Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 14
Increased Frequency of Circulating Classical Monocytes in Patients with Rosacea
Authors Gao C , Ge L, Chen D, Zhang M, Zhao L, Liu W, Chen S, Wang J, Zhou C, Zhao X, Li S, Song Z, Li J
Received 31 August 2021
Accepted for publication 19 October 2021
Published 9 November 2021 Volume 2021:14 Pages 1629—1636
DOI https://doi.org/10.2147/CCID.S336194
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey Weinberg
Cuie Gao,1 Lan Ge,1 Dewei Chen,2 Mengjie Zhang,2 Li Zhao,2 Wenying Liu,1 Shuguang Chen,1 Juan Wang,1 Cunjian Zhou,1 Xingwang Zhao,1 Shifei Li,1 Zhiqiang Song,1 Jian Li1
1Department of Dermatology, Southwest Hospital, Army Medical University, Chongqing, 400038, People’s Republic of China; 2Department of Pathophysiology, Army Medical University, Chongqing, 400038, People’s Republic of China
Correspondence: Jian Li; Zhiqiang Song
Department of Dermatology, Southwest Hospital, Army Medical University, Chongqing, 400038, People’s Republic of China
Tel +86 23 68754290
Fax +86 23 68755290
Email [email protected]; [email protected]
Purpose: Monocyte subsets, including classical, intermediate and non-classical monocytes, are involved in the pathogenesis of inflammatory or autoimmune diseases. The pathogenic role of monocytes in the peripheral blood mononuclear cells (PBMCs) of patients with rosacea remains unclear. This study aimed to assess frequencies of monocyte subsets in PBMCs from rosacea patients before and after clinical treatment.
Patients and Methods: We applied flow cytometry to examine frequencies of monocyte subsets in 116 patients with rosacea, while patients with 26 systemic lupus erythematosus (SLE), 28 acne and 42 normal healthy subjects without skin problems (HC) were recruited as controls. Expression of C–C chemokine receptor 2 (CCR2) on monocytes and plasma levels of CC-chemokine ligand 2 (CCL2), high mobility group box-1 (HMGB-1), interleukin-1 beta (IL-1β) and tumor necrosis factor alpha (TNF-α) were measured in HC and rosacea patients before and after treatment.
Results: The frequency of classical monocytes, but not intermediate or non-classical monocytes, was higher in rosacea as compared with HC, which decreased after treatment. Frequencies of monocyte subsets showed no gender difference, while increased with age in patients but not in HC. Frequencies of classical monocytes in patients with erythematotelangiectatic rosacea (ETR) and ETR-papulopustular rosacea (PPR) overlap were significantly higher than HC or patients with only PPR or phymatous rosacea (PhR). There was a significant higher expression of CCR2 in classical monocytes, with higher plasma levels of CCL2, HMGB-1, IL-1β and TNF-α in patients than in HC, which all significantly decreased after treatment.
Conclusion: Our data indicated a possible association between abnormal classical monocytes frequencies and rosacea.
