A total of 38 HT patients and 290 non-HT patients after AIS-LAA were admitted to the Neurology Department of Beijing Friendship Hospital between February 2018 and March 2019. The basic demographic and clinical characteristics and laboratory tests of the enrolled patients are shown in Table 1. Patients in the HT group were older (68.4±11.2 vs 67.0±11.0, P=0.572), contained a smaller proportion of males (68.4% vs 70.7%, P=0.774), had more severe admission NIHSS scores (6[4–8] vs 4[2–10], P=0.187), higher systolic blood pressure (mmHg) (150.9±16.7 vs 142.2±18.0, P=0.005) and higher diastolic blood pressure (mmHg) (85.5±11.2 vs 83.3±13.5, P=0.058), and infarct volume (mL) (9.6[6.8–12.2] vs 8.4[6.4–10.6], P=0.202); more patients in the HT group received anticoagulation (15.8% vs 5.2%, P=0.012) and thrombolysis (13.2% vs 4.5%, P=0.027) treatment compared with those in the non-HT group. Compared to the patients in the non-HT group, the patients in the HT group possessed a significantly higher white blood cell level (109/L)(7.52[6.00–10.01] vs 6.87[5.60–7.99], P=0.021), a significantly higher neutrophil count level (109/L)(5.20[3.58–8.61] vs 4.39[3.50–5.85], P=0.017), and a significantly lower ymphocyte count level (109/L)(1.45[1.18–1.78] vs 1.76[1.35–2.18], P=0.004). Both PLR (153.11[119.96–250.65] vs 118.77[88.96–150.36], P<0.001) and NLR (3.86[2.33–6.61] vs 2.56[2.05–3.71], P<0.001) were significantly higher in the patients with HT than those with non-HT. Figure 3 shows the violin plots of NLR and PLR among groups in mechanism subtypes of LAA (artery-to-artery embolization, in-situ thrombosis and branch atheromatous disease); we do not show hypoperfusion because of limited sample size. Both NLR and PLR in each subtype of LAA among groups were significantly different (P<0.005).
 |
Table 1 Baseline Characteristics of Patients with and without HT |
HT patients are divided into PH group and HI group. Table 2 shows the baseline data of HT patients. There is no difference in age, sex, admission NIHSS, infarct volume, systolic blood pressure, NLR and PLR between the two groups.
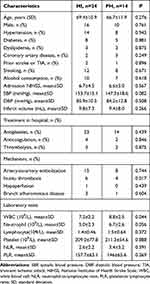 |
Table 2 Baseline Data of PH and HI |
PLR and HT
In binary logistic regression analysis, sex (aOR 0.594, 95% CI 0.262–1.343, P=0.210), age (aOR 1.008, 95% CI 0.979–1.038, P=0.592), systolic blood pressure (aOR 1.025, 95% CI 1.006–1.044, P=0.010), white blood cell (aOR 1.228, 95% CI1.000–1.456, P=0.050), neutrophil (aOR 1.162, 95% CI 1.038–1.386, P=0.016), lymphocyte (aOR 0.330, 95% CI 0.158–0.694, P=0.008), platelet (aOR 0.550, 95% CI 0.233–1.298, P=0.183), NLR (aOR 1.356, 95% CI 1.179–1.562, P<0.001) and PLR (aOR 3.872, 95% CI 2.237–5.706, P<0.001) were independently associated with HT after adjustment for possible confounders (Table 3).
 |
Table 3 Multivariate Analysis for the Association Between Indicators and HT |
The ROC curves predicting the value of NLR and PLR for HT after AIS-LAA are presented in Figure 4. The area under the curve (AUC) value of PLR (0.72, 95% CI (0.64–0.80), P<0.001) showed a greater discriminatory ability compared with NLR (0.67, 95% CI (0.58–0.76), P<0.001). The optimal cutoff value of PLR was 140.15, with a sensitivity of 66.9% and a specificity of 68%, but the optimal cutoff value of NLR was 3.28, with a sensitivity of 48.8% and a specificity of 75.5%. The binary logistic analysis, with PLR as a dichotomous variable, PLR≥140.15 (OR 4.104, 95% CI (2.049–8.219), P<0.001; aOR 4.721, 95% CI (2.204–9.110), P<0.001) had a significant association with HT after AIS-LAA (Table 4).
