Back to Journals » Infection and Drug Resistance » Volume 16
Increased Microbial Translocation is a Prognostic Biomarker of Different Immune Responses to ART in People Living with HIV
Authors Tian X, Xie Y, Chen J, Yin W, Zhao YL, Yao P, Dong M, Jin C , Wu N
Received 11 February 2023
Accepted for publication 10 June 2023
Published 17 June 2023 Volume 2023:16 Pages 3871—3878
DOI https://doi.org/10.2147/IDR.S404384
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Xuebin Tian,1,2,* Yiwen Xie,1,2,* Jingjing Chen,3,* Wanpeng Yin,1,2 Yu Long Zhao,1 Peng Yao,4 Mingqing Dong,4 Changzhong Jin,1,2 Nanping Wu1,2
1Cell Biology Research Platform, Jinan Microecological Biomedicine Shandong Laboratory, Jinan, Shandong, People’s Republic of China; 2State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, National Medical Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China; 3Hospital Office, Shandong Second Provincial General Hospital, Jinan, Shandong, People’s Republic of China; 4Department of Infectious Disease, Zhejiang Qingchun Hospital, Hangzhou, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Changzhong Jin; Nanping Wu, Email [email protected]; [email protected]
Background: Microbial translocation (MT) is a characteristic of human immunodeficiency virus (HIV) infection. Whether MT is also a biomarker of different immune responses to antiretroviral therapy (ART) received by people living with HIV (PLWH) is not known.
Methods: We examined the presence of MT in a cohort of 33 HIV-infected immunological responders (IRs) and 28 immunological non-responders (INRs) (≥ 500 and < 200 cluster of differentiation (CD)4+ T-cell counts/μL after 2 years of HIV-1 suppression, respectively) with no comorbidities. Plasma samples were used to measure the circulating levels of MT markers. All enrolled study participants had received 2 years of viral-suppression therapy.
Results: Levels of lipopolysaccharide (P = 0.0185), LPS-binding protein (P < 0.0001), soluble-CD14 (P < 0.0001), and endogenous endotoxin-core antibody (P < 0.0001) at baseline were significantly higher in INRs than in IRs and were associated with an increased risk of an immunological non-response, whereas the level of intestinal fatty acid-binding protein did not show this association. Analysis of receiver operating characteristic (ROC) curves demonstrated the utility of these individual microbial markers in discriminating INRs after ART in people living with HIV with high sensitivity, specificity, and area under the ROC curve.
Conclusion: INRs in HIV infection are characterized by increased MT at baseline. These markers could be used as a rapid prognostic tool for predicting immune responses in people infected with the HIV.
Keywords: HIV, immunological non-responders, microbial translocation
Introduction
Microbial translocation (MT), with resultant endotoxemia, typically results from intestinal dysbiosis and increased intestinal permeability, which leads to translocation of microbial products into the circulation.1,2 MT has also been described as a prominent feature of human immunodeficiency virus (HIV) infection.3,4 Activation of the immune system due to MT is a significant feature of HIV infection.5 Markers of activation of the innate immune system comprise circulating microbial products, such as lipopolysaccharide (LPS), LPS-binding protein (LBP), soluble cluster of differentiation (sCD)14, and endogenous endotoxin-core antibody (EndoCAb).2 Studies have demonstrated an increase in the level of these markers in several types of infections, including colorectal cancer2 and parasitic infections.6,7
HIV infection has become a manageable chronic disease thanks to antiretroviral therapy (ART), but the extent of immunological recovery varies greatly between individuals. Even under long-term suppressive ART, approximately 20% of people living with HIV (PLWH) fail to restore CD4+ T-cell counts to levels similar to those of healthy volunteers (HVs), and an increased prevalence of complications, morbidity, and mortality is observed among these patients.8 PLWH who fail to achieve normalization of CD4+ T-cell counts despite persistent virological suppression are considered to be “immunological non-responders” (INRs), who differ from “immunological responders” (IRs).4 INRs are defined as patients with absolute CD4+ T-cell count <200 cells/μL after years of ART. IRs are defined as patients with CD4+ T-cell count >500 cells/μL after receiving ART for years.9,10
Being an INR increases the susceptibility to opportunistic infections, which can lead to a higher risk of death than that for IRs with a restored CD4+ T-cell count.11 Opportunistic infections are common in INRs, and the incidence rate and mortality are also high. Therefore, biomarkers that predict different immune responses during the initial treatment phase could immensely advance clinical prognosis.