Keywords: monocytes, classical monocytes, CCR2, rosacea
Introduction
Rosacea is a chronic inflammatory skin disease that affects 0.09–22.41% of the general population,1 with the prevalence rate estimated to be 3.48% in China.2 The disease is conventionally classified into four main subtypes, including erythematotelangiectatic rosacea (ETR), papulopustular rosacea (PPR), phymatous rosacea (PhR), and ocular rosacea, with great overlaps and varied courses.3,4 The diverse clinical presentations of rosacea severely affect the aesthetic appearance and mental health of patients, causing significant impairment in the quality of life.5
The pathogenesis of rosacea remains incompletely elucidated. Evidences show that abnormal immune responses, especially the innate immunity and neurovascular disorders, may be related to the pathophysiology.6,7 In human, circulating monocytes are a group of innate immune cells that arise from hematopoietic stem cell precursors in the bone marrow, and eventually migrate into inflammatory sites where further mature to exert multiple functions, including homeostasis, immune defense and tissue repair.8,9 Monocytes were found to contribute to the pathogenesis of certain diseases, such as rheumatoid arthritis,10 asthma11,12 and multiple sclerosis.13
Circulating monocytes are a heterogeneous population, and can be divided into three subsets based on the expression of CD14 and CD16: classical (CD14++CD16−), intermediate (CD14++CD16+), and non-classical (CD14+CD16++) monocytes. Classical monocytes are the major subset and account for ~85% of circulating monocytes,9,14 which express high levels of chemokine receptor CCR2.15 They migrate into inflammatory sites through interactions between CCR2 and its ligand CCL2,16 and contribute to the release of damage-associated molecular patterns (DAMPs),17 such as HMGB-1 protein,18,19 which can bind multiple receptors to induce the recruitment of inflammatory cells and initiate a series of cascade reactions to release inflammatory cytokines like IL-1β and TNF-α.20–22 In CCR2 knockout mice, decreased monocyte recruitment and proinflammatory cytokines levels (such as IL-1β, TNF-α) were found in inflammatory sites, indicating the role of CCR2 in the targeted migration of monocytes.23,24 The pathogenic role of circulating monocytes and their subsets in rosacea remains poorly understood.
The current study was aimed to explore the potential role of monocytes dysregulation in rosacea, as compared to SLE, acne and healthy controls (HC) without skin problems. The concentrations of CCL2, HMGB-1, IL-1β and TNF-α in the plasma of rosacea patients and HC were also evaluated.
Patients and Methods
Participants and Blood Sampling
A total of 116 patients diagnosed with rosacea were recruited in the Southwest Hospital of the Army Medical University, Chongqing, China in 2019. The clinical diagnosis of rosacea subtypes was made according to the classification system of the National Rosacea Society.25 Samples of 26 stable SLE patients (SLE disease activity index, SLEDAI ≤ 4), 28 moderate acne patients and 42 healthy individuals (HC) without skin problems individuals were investigated. Diagnosis of SLE was based on the criteria of the American College of Rheumatology.26 Evaluation of the types and severity of acne was implemented in accordance with Chinese guidelines.27 Among 116 rosacea patients, 18 subjects received carvedilol 5 mg twice a day for one month and blood sample was checked again. Evaluation of rosacea was based on the Investigator’s Global Assessment (IGA) and Clinical Erythema Assessment (CEA) scores before and after treatment.28 The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Southwest Hospital (Approval number KY201969). All study participants agreed to participate in the study and signed an informed consent.
Peripheral blood samples from the participants were obtained and collected into vacuum tubes anticoagulated with EDTA-K2. PBMCs were prepared using density gradient centrifugation by laying blood samples on the Ficoll (TBD, Tianjin, China). Plasma samples were separated and stored at –80°C until used for enzyme-linked immunosorbent assays (ELISA).
Flow Cytometry
Isolated human PBMCs were stained with antibodies obtained from BioLegend (San Diego, CA, USA): CD3 (PerCP/Cyanine5.5), CD19 (PerCP/Cyanine5.5), CD20 (PerCP/Cyanine5.5), CD56 (PerCP/Cyanine5.5), CD66b (PerCP/Cyanine5.5), HLA-DR (PE/Cyanine7), CD14 (FITC), CD16 (Brilliant Violet 510™), CCR2 (APC). Flow cytometry was performed with Navios Flow Cytometer (Beckman Coulter, Miami, USA) and data were analyzed using Navios Software version 1.3. Total Lin− (CD3, CD19, CD20, CD56 and CD66b) HLA-DR+ cells were gated by the forward/side scatter showing Lin/HLA-DR expression patterns. Circulating classical monocytes (CD14++CD16−), intermediate monocytes (CD14++CD16+) and non-classical monocytes (CD14+CD16++) were gated from Lin−HLA-DR+ cells based on expression levels of CD14 and CD16. Specific gating strategies to identify circulating monocyte subsets and CCR2 expression in different subsets were shown in Figure S1.