 |
Table 4 Univariate and Multivariate Analysis for the Relationship Between NLR, PLR and HT, with NLR and PLR Dichotomized by the Optimal Cutoff Value in ROC Curves |
We performed univariate and multivariate analysis to identify whether an association existed between PLR and the risk of HT after AIS-LAA in different LAA mechanism subtypes. Table 5 summarizes univariate and multivariate analysis for the association between PLR and HT in each LAA subtype. PLR was observed to be a predictor of HT in AIS with artery-to-artery embolization (OR 1.632, 95% CI (1.207–2.940), P=0.016; aOR 1.702, 95% CI (1.300–3.218), P=0.024), in-situ thrombosis (OR 3.178, 95% CI (1.244–8.114), P<0.001; aOR 4.502, 95% CI (1.346–9.056), P<0.001) and branch atheromatous disease (OR 3.452, 95% CI (1.123–8.652), P<0.001; aOR3.240, 95% CI (1.099–8.357), P<0.001) (Table 5).
 |
Table 5 Univariate and Multivariate Analysis for the Association Between PLR and HT in Each Stroke with LAA Subtypes |
PLR and In-Hospital Mortality of HT After AIS-LAA
The difference between all-cause in-hospital mortality rates is statistically significant (p=0.012) in the two groups: 13.16% (n=5/38) of patients with HT versus 3.79% (n=11/290) of patients with non-HT. The causes of death were neurological in 5 patients in HT, including herniation of the brain in 2 (5.26%) and recurrent cerebral ischemia in 3 (7.89%). The cause of death in the non-HT group was as follows: 3 patients died from cerebral ischemia (1.03%), 4 patients died from pulmonary complications (1.38%), and 4 patients died from cardiovascular issues (1.38%). Compared to the non-in-hospital mortality patients in the HT group, patients who died in hospital in the HT group possessed significantly higher levels of PLR (191.72 [114.82–275.08] vs 126.77[109.47–144.07], P<0.001). Univariate and multivariate analysis showed that increased PLR predicts high in-hospital mortality of HT after AIS-LAA (OR 1.041, 95% CI (1.006–1.077), P=0.020; aOR 1.055, 95% CI (1.007–1.106), P=0.033) (multivariate analysis was adjusted for age, sex, systolic blood pressure, infarct volume, anticoagulation, and thrombolysis).
Discussion
To the best of our knowledge, PLR has not previously been assessed in HT after AIS. This is the first study to explore the association between PLR and HT in AIS-LAA, including in patients with artery-to-artery embolization, in-situ thrombosis and branch atheromatous disease, and increased PLR predicts high in-hospital mortality of HT in AIS-LAA. The present study indicated that PLR was associated with an increased risk of HT after AIS-LAA. It was reported that an increased NLR at admission had a direct relationship with HT in AIS.11–16 It was also reported that systemic immune-inflammation index (SII), based on peripheral neutrophil, lymphocyte, and platelet counts, was associated with hemorrhagic transformation in anterior circulation AIS patient with large-artery atherosclerosis.41 However, as documented in our study, we found that PLR had more clinical utility and more accuracy for HT in AIS-LAA patients than did NLR.
As we all know, AIS with cardio-embolism accounts for approximately 25% of HT after AIS in white people, but LAA is a major contributor to HT after AIS in Chinese people.38 In recent years, numerous studies have drawn more attention to inflammatory mechanisms in the pathogenesis of AIS.24 PLR calculated by platelet count to lymphocyte count ratio has been reported as an effective predictor for severe atherosclerosis.23 Both platelets and lymphocytes are predictors of prognosis in myocardial infarction and cerebral infarction.42,43 HT was attributed to blood–brain barrier permeability, hemostatic dysfunction, oxidative stress, inflammation, and immunologic disorder.5
Why is PLR level related to risk of HT? The roles of platelet and lymphocytes in AIS and damage to the blood–brain barrier (BBB) may be the underlying mechanisms.25 There is an autopsy study demonstrating that up-regulation of leukocytes and depletion of lymphopenia in 6 to 12 hours perfused cerebral infarction regions, which is associated with breakdown of BBB, endothelial activation, and complex neurohormonal responses.26–29 Increased marix metalloproteinase-9 (MMP-9) derived from peripheral leukocytes/neutrophils at 6–8 hours after AIS degrade components of BBB and ultimately result in HT.30,31 Lymphocytes, as a neuroprotective effector, contribute to neurological function improvement.32 Ischemic stroke induces lymphopenia by mechanisms involving complex neurohormonal responses and facilitates HT.33 AIS may lead to platelet function abnormality, and activated and hyper-responsive platelets may interact with platelet-binding T lymphocyte cells to produce cytokines, interferons, chemokines and altering adhesion molecules, and result in hampering AIS recovery.34–37 Moreover, some factors released after AIS, including reactive oxygen species, cathepsin G, protease, myeloperoxidase, elastase, chemokines and cytokines, may also elevate permeability of BBB and risk of HT.4
A number of studies have indicated that PLR is an independent predictor of the incidence and mortality of in-hospital and long-term major adverse cardiovascular events in patients with acute myocardial infarction.44,45 PLR is a combination of platelets and lymphocytes which provide the pathogenesis of AIS. Elevated level of PLR is associated with worse outcomes after intravenous thrombolysis in AIS, according to the study of Xu et al.17 The finding of our research has provided new support for the role of PLR in HT after AIS-LAA.