We hypothesize that enhanced systemic inflammation at baseline is an important factor contributing to the different immune responses in PLWH receiving ART. To test this hypothesis, we measured levels of MT markers at baseline in a cohort of individuals suffering from HIV in Hangzhou (China). Our findings reveal levels of MT markers at baseline to be predictors of immune status in HIV-1 infection.
Methods
Study Population
Sixty-one PLWH who were diagnosed by the Disease Control and Prevention Center of Zhejiang Province (33 IRs and 28 INRs) were recruited from the HIV clinic of the First Affiliated Hospital of Zhejiang University (Zhejiang, China) from November 2020 to October 2022. All individuals had started ART during the chronic phase of HIV infection. In the present study, IR and INR were defined as patients with an average of the last two CD4+ T-cell counts/µL ≥500 or <200 after 2 years of receiving complete viral-suppression therapy, respectively.
We excluded candidates with either of the following: (i) Age < 18 years; (ii) Showing the symptoms of an opportunistic infection; (iii) Infected with the hepatitis-B virus or hepatitis-C virus; (iv) Use of antibiotics, an immunosuppressive regimen, probiotics, prebiotics, or symbiotics in the previous 6 months; (v) Body mass index (BMI) >30 kg/m2.
Plasma specimens were prepared from the venous blood, 8 mL of blood was drawn into an EDTA collection tube. Within 3 hours of collection, well-mixed blood per subject was centrifuged at 3000 g for 10 minutes. Following centrifugation, plasma was collected and stored at −80°C until further analysis.
MT Markers
Plasma samples may contain endotoxin-inhibiting compounds. Therefore, to inactivate plasma proteins, plasma samples were heated to 75°C for 5 min. Commercially available (Quanzhou Ruixin Biotechnology, Fujian, China) enzyme-linked immunosorbent assay kits were used to measure plasma levels of LPS, LBP, sCD14, intestinal fatty acid-binding protein (iFABP), and EndoCAb.
Statistical Analyses
Geometric mean (GM) values were used for measurements of central tendency. Differences between the INR group and IR group were analyzed using the Mann–Whitney test with Holm’s correction for multiple comparisons. Receiver operator characteristic (ROC) curves were designed to test the power of each biomarker. Analyses were undertaken using MedCalc 20.100 (www.medcalc.org/). P < 0.05 was considered significant. A classification and regression tree (CART) model was employed to identify the cutoff for the levels of biomarkers which could distinguish HIV-infected INRs and IRs after ART. The analysis was done using R 4.1.2 (Institute for Statistical Computing, Vienna, Austria).
Results
Clinical Characteristics
The demographic and clinical characteristics of 33 IRs and 28 INRs are shown in Table 1. The median age of the study cohort was 43.5 (interquartile range [IQR] 33.75–55.75) years for the INR group and 42 (IQR 35–45) years for the IR group. There were no significant differences in age, BMI, or prevalence of tobacco smoking between the two groups (Table 1). There were no differences in the duration in starting ART between the two groups. Nadir and current CD4+ T cell counts are significantly higher in the IR group than the INR group. As expected, the amount of the CD4/CD8 ratio in the INR group is lower than those in the IR group (P < 0.001). The HIV RNA load was undetectable (<20 copies/mL) in all samples from patients.
 |
Table 1 Clinical Characteristics of the Study Cohort |
HIV-Infected INRs Were Characterized by Increased Levels of MT Markers
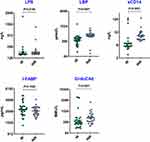
We wished to assess MT at baseline in HIV-infected INRs and IRs, so we measured levels of MT markers at baseline (pre-treatment). Levels of LPS (GM of 187.3 ng/L in INRs versus 170.3 ng/L in IRs), LBP (GM of 37,424 μmol/L in INRs versus 24,821 μmol/L in IRs), sCD14 (GM of 22.03 ng/mL in INRs versus 10.29 ng/mL in IRs), and EndoCAb (GM of 432.1 MMU/L in INRs versus 326.6 MMU/L in IRs) were significantly higher in INRs compared with those in IRs (Figure 1). However, a significant difference in the iFABP level was not seen. Thus, HIV-infected INRs were associated with increased levels of MT markers at baseline.