Enzyme-Linked Immunosorbent Assays (ELISA)
Plasma levels of CCL2 (Dakewe, Beijing, China), HMGB-1 (Elabscience, Wuhan, China), IL-1β (Elabscience) and TNF-α (Elabscience) were measured using ELISAs according to instructions of the manufacturers.
Statistical Analysis
GraphPad Prism version 5 (GraphPad Software, San Diego, CA, USA) and SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) were used to analyze relevant experimental data. Results were expressed as mean ± standard error of the mean (SEM). The unpaired Student’s t-test and paired Student’s t-test were used to compare the two groups, and before and after treatment of the treated group, respectively. p < 0.05 was considered as statistically significant.
Results
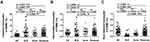
Frequencies of Monocyte Subsets in 3 Inflammatory Diseases
Frequencies of circulating classical or intermediate monocytes were higher in SLE (p < 0.001 or p = 0.0465) and acne (p < 0.001 or p = 0.0036) patients than those in HC, while the frequency of non-classical monocytes significantly reduced (p < 0.001) (Figure 1). The frequency of classical monocytes was higher in rosacea patients than in HC (p < 0.001), with no significant difference in intermediate or non-classical monocytes (Figure 1).
Compared with SLE, frequencies of classical and non-classical monocytes decreased (p = 0.0028) and increased (p < 0.001) in the rosacea patients, respectively. There are no difference in intermediate monocytes (Figure 1).
Compared with acne, the frequency of non-classical monocytes increased (p = 0.0101) in patients with rosacea, without difference in classical or intermediate monocytes (Figure 1).
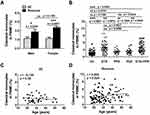
Effects of Gender, Age and Subtypes on Frequencies of Monocyte Subsets in Rosacea Patients
A total of 116 patients (24 males, 92 females; average age, 29.85 ± 10.28 years) with rosacea were enrolled in this study. Among them, there were 51 cases with ETR, 19 cases with PPR, 17 cases with PhR and 29 cases with ETR + PPR. In addition, 42 healthy controls without any skin problem individuals (20 males, 22 females; average age, 30.07 ± 9.46 years) were also selected as controls. Specific demographic and clinical data of rosacea patients and HC are summarized in Tables 1 and 2.
 |
Table 1 Demographics Characteristics of Patients with Rosacea and Healthy Controls (HC) |
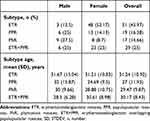 |
Table 2 Demographics and Clinical Characteristics of Patients with Different Subtypes of Rosacea |
Frequencies of classical monocytes were higher in both male (p = 0.0232) and female (p < 0.001) patients than in HC, without gender difference (Figure 2A). No significant difference was observed in the frequencies of intermediate or non-classical monocytes in patients with rosacea compared with HC, independent of the gender grouping (Figure S2A and B). Frequencies of monocyte subsets did not change with age in HC (Figures 2C, S2E and F), but increased with age in rosacea patients (classical, intermediate and nonclassical, p = 0.024, 0.007 and 0.000, respectively) (Figures 2D, S2G and H).
The frequencies of classical monocytes were significantly higher in subtypes ETR (p = 0.0015 or 0.0056) and ETR+PPR (p = 0.0010 or 0.0042) than those in PPR or PhR, while no difference between ETR and ETR+PPR or between PPR and PhR (Figure 2B). There were no significant difference in the frequencies of intermediate and non-classical monocytes in all the subtypes except that frequencies of intermediate monocytes in ETR (p = 0.0069) and ETR+PPR (p = 0.01) were higher than those in PhR (Figure S2C and D).