Higher PLR on admission may indicate HT after AIS-LAA. In our study, we found PLR was an independent predictor of HT, and the risk of HT increased 4.719-fold when PLR 140.15. PLR takes more clinical weight of parameters into consideration than NLR. Among the mechanism subtypes of LAA, HT after AIS patients with artery-to-artery embolization, in-situ thrombosis or branch atheromatous disease had higher PLR levels. We considered that PLR is a sensitive indicator of HT after AIS-LAA. Meanwhile, artery-to-artery embolization, in-situ thrombosis and branch atheromatous disease were independent risk factors for HT after AIS.
140.15. PLR takes more clinical weight of parameters into consideration than NLR. Among the mechanism subtypes of LAA, HT after AIS patients with artery-to-artery embolization, in-situ thrombosis or branch atheromatous disease had higher PLR levels. We considered that PLR is a sensitive indicator of HT after AIS-LAA. Meanwhile, artery-to-artery embolization, in-situ thrombosis and branch atheromatous disease were independent risk factors for HT after AIS.
There is a trend that in-hospital mortality in HT after AIS-LAA is significantly higher than that in AIS-LAA, and the causes of recurrent cerebral ischemia in the HT group stop anti-thrombotic treatment. We also identified that increased PLR was an independent predictor of high in-hospital mortality in HT after AIS-LAA patients. Thus, the PLR plays multiple roles in patients with AIS-LAA as a predictor of HT and as a predictor of in-hospital mortality of HT after AIS-LAA.
Our study suggests that the PLR is a sensitive biomarker for HT after AIS-LAA, and predicts in-hospital mortality in patients with HT after AIS-LAA. However, there are several limitations in our study. First, the single-center retrospective observation study leads to selection bias because of limited sample size. In our study, we could not analyse the relationship between PLR and HT in the subgroup with hypoperfusion because of low case numbers. Meanwhile, patients in the HT group were older, contained a smaller proportion of males, had more severe admission NIHSS scores but showed no significant differences. We should enlarge the patient sample size to test this trend. Second, we need to conduct a follow-up study to confirm the relationship between PLR and HT. Third, our further study needs to observe the dynamic relationship of PLR in HT after AIS.
Conclusion
In conclusion, high PLR is associated with greater risk of HT in Chinese patients with AIS-LAA, including in artery-to-artery embolization, in-situ thrombosis or branch atheromatous disease, and increased PLR predicts high in-hospital mortality in patients with HT after AIS-LAA. However, further investigation is needed into dynamic changes of PLR on the risk of HT after AIS-LAA in a larger cohort.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Adams HP
2. Rovira A, Grive E, Rovira A, Alvarez-Sabin J. Distribution territories and causative mechanisms of ischemic stroke. Eur Radiol. 2005;15:416–426.
3. Von Kummer R, Broderick JP, Campbell BCV, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46(10):2981–2986. doi:10.1161/STROKEAHA.115.010049
4. Jickling GC, Liu D, Stamova B, et al. Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab. 2014;34:185–199. doi:10.1038/jcbfm.2013.203
5. Lu GF, He QW, Shen Y, Cao F. Potential biomarkers for predicting hemorrhagic transformation of ischemic stroke. Int J Neurosci. 2018;128:179–189. doi:10.1080/00207454.2017.1349766
6. Lee SJ, Lee DG. Distribution of atherosclerotic stenosis determining early neurologic deterioration in acute ischemic stroke. PloS One. 2017;12(9):e0185314. doi:10.1371/journal.pone.0185314
7. Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124(2):315–327. doi:10.1161/CIRCRESAHA.118.313591
8. Schaftenaar F, Frodermann V, Kuiper J, Lutgens E. Atherosclerosis: the interplay between lipids and immune cells. Curr Opin Lipidol. 2016;27(3):209–215.
