Back to Journals » Cancer Management and Research » Volume 15
Increased Platelet Distribution Width Predicts 3-Year Recurrence in Patients with Hepatocellular Carcinoma After Surgical Resection
Authors Li H, Liu J, Yan S, Rao C, Wang L
Received 3 March 2023
Accepted for publication 2 June 2023
Published 14 June 2023 Volume 2023:15 Pages 501—509
DOI https://doi.org/10.2147/CMAR.S408548
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Huiming Li,1 Jun Liu,1 Shaoying Yan,1 Chunmei Rao,2 Ling Wang3
1Department of Laboratory Medicine, The First Affiliated Hospital of Nanchang University, Nanchang, 330006, People’s Republic of China; 2Department of Laboratory Medicine, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai, 200438, People’s Republic of China; 3Department of Nuclear Medicine, The First Affiliated Hospital of Nanchang University, Nanchang, 330006, People’s Republic of China
Correspondence: Ling Wang, Email [email protected]
Background: Platelet distribution width (PDW) is a marker of platelet anisocytosis that increases with platelet activation. The clinical implications of PDW in HCC are not well-defined. This study aimed to determine whether PDW could predict recurrence in patients with HCC after resection.
Methods: Between January and December 2008, 471 patients with HCC were recruited retrospectively. The clinicopathological characteristics of patients with HCC were analyzed based on the relationship between the two PDW groups. Kaplan-Meier curves and multivariate Cox regression analyses were used to evaluate the relationship between PDW and disease-free survival (DFS). A novel nomogram was developed based on the identified independent risk factors. Its accuracy was evaluated using a calibration curve and concordance index. The predictive value was evaluated using a receiver operating characteristic (ROC) curve.
Results: PDW was significantly associated with direct bilirubin, total bilirubin, urea, and prothrombin time. Patients with PDW ≥ 17.1 were a significantly shorter DFS than those with PDW < 17.1 (17.98% vs 49.83%, p< 0.001). Multivariate analysis determined that alpha-fetoprotein (AFP), carcinoembryonic antigen, microvascular invasion (MVI), tumor size, and tumor number were the independent variables associated with DFS. Patients with PDW ≥ 17.1 had a hazard ratio of 1.381 (95% confidence interval: 1.069– 1.783, p = 0.014) for DFS. AFP, PDW, MVI, tumor size, and tumor number were identified as preoperative independent risk factors for DFS and used to establish the nomogram. Calibration curve analysis revealed that the standard curve fitted well with the predicted curve. ROC curve analysis demonstrated the high efficiency of the nomogram.
Conclusion: Increased PDW may predict recurrence-free survival in patients with HCC. Our nomogram model also performed well in predicting patient prognoses.
Keywords: hepatocellular carcinoma, platelet distribution width, recurrence, nomogram
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy and third most common cause of cancer-related deaths globally.1,2 Although reasonable treatment methods have been developed, the prognosis of patients with HCC remains poor. HCC is a highly invasive tumor with frequent intrahepatic and distant metastases, which is the main reason for the high 3-year recurrence and poor 5-year survival rate of HCC after surgical resection.3,4 Thus, it is critical to identify new biomarkers to assist in early warning of postoperative metastasis of HCC.
Platelet distribution width (PDW) signifies the size distribution of platelets, which is a marker of platelet anisocytosis that increases with platelet activation.5,6 The interaction of tumor cells with platelets is a prerequisite for successful hematogenous metastatic dissemination.7 Aberrant platelet activation or aggregation frequently occurs in the vasculature of patients with cancer, especially those with metastatic tumors.8 Platelets within the tumor microenvironment can regulate cancer cell survival and hematogenous metastasis.9 Platelets have also been involved in mechanisms leading to carcinogenesis, tumor growth, and tumor angiogenesis and in the outcome of therapy.10 Elevated PDW levels are associated with poor prognosis in various tumors, such as gastric cancer, breast cancer, and non-small cell lung cancer.11–13 However, the clinical implications of PDW in HCC have not been well defined. This study aimed to determine whether PDW could predict recurrence in patients with HCC after resection.
Patients and Methods
Patients
The hospital records of all patients who had a preoperative diagnosis of HCC and underwent hepatic resection at the Department of Liver Surgery, Eastern Hepatobiliary Surgery Hospital, China, between January 2008 and December 2008 were reviewed. The present study is a retrospective study and was reviewed and approved by the Institutional Ethics Review Board of Eastern Hepatobiliary Surgery Hospital.