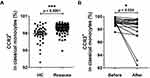
Frequencies of Monocyte Subsets, Expression of CCR2 in Subsets and Plasma Levels of HMGB-1, CCL2, IL-1β and TNF-α Before and After Treatment in Rosacea Patients
After treatment with carvedilol, IGA score of rosacea patients decreased from 1.56 ± 0.36 to 0.67 ± 0.18 (p = 0.0355) and CEA score decreased from 2.33 ± 0.18 to 1.11 ± 0.11 (p < 0.001). In addition, the frequency of classical monocytes decreased significantly (p < 0.001), without difference in intermediate or non-classical monocytes (Figure 3).
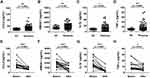
Before treatment, the expression of CCR2 in classical (p < 0.001) or intermediate (p < 0.001) monocytes, but not in non-classical monocytes, were higher in rosacea patients than in HC (Figures 4A, S3A and B). Expression of CCR2 in classical monocytes significantly decreased after treatment (p = 0.034), without significant difference observed in the intermediate or non-classical monocytes (Figures 4B, S3C and D).
The plasma levels of CCL2 (p = 0.0023), HMGB-1 (p = 0.001), IL-1β (p =0.0393) and TNF-α (p = 0.0069) were significantly higher in rosacea patients than those in HC (Figure 5A–D), which all decreased after treatment (p < 0.001, p = 0.0003, p < 0.001 and p = 0.010, respectively) (Figure 5E–H).
Discussion
Monocytes are heterogeneous cells in their function, which can be divided into classical, intermediate and non-classical monocytes. The classical monocytes are recruited to inflammatory sites and play a regulatory role in inflammation. The intermediate monocytes show pro-inflammatory properties, especially in patients with acute inflammation. The non-classical monocytes are considered a group of patrolling monocytes to maintain vascular homeostasis.29,30 In the current study, alterations in the frequencies of monocyte subsets were demonstrated in three inflammatory diseases (SLE, acne and rosacea) as compared with healthy controls, but differentiation between them was noted.
Our findings of imbalance in classical but not in intermediate or non-classical monocytes supported the clinical observation that the inflammation in rosacea is mostly chronic. Imbalance in classical monocytes may affect the process of differentiation of intermediate and non-classical monocytes or monocyte-derived dendritic cells, which subsequently exert impact on the adaptive immune response,31,32 the potential role of classical monocytes in the initiation and progression of rosacea was supported by our results.
In study of alterations in human immunity with age,33–35 most studies have focused on the role of acquired immune cells such as T and B cells in aging,36–38 but the inflammageing phenomenon in monocytes and macrophages also contributes to immunosenescence.36–38 Our results supported previous reports that frequencies of monocyte subsets did not change with age in HC.39,40 On the other hand, increase of different monocyte subsets with age in rosacea patients indicated that the occurrence of rosacea might partially be associated with immunosenescence.
Neurogenic inflammation in controlling vascular response was proposed to involve the pathophysiology of rosacea, which may reflect the clinical features of flushing, erythema, and induction of leukocyte infiltration.41,42 On the other hand, it has been demonstrated that immune mechanisms including classical monocytes are associated with arterial hypertension and atherosclerosis related to vascular inflammation.43,44 Our results provided further evidence that circulating classical monocytes were more closely associated with ETR and ETR overlapping PPR than PPR alone in rosacea. In addition, our results showed that IGA and CEA scores and the frequency of classical monocytes and expression of CCR2 on their surface of patients with rosacea decreased after treatment with carvedilol, which also supported the report that carvedilol may be an effective treatment mean for rosacea, especially flushing and erythema.45,46
Finally, our results indicated that increase of CCL2 in plasma might promote the recruitment of CCR2+ monocytes to inflammatory skin, leading to the release of damage-associated molecular patterns and inflammatory cytokines HMGB-1, IL-1β and TNF-α, as demonstrated in the current study. Our findings supported the report on the increased expression of CCR2 and its ligand CCL2 in rosacea skin.47
There are several limitations in the current study: (1) lack of dynamic study in the same individuals with different diseases under examination, (2) small number of healthy controls and patients treated with carvedilol, respectively, (3) lack of placebo control group in carvedilol treatment, (4) detection of circulating monocyte subsets and their expression of cell surface marker cannot truly reflect the situ facial conditions, (5) the current classification of clinical subtypes shows varying overlaps and dynamic changes, while PPR alone without ETR remains a debating entity, and PhR is usually accompanied by ETR.48
Conclusion
In conclusion, this study provided primary findings about the frequencies in circulating monocyte subsets and expression of cell surface marker (CCR2) in rosacea patients. Their operation in situ remains to be determined. Further studies are required to explore their pathogenic role in and causative association with the disease.