9. Song QH, Pan RS, Jin YX, et al. Lymphocyte-to-monocyte ratio and risk of hemorrhagic transformation in patients with acute ischemic stroke. Neurol Sci. 2020;41(9):2511–2520. doi:10.1007/s10072-020-04355-z
10. He WW, Ruan YT, Yuan CX, et al. High neutrophil-to-platelet ratio is associated with hemorrhagic transformation in patients with acute ischemic stroke. Front Neurol. 2019;10:1310. doi:10.3389/fneur.2019.01310
11. Zhang WB, Zeng YY, Wang F, Cheng L, Tang WJ, Wang XQ. A high neutrophil-to-lymphocyte ratio predicts hemorrhagic transformation of large atherosclerotic infarction in patients with acute ischemic stroke. Aging. 2020;12(3):2428–2439. doi:10.18632/aging.102752
12. Zhang RR, Wu XD, Hu WJ, et al. Neutrophil‐to‐lymphocyte ratio predicts hemorrhagic transformation in ischemic stroke: a meta‐analysis. Brain Behav. 2019;9(9):e01382. doi:10.1002/brb3.1382
13. Song QH, Li YS, Wang YN, Wei CC, Liu JF, Liu M. Increased neutrophil-to-lymphocyte ratios are associated with greater risk of hemorrhagic transformation in patients with acute ischemic stroke. Curr Neurovasc Res. 2018;15(4):326–335. doi:10.2174/1567202616666181204122457
14. Gong PY, Xie Y, Jiang T, et al. Neutrophil-lymphocyte ratio predicts post-thrombolysis early neurological deterioration in acute ischemic stroke patients. Brain Behav. 2019;9(10):e01426. doi:10.1002/brb3.1426
15. Duan ZH, Wang HM, Wang Z, et al. Neutrophil-lymphocyte ratio predicts functional and safety outcomes after endovascular treatment for acute ischemic stroke. Cerebrovasc Dis. 2018;45(5–6):221–227. doi:10.1159/000489401
16. Brooks SD, Spears C, Cummings C, et al. Admission neutrophil‐lymphocyte ratio predicts 90 day outcome after endovascular stroke therapy. J Neurointerv Surg. 2014;6(8):578–583. doi:10.1136/neurintsurg-2013-010780
17. Xu JH, He XW, Li Q, et al. Higher platelet-to-lymphocyte ratio is associated with worse outcomes after intravenous thrombolysis in acute ischaemic stroke. Front Neurol. 2019;10:1192. doi:10.3389/fneur.2019.01192
18. Maimaiti A, Li Y, Wang YT, et al. Association of platelet-to-lymphocyte count ratio with myocardial reperfusion and major adverse events in patients with acute myocardial infarction: a two-center retrospective cohort study. BMJ Open. 2019;9(9):e025628. doi:10.1136/bmjopen-2018-025628
19. Zhang YF, Jiang L, Yang P, Zhang YZ. Comparison of lymphocyte count, neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in predicting the severity and the clinical outcomes of acute cerebral infarction patients. Clin Lab. 2019;65(7). doi:10.7754/Clin.Lab.2019.190102
20. Huang GQ, Chen HJ, Wang QZ, et al. High platelet-to-lymphocyte ratio are associated with post-stroke depression. J Affect Disord. 2019;246:105–111. doi:10.1016/j.jad.2018.12.012
21. Hatano S. Experience from a multicenter stroke register: a preliminary report. Bull World Health Organ. 1976;54(5):541–553.
22. de Los Rios la Rosa F, Khoury J, Kissela BM, et al. Eligibility for intravenous recombinant tissue-type plasminogen activator within a population: the effect of the European Cooperative Acute Stroke Study (ECASS) III trial. Stroke. 2012;43(6):1591–1595. doi:10.1161/STROKEAHA.111.645986
23. Yuksel M, Yildiz A, Oylumlu M, et al. The association between platelet/lymphocyte ratio and coronary artery disease severity. Anatol J Cardiol. 2015;15:640–647. doi:10.5152/akd.2014.5565
24. Rust R, Grönnert L, Schwab ME. Inflammation after stroke: a local rather than systemic response? Trends Neurosci. 2018;41(12):877–879. doi:10.1016/j.tins.2018.09.011
25. Xu XR, Zhang D, Oswald BE, et al. Platelets are versatile cells: new discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond. Crit Rev Clin Lab Sci. 2016;53:409–430. doi:10.1080/10408363.2016.1200008
26. Mercuri M, Cinffetti G, Lombardini R, Neri C, Robinson M. Do leukocytes have a role in the cerebrovascular no-reflow phenomenon? J Neurol Neurosurg Psychiatry. 1990;53(6):536.