Patient data, including age, sex, hepatitis B surface antigen (HBsAg), liver cirrhosis, tumor size, tumor number, Child-Pugh grade, and microvascular invasion (MVI), were collected from medical records. Blood test information was obtained from the Department of Clinical Laboratory of the Eastern Hepatobiliary Surgery Hospital. Laboratory blood tests including HBsAg, serum alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), carbohydrate antigen 19-9, serum albumin (ALB), serum total bilirubin (TBIL), alanine aminotransferase (ALT), Prealbumin (PA) and prothrombin time (PT) were performed before hepatic resection.
Follow-Up
All patients were followed up with monthly computed tomography (CT) or magnetic resonance imaging (MRI) scans for 1 year. CT or MRI was performed once every 6 months thereafter. Tumor recurrence was suspected when there was a progressive elevation of serum AFP levels and when diagnosed by dynamic CT scan or MRI.
Statistical Analysis
Data are expressed as the mean ± standard deviation or median (range). Statistical analyses were performed using the Statistical Program software (version 22.0; IBM Corp., Armonk, NY, USA). The cut-off value of PDW was determined using a receiver operating characteristic (ROC) curve with the best accuracy (the greatest sensitivity and specificity) using MedCalc version 15.2.2. Quantitative values were compared using Student’s t-test or Mann–Whitney nonparametric U-test. Categorical variables were tested using the Chi-square or Fisher’s exact test. Cumulative recurrence rates were calculated using the Kaplan-Meier method, and differences were compared using the Log rank test. Univariate and multivariate analyses were performed using the Cox proportional hazard regression model. A nomogram was formulated based on the results of multivariate logistic regression analysis using the rms package of R, version 4.2.1 (http://www.r-project.org/). The predictive performance of the nomogram was measured using the concordance index (C-index) and calibration with 1000 bootstrap samples to decrease overfitting bias. Statistical significance was defined as a p value of < 0.05.
Results
In total, 471 patients were included in the study cohort. The mean age was 52.4 ± 11.3 years. Four hundred and two (85.2%) patients were male and 70 (14.8%) were female.
According to the ROC curve analysis, we determined that the optimal cut-off value of 17.1 for PDW, and the sensitivity and specificity for the diagnosis of HCC recurrence 3 years after operation were 47.0% and 84.6%, respectively (area under the curve = 0.681, 95% confidence interval [CI]: 0.637–0.723, p < 0.001) (Figure 1). Therefore, all patients were divided into two groups: patients with PDW < 17.1 and patients with PDW ≥ 17.1. As shown in Table 1, there were 293 (62%) patients in the group with PDW < 17.1 and 178 (38%) patients with PDW ≥ 17.1. The current results showed that PDW was significantly associated with direct bilirubin, platelet count, PT, TBIL, Crea, and MVI. The low platelet count and proportion of MVI were higher in the PDW > 17.1 group, and the proportion of MVI was also higher. Nevertheless, there were no significant differences between the groups in terms of sex, age, AFP, ALB, tumor size, and HBsAg.
 |
Table 1 Baseline Characteristics of HCC Patients in PDW Group |
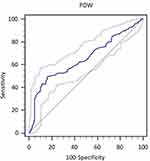 |
Figure 1 The ROC curve of HCC recurrence diagnosed by PDW. |
After a follow-up of 36 months, 293 (62.21%) patients experienced recurrence. Patients with PDW ≥ 17.1 had significantly shorter disease-free survival (DFS) than those with PDW < 17.1 (17.98% vs 49.83%, p < 0.001). The Kaplan-Meier curves of normal versus elevated PDW showed significant separation (Figure 2).
 |
Figure 2 The correlation between PDW levels and disease free survival in patients with HCC. The cumulative recurrence was significantly higher in patients with PDW≥17.1 groups. |
Cox univariate and multivariate analyses were used to analyze the risk factors for the postoperative recurrence of HCC. AFP, CEA, PDW, tumor size, PA, tumor number, MVI, and cirrhosis were identified as significant factors with p value < 0.10 in univariate analysis. These variables were then included in the multivariate analysis. Multivariate analysis determined that AFP, CEA, PDW, MVI, tumor size, and tumor number were the independent variables associated with DFS. Interestingly, patients with PDW ≥ 17.1 had a hazard ratio of 1.381 (95% CI: 1.069–1.783, p = 0.014] for DFS (Table 2).