Abbreviations
PBMCs, peripheral blood mononuclear cells; SLE, systemic lupus erythematosus; HC, healthy control; CCR2, C–C chemokine receptor 2; CCL2, CC-chemokine ligand 2; HMGB-1, high mobility group box 1; IL-1β, interleukin-1 beta; TNF-α, tumor necrosis factor alpha; ETR, erythematotelangiectatic rosacea; PPR, papulopustular rosacea; PhR, phymatous rosacea; DAMP, damage-associated molecular pattern; SLEDAI, SLE disease activity index; IGA, Investigator’s Global Assessment; CEA, Clinical Erythema Assessment; ELISA, Enzyme-linked immunosorbent assays; SEM, standard error of the mean.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81703154). We wish to thank Professor Bing Ni (Department of Pathophysiology, Army Medical University, China) for suggestions regarding experimental methods and research ideas. Zhiqiang Song and Jian Li are co-correspondence authors for this study.
Disclosure
All authors declare that they have no conflicts of interest.
References
1. Gether L, Overgaard LK, Egeberg A, Thyssen JP. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179(2):282–289. doi:10.1111/bjd.16481
2. Li J, Wang B, Deng Y, et al. Epidemiological features of rosacea in Changsha, China: a population-based, cross-sectional study. J Dermatol. 2020;47(5):497–502. doi:10.1111/1346-8138.15301
3. Tan J, Almeida LM, Bewley A, et al. Updating the diagnosis, classification and assessment of rosacea: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176(2):431–438. doi:10.1111/bjd.15122
4. Schaller M, Almeida LMC, Bewley A, et al. Recommendations for rosacea diagnosis, classification and management: update from the global ROSacea COnsensus 2019 panel. Br J Dermatol. 2020;182(5):1269–1276. doi:10.1111/bjd.18420
5. Wu Y, Fu C, Zhang W, Li C, Zhang J. The dermatology life quality index (DLQI) and the hospital anxiety and depression (HADS) in Chinese rosacea patients. Psychol Health Med. 2018;23(4):369–374. doi:10.1080/13548506.2017.1361540
6. Holmes AD, Steinhoff M. Integrative concepts of rosacea pathophysiology, clinical presentation and new therapeutics. Exp Dermatol. 2017;26(8):659–667. doi:10.1111/exd.13143
7. Kulkarni NN, Takahashi T, Sanford JA, et al. Innate immune dysfunction in rosacea promotes photosensitivity and vascular adhesion molecule expression. J Invest Dermatol. 2020;140(3):645–655.e646. doi:10.1016/j.jid.2019.08.436
8. Lee J, Breton G, Oliveira TY, et al. Restricted dendritic cell and monocyte progenitors in human cord blood and bone marrow. J Exp Med. 2015;212(3):385–399. doi:10.1084/jem.20141442
9. Ziegler-Heitbrock L, Ancuta P, Crowe S, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116(16):e74–e80. doi:10.1182/blood-2010-02-258558
10. Yoon BR, Yoo SJ, Choi Y, et al. Functional phenotype of synovial monocytes modulating inflammatory T-cell responses in rheumatoid arthritis (RA). PLoS One. 2014;9(10):e109775. doi:10.1371/journal.pone.0109775
11. Moniuszko M, Bodzenta-Lukaszyk A, Kowal K, Lenczewska D, Dabrowska M. Enhanced frequencies of CD14++CD16+, but not CD14+CD16+, peripheral blood monocytes in severe asthmatic patients. Clin Immunol. 2009;130(3):338–346. doi:10.1016/j.clim.2008.09.011
