Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Increased Expression of Toll-Like Receptor (TLR) 2 and TLR6 on Peripheral Blood Monocytes by Induction of Staphylococcal Enterotoxin B During Exacerbation of Atopic Dermatitis Patients
Authors Salim F , Gunawan H , Suwarsa O , Sutedja E
Received 16 December 2022
Accepted for publication 24 January 2023
Published 31 January 2023 Volume 2023:16 Pages 301—307
DOI https://doi.org/10.2147/CCID.S401815
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Anne-Claire Fougerousse
Fitria Salim,1,2 Hendra Gunawan,3 Oki Suwarsa,3 Endang Sutedja3
1Doctoral Study Program, Faculty of Medicine, Universitas Padjadjaran, Bandung, West Java, Indonesia; 2Department of Dermatology and Venereology, Faculty of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia; 3Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran–Hasan Sadikin General Hospital, Bandung, West Java, Indonesia
Correspondence: Fitria Salim, Department of Dermatology and Venereology, Faculty of Medicine, Universitas Syiah Kuala – Zainoel Abidin General Hospital, Kopelma Darussalam, Banda Aceh, Aceh, 23111, Indonesia, Tel +65151977, Fax +65152053, Email [email protected]
Background: Atopic dermatitis (AD) is a chronic and recurrent inflammatory skin disease that can be triggered by various precipitating factors, including colonization by Staphylococcus aureus (S. aureus). The toll-like receptor (TLR), which belongs to the family of pattern recognition receptors (PRR), can recognize components of S. aureus, such as staphylococcal enterotoxin B (SEB). This receptor is known to be expressed on monocytes. However, the understanding of the role of SEB in the pathogenesis of AD through the TLR pathway, especially TLR2 and TLR6, is not widely known.
Purpose: To investigate the expression of TLR2 and TLR6 on peripheral blood monocytes induced by SEB during AD exacerbations.
Patients and Methods: Twenty AD patients and 20 healthy subjects as a control group were selected. A 5 mL blood sample from each subject was taken for monocyte culture, which was induced by SEB for three days, and the outcomes were assessed by flow cytometry to evaluate TLR2 and TLR6 expression.
Results: The expression of TLR2 on peripheral blood monocytes in AD patients was increased compared to healthy controls (p = 0.000), but not for the expression of TLR6 (p = 0.304). In the AD group, TLR2 and TLR6 expression on peripheral blood monocytes after being induced by SEB was significantly increased compared to before induction (p = 0.025 and p = 0.023, respectively), but not in the control group (p = 0.737 and p = 0.100, respectively).
Conclusion: There is significantly increased expression of TLR2 and TLR6 on peripheral blood monocytes induced by SEB during exacerbation in AD patients.
Keywords: atopic dermatitis, monocyte, staphylococcal enterotoxin B, TLR2, TLR6
Introduction
Atopic dermatitis (AD) is a common, chronic, and relapsing inflammatory skin disease with an increasing incidence during the past few decades, especially in developing countries.1,2 The etiology and pathogenesis of AD are influenced by numerous factors, both intrinsic and extrinsic, as well as the interaction of these two factors. Intrinsic factors include genetics, impaired skin barrier function, and dysregulation of immune factors, while extrinsic factors come from the environment, namely inhaled and food allergens, infections, irritants, and pollutants.3,4 Impaired barrier function will increase the chance of exposure to contactants (irritants and allergens) and facilitate the colonization and infection of pathogenic bacteria, particularly Staphylococcus aureus (S. aureus).5,6
S. aureus produces enterotoxins with superantigenic character, such as staphylococcal enterotoxin B (SEB). SEB plays a major role in the exacerbation and severity of AD.7 This is due to the binding of toll-like receptors (TLRs) on the dendritic cell membrane with the SEB pathogen, which will activate the innate immune response through the activation of nuclear factor kappa B (NF-kB), the main regulator of the inflammatory response, which produces various cytokines and chemokines.8,9