Biomarkers Used to Discriminate INRs from IRs After ART in PLWH
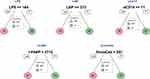
A CART model was employed to identify the cutoff of the levels of biomarkers which could be used to distinguish HIV-infected INRs from IRs after ART. As the input for tree construction, we used data on all markers and selected the most relevant biomarker that classified the group more accurately (Figure 2). Briefly, the dataset formed a “parent node”, which contained the whole population. The best peak to separate the dataset was selected. LPS (cutoff = 164 ng/L; area under the curve (AUC) = 0.682), LBP (<373 μmol/L; 0.876), sCD14 (<11 ng/mL; 0.883), and EndoCAb (257 MMU/L; 0.800) could be employed to distinguish HIV-infected INRs from IRs (Figure 2). However, the level of I-FABP (cutoff = 2712 pg/mL; AUC = 0.601) could not be used to distinguish HIV-infected INRs from IRs. Analysis of the CART model demonstrated that levels of LPS, LBP, and sCD14 (especially LBP) could be sensitive prognostic biomarkers to identify HIV-infected INRs.
HIV-Infected INRs Carry a “Signature” of MT Markers
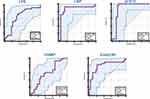
We wondered if we could utilize MT markers to predict INRs versus IRs. Hence, we carried out analysis of ROC curves on levels of MT markers. LPS (sensitivity = 75%; specificity = 64.29%; AUC = 0.682), LBP (92.86%; 85.71%; 0.876), sCD14 (100%; 82.14%; 0.883), and EndoCAb (82.14%; 78.79%; 0.800) showed significantly high AUC, sensitivity, and specificity (especially LBP) (Figure 3). However, I-FABP (sensitivity = 92.86%; specificity = 33.33%; AUC = 0.601) did not allow discrimination between groups.
Taken together, these data strongly suggested that measurement of the level of at least two biomarkers before treatment could help to predict HIV-infected INRs in our study cohort.
Discussion
The hallmark of HIV/AIDS, in the context of uncontrolled HIV-1 replication, is progressive depletion of CD4+ T cells. Direct viral cytopathic effects, apoptosis, immune-mediated lysis, and pyroptosis are among the mechanisms evoked to explain such depletion.12 Irrespective of the specific role of each of these mechanisms, evidence suggests that ART could reduce viral replication significantly in PLWH, increase the CD4+ T-cell count, and reconstruct immune functions.13 However, INRs represent a special population with poor outcomes, with no obvious recovery of the CD4 count following stable ART. Such suboptimal recovery of the CD4+ T-cell count has been demonstrated to be associated with a substantial increase in the risk of acquired immunodeficiency syndrome (AIDS)-related and non-AIDS-related morbidity and death. Hence, investigating the underlying mechanisms of INRs and developing relevant interventions are rational approaches.14
Several factors are related to the failure of reconstitution of the immune system in PLWH. Compared with the IRs, the morbidity and mortality of non-AIDS-related diseases (eg, cardiovascular disease, non-AIDS-related tumors, and HIV-related neurocognitive disorders) significantly increased in INRs.15–17 Therefore, early prediction or diagnostic biomarkers of INR should be explored urgently to provide a foundation for clinical diagnosis, and the prevention and treatment of patients’ clinical diseases. Several studies have revealed the spectrum of immune-system biomarkers of HIV infection.3,18 However, few reports have focused on the biomarkers in HIV-infected INRs. Therefore, searching for a marker associated with poor reconstitution of the immune system is important to improve the prognosis for HIV-1-infected patients.
We investigated whether levels of MT markers could be used to differentiate between HIV-infected INRs and IRs. We found that levels of MT markers such as LPS, sCD14, LBP, and EndoCAb were (i) associated with an increased risk of HIV-infected INRs; (ii) unique correlates of risk that could serve as predictors of unfavorable treatment at baseline before initiation of ART.