12. Niessen NM, Baines KJ, Simpson JL, et al. Neutrophilic asthma features increased airway classical monocytes. Clin Exp Allergy. 2021;51(2):305–317. doi:10.1111/cea.13811
13. Gjelstrup MC, Stilund M, Petersen T, Møller HJ, Petersen EL, Christensen T. Subsets of activated monocytes and markers of inflammation in incipient and progressed multiple sclerosis. Immunol Cell Biol. 2018;96(2):160–174. doi:10.1111/imcb.1025
14. Wong KL, Tai JJ, Wong WC, et al. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118(5):e16–e31. doi:10.1182/blood-2010-12-326355
15. Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19(1):71–82. doi:10.1016/S1074-7613(03)00174-2
16. Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–964. doi:10.1038/nri1733
17. Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Nat Rev Immunol. 2007;28(10):429–436. doi:10.1016/j.it.2007.08.004
18. Tang D, Kang R, Zeh HJ, Lotze MT. High-mobility group box 1, oxidative stress, and disease. Antioxid Redox Signal. 2011;14(7):1315–1335. doi:10.1089/ars.2010.3356
19. Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–162. doi:10.1146/annurev-immunol-030409-101323
20. Yu M, Wang H, Ding A, et al. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26(2):174–179. doi:10.1097/01.shk.0000225404.51320.82
21. Venereau E, Schiraldi M, Uguccioni M, Bianchi ME. HMGB1 and leukocyte migration during trauma and sterile inflammation. Mol Immunol. 2013;55(1):76–82. doi:10.1016/j.molimm.2012.10.037
22. Park JS, Gamboni-Robertson F, He Q, et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290(3):C917–924. doi:10.1152/ajpcell.00401.2005
23. Varvel NH, Neher JJ, Bosch A, et al. Infiltrating monocytes promote brain inflammation and exacerbate neuronal damage after status epilepticus. Proc Natl Acad Sci U S A. 2016;113(38):E5665–E5674. doi:10.1073/pnas.1604263113
24. França CN, Izar MCO, Hortêncio MNS, et al. Monocyte subtypes and the CCR2 chemokine receptor in cardiovascular disease. Clin Sci. 2017;131(12):1215–1224. doi:10.1042/CS20170009
25. Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78(1):148–155. doi:10.1016/j.jaad.2017.08.037
26. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi:10.1002/art.1780400928
27. Acne Group, Combination of Traditional and Western Medicine Dermatology, Acne Group, Chinese Society of Dermatology, Acne Group, Chinese Dermatologist Association, Chinese Non-government Medical Institutions Association. Chinese guidelines for the management of acne vulgaris: 2019 update#. Int J Dermatol Vener. 2019;2(3):129–137. doi:10.1097/JD9.0000000000000043
28. Bribeche MR, Fedotov VP, Gladichev VV, Pukhalskaya DM, Kolitcheva NL. Clinical and experimental assessment of the effects of a new topical treatment with praziquantel in the management of rosacea. Int J Dermatol. 2015;54(4):481–487. doi:10.1111/ijd.12552
29. Yang J, Zhang L, Yu C, Yang XF, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. 2014;2(1):1. doi:10.1186/2050-7771-2-1
30. Narasimhan PB, Marcovecchio P, Hamers AAJ, Hedrick CC. Nonclassical monocytes in health and disease. Annu Rev Immunol. 2019;37:439–456. doi:10.1146/annurev-immunol-042617-053119
31. Kwissa M, Nakaya HI, Onlamoon N, et al. Dengue virus infection induces expansion of a CD14(+)CD16(+) monocyte population that stimulates plasmablast differentiation. Cell Host Microbe. 2014;16(1):115–127. doi:10.1016/j.chom.2014.06.001