 |
Table 2 The Univariate and Multivariate Analysis of Disease-Free Survival in HCC Patients |
AFP, PDW, MVI, tumor size, and tumor number were identified as independent risk factors in multivariate analysis (Table 2). We created a nomogram model to precisely predict the 3-year recurrence risk using these factors (Figure 3). In the nomogram, each predictor had a parallel score within the range of 0–100 for its contribution to 3-year recurrence, which could be used to predict the risk of 3-year recurrence. The total points for the nomogram were calculated by summing the points for each factor, and the probability of 3-year recurrence was estimated from the total number of points. The resulting model was internally validated using bootstrap validation. The nomogram demonstrated good accuracy in estimating the risk of 3-year recurrence risk, with an unadjusted C-index of 0.663 (Figure 4). In addition, calibration plots showed good agreement on recurrence after surgical resection between the risk estimation by the nomogram and the follow-up results. The predictive model of the nomogram had good predictive ability.
 |
Figure 3 Nomogram predicting 3-year recurrence in HCC after surgery. |
Discussion
The relationship between platelet and tumor metastasis has been widely studied. However, little is known regarding the role of platelet in HCC with tumor cell growth and metastasis, and in the present study, we revealed that increased PDW was associated with MVI. MVI is an important risk factor for recurrence and poor prognosis in HCC.14
In addition, elevated PDW was an independent indicator of DFS in patients with HCC. AFP, PDW, MVI, tumor size, and tumor number were identified as having good predictive abilities in the nomogram model.
Increased platelet count is associated with poor prognosis in patients with a wide spectrum of malignancies, such as pancreatic, gastric, colorectal, endometrial, and ovarian cancers. Several reports have mentioned that PDW is an indicator of platelet activation.5,6
Recently, several studies have investigated the relationship between activated platelets and cancer metastasis. Li et al enriched nanospheres in primary tumors, lungs, and metastatic tumors using a P-selectin-targeted peptide modified to capture activated platelets, which effectively inhibited almost every key and continuous step of the metastasis cascade, delaying the progress of epithelial-mesenchymal transformation (EMT) in the tumor.15 Xu et al designed tumor microenvironment-responsive nitric oxide-releasing nanocarriers, which could inhibit the interaction of platelets and tumor cells, successfully blocking tumor-specific platelet functions, suppressing the EMT process, preventing the adhesion of platelets around CTCs, and reducing distant metastasis.16 These studies partially demonstrated a relationship between activated platelets and tumor metastasis.
Our study was a retrospective clinical study and did not investigate the specific mechanism of activated platelets in tumor metastasis. However, little is known regarding the transfer of platelets and HCC. There is no doubt that the role of serotonin in the interaction between platelets and tumor cells should be affirmed. Platelets significantly support tumor cell migration by binding the adhesion receptors GPIIb/IIIa, GPIb-IX-V, and p-selectin, thus protecting them from immunosurveillance and shear forces of the blood flow and supporting their blockage against the vascular wall.17 Serotonin is strongly enhanced in HCC, and most of it circulates in the blood and is transported by platelet-dense granules. In vitro experiments have shown that serotonin induces the proliferation of three different HCC cell lines, while inhibiting the serotonin signaling pathway inhibits the growth of two tumor mouse models.18,19 Transforming growth factor-beta (TGFβ) from platelet origin has been found to induce TGFβ/Smad and NF-κB synergistic signaling cascades in tumor cells, promoting epithelial-mesenchymal conversion to pre-metastatic phenotypes and allowing extravasation and metastasis.20
This study had several limitations. First, the mechanisms underlying the involvement of PDW in HCC should be clarified. Second, internal and external validation should be included in our research. Finally, the patients were from a single center. Multicenter prospective studies with larger sample sizes are required to verify our results.
In conclusion, increased PDW may predict recurrence-free survival in patients with HCC. Our nomogram model also performed well in predicting patient prognoses.
Abbreviations
HCC, Hepatocellular carcinoma; PDW, Platelet distribution width; HBsAg, hepatitis B surface antigen; AFP, alphafetoprotein; ALB, albumin; ALT, alanine aminotransferase; CA 19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; TBIL, total bilirubin; DBIL, Direct bilirubin; HBsAg, Hepatitis B surface antigen; PA, prealbumin; PT, prothrombin time; MVI, microvascular invasion.
Ethics Approval and Informed Consent
This study was ethically approved by the institutional review board of Eastern Hepatobiliary Surgery Hospital. To protect patient privacy, all data was anonymous, the requirement for informed consent was waived. This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
This study was supported by Health Committee of Jiangxi Province (Nos. 202130329), Key projects of Jiangxi Provincial Department of Education (Nos. GJJ210135); Jiangxi Traditional Chinese Medicine Science and Technology Plan Project (2022B921); Jiangxi Traditional Chinese Medicine Science and Technology Plan Project (2022B966);
Disclosure
All authors declare no conflict of interest.