Translocation of bacterial products results in an increased level of LPS in the circulation without leading to overt bacteremia.19 During chronic infectious diseases, the pro-inflammatory response may disrupt the integrity of the mucosal barrier and result in the translocation of gastrointestinal bacteria, thereby leading to an increased circulating level of LPS.20 A recent study showed that an increased circulating level of LPS differed in INRs compared with that in IRs and HVs.21 sCD14 is one of the main elements of the innate immune system. It functions as a coreceptor for bacterial LPS and is present in membrane-bound and soluble forms.22 Few studies have reported that the systemic level of sCD14 is increased notably in INRs compared with that in HVs.21 LBP is an acute-phase reactant that facilitates immune responses triggered by microbial products.23 ToVinh et al reported an increased plasma level of LBP in PLWH, thereby indicating that translocation of microbial products is associated with chronic activation of the immune system.18 LBP is an acute-phase serum protein that regulates the LPS-induced immune response, and also enhances the inflammatory response to LPS.24 EndoCAb is used as an alternate measure of the circulating level of LPS. The iFABP level may also suggest disruption in epithelial integrity associated with chronic intestinal infections.25 We did not observe significant alteration in the circulating level of iFABP in HIV-infected INRs versus IRs.
Our findings indicate that levels of LPS, LBP, sCD14, and EndoCAb could be good prognostic biomarkers for monitoring of HIV treatment which, in turn, would help with clinical decision-making by identifying patients who respond favorably to ART. Hence, these microbial markers of the response of the immune system act as unique correlates of risk that could function as predictors of adverse treatment outcomes at baseline. Our study has several limitations. The low number of participants caused limitations to the explanation of our findings. Furthermore, since the fecal microbiome plays an important role in modulating the systemic immune system, the microbiome differences could be a driving factor for microbial translocation between the INR group and IR group. There should be more studies on identifying bacterial pathways and the resulting metabolites that promote microbial translocation. Bearing these limitations in mind, our results should be interpreted with caution.
Our study delivered encouraging results to unravel the effect of unique prognostic disease biomarkers in patients with HIV infection. Validation of these findings in other cohorts and other populations outside of China could aid development of a simple point-of-care rapid diagnostic test for patients with HIV infection.
Data Sharing Statement
All the reported data are available within the article.
Ethics Statement
This study was performed in line with the principles of the Declaration of Helsinki. This study was approved by the Institutional Review Committee of the First Affiliated Hospital of Zhejiang University (ethical number 2020-IIT54). Written informed consent was obtained from all participants. All the methods were performed in accordance with the relevant institutional ethical committee guidelines.
Acknowledgments
The authors acknowledge all the participants for their involvement in the study.
Funding
This work was supported by the Research Project of Jinan Microecological Biomedicine Shandong Laboratory (grant numbers JNL-20220038C, JNL-2022005B).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Blobaum L, Witkowski M, Wegner M, et al. Intestinal barrier dysfunction and microbial translocation in patients with first-diagnosed atrial fibrillation. Biomedicines. 2023;11(1):176. doi:10.3390/biomedicines11010176
2. Shi M, Zong X, Hur J, et al. Circulating markers of microbial translocation and host response to bacteria with risk of colorectal cancer: a prospective, nested case-control study in men. EBioMedicine. 2023;91:104566. doi:10.1016/j.ebiom.2023.104566
3. He J, Shi R, Duan S, et al. Microbial translocation is associated with advanced liver fibrosis among people with HIV. HIV Med. 2022;23(9):947–958. doi:10.1111/hiv.13279
4. Xie Y, Sun J, Wei L, et al. Altered gut microbiota correlate with different immune responses to HAART in HIV-infected individuals. BMC Microbiol. 2021;21(1):11. doi:10.1186/s12866-020-02074-1
5. Cribbs SK, Crothers K, Morris A. Pathogenesis of HIV-related lung disease: immunity, infection, and inflammation. Physiol Rev. 2020;100(2):603–632. doi:10.1152/physrev.00039.2018