27. Arumugam TV, Granger DN, Mattson MP. Stroke and T-cells. Neuromolecular Med. 2015;7(3):229–242. doi:10.1385/NMM:7:3:229
28. Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol. 2006;66(3):232–245.
29. Perera MN, Ma HK, Arakawa S, et al. Inflammation following stroke. J Clin Neurosci. 2006;13(1):1–8. doi:10.1016/j.jocn.2005.07.005
30. Jin R, Yang G, Li G. Molecular insights and therapeutic targets for blood-brain barrier disruption in ischemic stroke: critical role of matrix metalloproteinases and tissue-type plasminogen activator. Neurobiol Dis. 2010;38:376–385. doi:10.1016/j.nbd.2010.03.008
31. Turner RJ, Sharp FR. Implications of MMP9 for blood brain barrier disruption and hemorrhagic transformation following ischemic stroke. Front Cell Neurosci. 2016;10:56. doi:10.3389/fncel.2016.00056
32. Macrez R, Ali C, Toutirais O, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. 2011;10(5):471–480. doi:10.1016/S1474-4422(11)70066-7
33. Salas-Perdomo A, Miró-Mur F, Urra X, et al. T cells prevent hemorrhagic transformation in ischemic stroke by P-selectin binding. Arterioscler Thromb Vasc Biol. 2018;38(8):1761–1771. doi:10.1161/ATVBAHA.118.311284
34. Yip HK, Chen SS, Liu JS, et al. Serial changes in platelet activation in patients after ischemic stroke: role of pharmacodynamic modulation. Stroke. 2004;35(7):1683–1687. doi:10.1161/01.STR.0000131658.80951.c3
35. Fateh-Moghadam S, Htun P, Tomandl B, et al. Hyperresponsiveness of platelets in ischemic stroke. Thromb Haemost. 2007;97(6):974–978. doi:10.1160/TH06-12-0725
36. Marquardt L, Ruf A, Mansmann U, et al. Course of platelet activation markers after ischemic stroke. Stroke. 2002;33(11):2570–2574. doi:10.1161/01.STR.0000034398.34938.20
37. Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113(17):2105–2112. doi:10.1161/CIRCULATIONAHA.105.593046
38. Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008;39:2396–2399. doi:10.1161/STROKEAHA.107.505776
39. Bang OY. Intracranial atherosclerosis: current understanding and perspectives. J Stroke. 2014;16(1):27. doi:10.5853/jos.2014.16.1.27
40. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9(7):689–701. doi:10.1016/S1474-4422(10)70104-6
41. Yang Y, Han YF, Sun WD, Zhang YB. Increased systemic immune-inflammation index predicts hemorrhagic transformation in anterior circulation acute ischemic stroke due to large-artery atherosclerotic. Int J Neurosci. 2021;Jul 22:1–7. doi:10.1080/00207454.2021.1953021
42. Yang M, Pan Y, Li Z, et al. Platelet count predicts adverse clinical outcomes after ischemic stroke or TIA: subgroup analysis of CNSR II. Front Neurol. 2019;10:370. doi:10.3389/fneur.2019.00370
43. Zierath D, Tanzi P, Shibata D, Becker KJ. Cortisol is more important than metanephrines in driving changes in leukocyte counts after stroke. J Stroke Cerebrovasc Dis. 2018;27:555–562. doi:10.1016/j.jstrokecerebrovasdis.2017.09.048
44. Cetin EHO, Cetin MS, Aras D, et al. Platelet to lymphocyte ratio as a prognostic marker of in-hospital and long term major adverse cardiovascular events in ST-segment elevation myocardial infarction. Angiology. 2016;67:336–345. doi:10.1177/0003319715591751
45. Bodi V, Sanchis J, Nunez J, et al. Post-reperfusion lymphopenia and microvascular obstruction in ST-segment elevation acute myocardial infarction. Rev Esp Cardiol. 2009;62:1109–1117.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