32. Wolf AA, Yáñez A, Barman PK, Goodridge HS. The ontogeny of monocyte subsets. Front Immunol. 2019;10:1642. doi:10.3389/fimmu.2019.01642
33. Alpert A, Pickman Y, Leipold M, et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat Med. 2019;25(3):487–495. doi:10.1038/s41591-019-0381-y
34. Mogilenko DA, Shpynov O, Andhey PS, et al. Comprehensive profiling of an aging immune system reveals clonal GZMK+ CD8+ T cells as conserved Hallmark of inflammaging. Immunity. 2021;54(1):99–115.e112. doi:10.1016/j.immuni.2020.11.005
35. Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 2013;14(5):428–436. doi:10.1038/ni.2588
36. Akbar AN, Henson SM, Lanna A. Senescence of T lymphocytes: implications for enhancing human immunity. Trends Immunol. 2016;37(12):866–876. doi:10.1016/j.it.2016.09.002
37. Ma S, Wang C, Mao X, Hao Y. B cell dysfunction associated with aging and autoimmune diseases. Front Immunol. 2019;10:318. doi:10.3389/fimmu.2019.00318
38. De Maeyer RPH, Chambers ES. The impact of ageing on monocytes and macrophages. Immunol Lett. 2021;230:1–10. doi:10.1016/j.imlet.2020.12.003
39. Costantini A, Viola N, Berretta A, et al. Age-related M1/M2 phenotype changes in circulating monocytes from healthy/unhealthy individuals. Aging. 2018;10(6):1268–1280. doi:10.18632/aging.101465
40. Seidler S, Zimmermann HW, Bartneck M, Trautwein C, Tacke F. Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunol. 2010;11:30. doi:10.1186/1471-2172-11-30
41. Schwab VD, Sulk M, Seeliger S, et al. Neurovascular and neuroimmune aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15(1):53–62. doi:10.1038/jidsymp.2011.6
42. Sulk M, Seeliger S, Aubert J, et al. Distribution and expression of non-neuronal transient receptor potential (TRPV) ion channels in rosacea. J Invest Dermatol. 2012;132(4):1253–1262. doi:10.1038/jid.2011.424
43. Wenzel U, Turner JE, Krebs C, Kurts C, Harrison DG, Ehmke H. Immune mechanisms in arterial hypertension. J Am Soc Nephrol. 2016;27(3):677–686. doi:10.1681/ASN.2015050562
44. Delaney JAC, Olson NC, Sitlani CM, et al. Natural killer cells, gamma delta T cells and classical monocytes are associated with systolic blood pressure in the multi-ethnic study of atherosclerosis (Mesa). BMC Cardiovasc Disord. 2021;21(1):45. doi:10.1186/s12872-021-01857-2
45. Zhang H, Tang K, Wang Y, Fang R, Sun Q. Rosacea treatment: review and update. Dermatol Ther. 2021;11(1):13–24. doi:10.1007/s13555-020-00461-0
46. Logger JGM, Olydam JI, Driessen RJB. Use of beta-blockers for rosacea-associated facial erythema and flushing: a systematic review and update on proposed mode of action. J Am Acad Dermatol. 2020;83(4):1088–1097. doi:10.1016/j.jaad.2020.04.129
47. Buhl T, Sulk M, Nowak P, et al. Molecular and morphological characterization of inflammatory infiltrate in rosacea reveals activation of Th1/Th17 pathways. J Invest Dermatol. 2015;135(9):2198–2208. doi:10.1038/jid.2015.141
48. Plewig G, Melnik B, Chen WC. Rosacea clinic and classification. In: Plewig G, Melnik B, Chen WC, editors. Plewig and Kligman´s Acne and Rosacea.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.