References
1. Erstad D, Tanabe K. Prognostic and therapeutic implications of microvascular invasion in hepatocellular carcinoma. Ann Surg Oncol. 2019;26(5):1474–1493. doi:10.1245/s10434-019-07227-9
2. He Q, Lin Z, Wang Z, et al. SIX4 promotes hepatocellular carcinoma metastasis through upregulating YAP1 and c-MET. Oncogene. 2020;39(50):7279–7295. doi:10.1038/s41388-020-01500-y
3. Yang J, Hainaut P, Gores G, Amadou A, Plymoth A, Roberts L. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi:10.1038/s41575-019-0186-y
4. Xu T, Ren L, Liao M, et al. Preoperative radiomics analysis of contrast-enhanced CT for microvascular invasion and prognosis stratification in hepatocellular carcinoma. J Hepatocell Carcinoma. 2022;9:189–201. doi:10.2147/JHC.S356573
5. Zuo X, Kong W, Feng L, Zhang H, Meng X, Chen W. Elevated platelet distribution width predicts poor prognosis in hepatocellular carcinoma. Cancer Biomark. 2019;24(3):307–313. doi:10.3233/CBM-182076
6. Osselaer JC, Jamart J, Scheiff JM. Platelet distribution width for differential diagnosis of thrombocytosis. Clin Chem. 1997;43(6 Pt 1):1072–1076. doi:10.1093/clinchem/43.6.1072
7. Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol. 2018;11(1):125. doi:10.1186/s13045-018-0669-2
8. Mezouar S, Frère C, Darbousset R, et al. Role of platelets in cancer and cancer-associated thrombosis: experimental and clinical evidences. Thromb Res. 2016;139:65–76. doi:10.1016/j.thromres.2016.01.006
9. Zhuang M, Xin G, Wei Z, et al. Dihydrodiosgenin inhibits endothelial cell-derived factor VIII and platelet-mediated hepatocellular carcinoma metastasis. Cancer Manag Res. 2019;11:4871–4882. doi:10.2147/CMAR.S202225
10. Santilli F, Boccatonda A, Davì G. Aspirin, platelets, and cancer: the point of view of the internist. Eur J Intern Med. 2016;34:11–20. doi:10.1016/j.ejim.2016.06.004
11. Cui M, Li N, Liu X, et al. Platelet distribution width correlates with prognosis of non-small cell lung cancer. Sci Rep. 2017;7(1):3456. doi:10.1038/s41598-017-03772-z
12. Li N, Diao Z, Huang X, et al. Increased platelet distribution width predicts poor prognosis in melanoma patients. Sci Rep. 2017;7(1):2970. doi:10.1038/s41598-017-03212-y
13. Huang Y, Cui M, Huang Y, et al. Preoperative platelet distribution width predicts breast cancer survival. Cancer Biomark. 2018;23(2):205–211. doi:10.3233/CBM-181267
14. Krishnan M, Rajan Kd A, Park J, et al. Genomic analysis of vascular invasion in HCC reveals molecular drivers and predictive biomarkers. Hepatology. 2021;73(6):2342–2360. doi:10.1002/hep.31614
15. Li S, Li L, Lin X, Chen C, Luo C, Huang Y. Targeted inhibition of tumor inflammation and tumor-platelet crosstalk by nanoparticle-mediated drug delivery mitigates cancer metastasis. ACS Nano. 2021;16(1):50–67.
16. Xu Y, Liu J, Liu Z, et al. Blockade of platelets using tumor-specific NO-releasing nanoparticles prevents tumor metastasis and reverses tumor immunosuppression. ACS Nano. 2020;14(8):9780–9795. doi:10.1021/acsnano.0c01687
17. Lavergne M, Janus-Bell E, Schaff M, Gachet C, Mangin PJC. Platelet integrins in tumor metastasis: do they represent a therapeutic target? Cancers. 2017;9(10). doi:10.3390/cancers9100133
18. Herr N, Bode C, Duerschmied D. The effects of serotonin in immune cells. Front Cardiovasc Med. 2017;4:48. doi:10.3389/fcvm.2017.00048
19. Liang C, Chen W, Zhi X, et al. Serotonin promotes the proliferation of serum-deprived hepatocellular carcinoma cells via upregulation of FOXO3a. Mol Cancer. 2013;12(1):14. doi:10.1186/1476-4598-12-14
20. He AD, Xie W, Song W, et al. Platelet releasates promote the proliferation of hepatocellular carcinoma cells by suppressing the expression of KLF6. Sci Rep. 2017;7(1):3989. doi:10.1038/s41598-017-02801-1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