Human TLRs are transmembrane glycoproteins composed of extracellular leucine-rich repeats (LRRs), a transmembrane domain, and a cytoplasmic tail containing a Toll/IL-1 receptor (TIR) domain. Each TLR can recognize a distinct set of pathogen-associated molecular patterns (PAMPs) either through direct or indirect interaction.10 TLR is essential for both initiating innate immune responses and regulating adaptive immunity. Regulation of TLR expression may be important in the pathogenesis of inflammatory skin diseases such as psoriasis and AD.11 TLR2 is functionally active as a heterodimer in cooperation with TLR1 or TLR6 to achieve specificity and discriminate subtle differences in the repertoire of bacterial lipoproteins. TLR2 has emerged as a principle of receptor in combating Gram-positive bacteria, especially S. aureus.12 TLR2 forms heterodimers with TLR1 and TLR6 to interact with a rather broad spectrum of ligands. TLR2 can bind ligands of lipoproteins/lipopeptides, peptidoglycans, lipopolysaccharides, and lipoarabinomannan that are components of Gram-positive bacteria.13
TLR6 is known to be specific for diacilated lipopeptides such as lipoteichoic acid that can be found on the cell wall of Gram-positive bacteria. After ligand recognition, the TLR6 receptor dimerizes with TLR2. Ligand-mediated dimerization is crucial for recruiting the adaptor proteins, which are necessary for transmitting the signal inside the cell.14 The TLR2/6 heterodimer, just as most of the TLRs, generally induces the myeloid differentiation protein 88 (MyD88)-dependent intracellular signaling pathway, which leads to nuclear translocation of NF-kB, resulting in the production of pro-inflammatory cytokines, and MyD88 also activates mitogen-activated protein kinases (MAPKs).10
TLR distribution can be found on various immune cells, such as the expression of TLR2 and TLR6, which can be detected on monocytes that recognize S. aureus secreted toxins and contribute to the priming of naive T cells into Th2 cells through activation and overexpression of TLR2.9 TLRs induce the expression of antimicrobial effector molecules and the release of various proinflammatory and immunomodulatory cytokines, which lead to the activation of adaptive immune responses.13 This study aimed to determine the expression of TLR2 and TLR6 on peripheral blood monocytes induced by SEB during the exacerbation of AD patients.
Materials and Methods
Study Population and Inclusion Criteria
The study was approved by the Institutional Review Board of the Health Research Ethics Committee in Universitas Padjadjaran, Bandung, West Java, Indonesia number 241/UN6.KEP/EC/2020 and conducted in accordance with the latest version of the Declaration of Helsinki. Each subject gave written informed consent prior to enrollment, and for participants under 18 years of age, informed consent was obtained from the parents or guardian at the time of data collection.
Twenty AD patients with an exacerbation and twenty healthy individuals were chosen as subjects. The AD patients were clinically diagnosed by dermatologists, then evaluated the severity of their condition using the Scoring Atopic Dermatitis (SCORAD) index.14 The inclusion criteria were AD patients older than two years old, and the exclusion criteria were subjects who had the other skin diseases and had received a topical or oral corticosteroids, immunosuppressants, or antibiotics within the previous 14 days. For healthy individuals, exclusion criteria were having previous skin diseases, including AD, allergies or significant underlying diseases, and a history of taking medications, including corticosteroids, immunosuppressants, and antibiotics.
Monocyte Isolation from PBMC Culture
About 5 mL of blood was collected in a tube with heparin as an anticoagulant. Monocytes were separated from the other blood components using the Ficoll-Hypaque density gradient method. The blood was diluted in 5 mL of Hank’s solution (Sigma-Aldrich, Missouri, USA) in a 1:1 proportion and mixed well. About 5 mL of Ficoll-Hypaque (Sigma-Aldrich, Missouri, USA) was carefully added to the bottom of the tube, such that the blood remained on top and was not mixed. The tubes were centrifuged for 30 minutes at 400 g. The upper solutions were discarded, leaving approximately 1.5 cm above the white ring of monocytes. Next, the monocytes were harvested and transferred to another tube. Then, a wash was performed by adding Hank’s solution to a final volume of 15 mL. After that, the tubes were centrifuged for 15 minutes at 500 g and the supernatants were discarded. This washing procedure was repeated twice. The pellet was resuspended in 1 mL of RPMI medium (Sigma-Aldrich, Missouri, USA) containing 15% human serum. The monocytes were cultured with medium and 15% human serum. The monocytes were either left unstimulated or were stimulated with SEB (R&D Systems, Minneapolis, USA) 1 μg/mL and incubated at 37 °C in a 5% CO2 incubator for three days.