6. Ferreira GR, Santos-Oliveira JR, Silva-Freitas ML, et al. Biomarkers of disease severity in patients with visceral leishmaniasis co-infected with HIV. Cytokine. 2022;149:155747. doi:10.1016/j.cyto.2021.155747
7. Rajamanickam A, Munisankar S, Menon PA, et al. Helminth mediated attenuation of systemic inflammation and microbial translocation in helminth-diabetes comorbidity. Front Cell Infect Microbiol. 2020;10:431. doi:10.3389/fcimb.2020.00431
8. Ruiz-Briseno MDR, De Arcos-Jimenez JC, Ratkovich-Gonzalez S, et al. Association of intestinal and systemic inflammatory biomarkers with immune reconstitution in HIV+ patients on ART. J Inflamm. 2020;17:32. doi:10.1186/s12950-020-00262-4
9. Garcia M, Jimenez-Sousa MA, Blanco J, et al. CD4 recovery is associated with genetic variation in IFNgamma and IL19 genes. Antiviral Res. 2019;170:104577. doi:10.1016/j.antiviral.2019.104577
10. Zhang LX, Song JW, Zhang C, et al. Dynamics of HIV reservoir decay and naïve CD4 T-cell recovery between immune non-responders and complete responders on long-term antiretroviral treatment. Clin Immunol. 2021;229:108773. doi:10.1016/j.clim.2021.108773
11. Abate D, Abate D, Abate D, et al; C.Global Burden of Disease Cancer. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5(12):1749–1768. doi:10.1001/jamaoncol.2019.2996
12. Lisco A, Wong CS, Lage SL, et al. Identification of rare HIV-1-infected patients with extreme CD4+ T cell decline despite ART-mediated viral suppression. JCI Insight. 2019;4. doi:10.1172/jci.insight.127113
13. Wang XM, Zhang JY, Xing X, et al. Global transcriptomic characterization of T cells in individuals with chronic HIV-1 infection. Cell Discov. 2022;8:29. doi:10.1038/s41421-021-00367-x
14. Yang X, Su B, Zhang X, et al. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: challenges of immunological non-responders. J Leukoc Biol. 2020;107(4):597–612. doi:10.1002/JLB.4MR1019-189R
15. Engsig FN, Zangerle R, Katsarou O, et al. Long-term mortality in HIV-positive individuals virally suppressed for >3 years with incomplete CD4 recovery. Clin Infect Dis. 2014;58(9):1312–1321. doi:10.1093/cid/ciu038
16. Pacheco YM, Jarrin I, Rosado I, et al. Increased risk of non-AIDS-related events in HIV subjects with persistent low CD4 counts despite cART in the CoRIS cohort. Antiviral Res. 2015;117:69–74. doi:10.1016/j.antiviral.2015.03.002
17. Takuva S, Maskew M, Brennan AT, et al. Poor CD4 recovery and risk of subsequent progression to AIDS or death despite viral suppression in a South African cohort. J Int AIDS Soc. 2014;17(1):18651. doi:10.7448/IAS.17.1.18651
18. ToVinh M, Horr G, Hoffmeister C, et al. HIV-associated microbial translocation may affect cytokine production of CD56bright NK cells via stimulation of monocytes. J Infect Dis. 2023;227(4):577–582. doi:10.1093/infdis/jiac485
19. Arab JP, Martin-Mateos RM, Shah VH. Gut-liver axis, cirrhosis and portal hypertension: the chicken and the egg. Hepatol Int. 2018;12(S1):24–33. doi:10.1007/s12072-017-9798-x
20. Merlini E, Cozzi-Lepri A, Castagna A, et al. Inflammation and microbial translocation measured prior to combination antiretroviral therapy (cART) and long-term probability of clinical progression in people living with HIV. BMC Infect Dis. 2021;21(1):557. doi:10.1186/s12879-021-06260-y
21. Luo Z, Health SL, Li M, et al. Variation in blood microbial lipopolysaccharide (LPS) contributes to immune reconstitution in response to suppressive antiretroviral therapy in HIV. EBioMedicine. 2022;80:104037. doi:10.1016/j.ebiom.2022.104037
22. Ranoa DRE, Kelley SL, Tapping RI. Human lipopolysaccharide-binding protein (LBP) and CD14 independently deliver triacylated lipoproteins to toll-like receptor 1 (TLR1) and TLR2 and enhance formation of the ternary signaling complex. J Biol Chem. 2013;288(14):9729–9741. doi:10.1074/jbc.M113.453266
23. Ha EK, Kim JH, Yon DK, et al. Association of serum lipopolysaccharide-binding protein level with sensitization to food allergens in children. Sci Rep. 2021;11:2143. doi:10.1038/s41598-020-79241-x
24. Gabarin RS, Li M, Zimmel PA, et al. Intracellular and extracellular lipopolysaccharide signaling in sepsis: avenues for novel therapeutic strategies. J Innate Immun. 2021;13(6):323–332. doi:10.1159/000515740
25. Cao VT, Carter MC, Brenchley JM, et al. sCD14 and intestinal fatty acid binding protein are elevated in the serum of patients with idiopathic anaphylaxis. J Allergy Clin Immunol Pract. 2023. doi:10.1016/j.jaip.2023.03.037
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.