Flow Cytometry
Take 100 mL of PBMCs that have been separated from the supernatant, then homogenized and incubated with 10 mL of TLR2/CD282 or TLR6/CD286 and CD14 antibodies (to separate monocytes from other mononuclear cells) for 15 minutes in the refrigerator (dark at 40 °C). Next, wash with 1% PBS or BSA twice. Then, centrifuge at 1500 rpm for 5 minutes without braking and discard the supernatant by pouring. After that, each tube was resuspended in 1% PBS or BSA. Finally, read using a flow cytometer with a reading duration of 10.000 events.
Statistical Analysis
Results were expressed as mean, standard deviation, and median. Statistically significant differences were defined as a p value less than 0.05. The Wilcoxon test was used to compare differences between SEB exposed and without exposure. The Mann–Whitney test was used to compare differences between the AD and healthy control.
Result
Demographics of Study Participants
The majority of the study participants with AD were female (75%), older than 18 years (75%), with a senior high school degree (30%), and were general employees (30%). While most of the healthy control group were females (65%), aged more than 18 years (95%), with an age range of 16–49 years old, with bachelor’s degrees (60%), and worked as general employees (45%), based on these comparability results (Table 1), the characteristics of age and gender in the two study groups showed no significant difference (p > 0.05).
 |
Table 1 Demographics of Study Participants |
Clinical characteristics of AD patients showed that the most common AD duration was more than six months (55%) and they had an AD exacerbation between 2 and 18 years of age (60%). Moderate AD was the most frequently found (70%), even though all participants in this study had a history of previous treatment (Table 2).
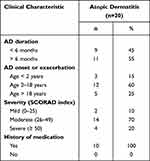 |
Table 2 Clinical Characteristics of Atopic Dermatitis Patients |
TLR2 Expression on Peripheral Blood Monocytes Induced by SEB During AD Exacerbation and Healthy Controls
The results of TLR2 expression on peripheral blood monocytes of AD patients and healthy controls before and after induction of SEB can be seen in Table 3. The mean expression of TLR2 on peripheral blood monocytes of AD patients after induction of SEB (45,489) was higher than without exposure (38,848) with p value 0.025.
 |
Table 3 Expression of TLR2 on Peripheral Blood Monocytes by Induction of SEB in AD Patients and Healthy Individuals |
The mean of TLR2 expression on peripheral blood monocytes was higher in healthy controls without exposure (26,843) than in SEB-induced (22,949) with p value 0.737. The comparison of TLR2 expression on peripheral blood monocytes of AD patients to healthy controls, without exposure or after SEB stimulation, were significant elevations (p = 0.000).
TLR6 Expression on Peripheral Blood Monocytes Induced by SEB During AD Exacerbation and Healthy Controls
The results of TLR6 expression on peripheral blood monocytes of AD patients and healthy controls before and after induction of SEB can be seen in Table 4. The mean expression of TLR6 on peripheral blood monocytes of AD patients after SEB induction (9.532) was higher than without exposure (7.912) with p value 0.023.
 |
Table 4 Expression of TLR6 on Peripheral Blood Monocytes by Induction of SEB in AD Patients and Healthy Individuals |
The mean of TLR6 expression on peripheral blood monocytes of healthy controls after induction of SEB (6054) was higher than without exposure (5387) with p value 0.100. There was no significant increase in TLR6 expression on peripheral blood monocytes of AD patients compared to healthy controls before induction of SEB (p = 0.304), but there was a significant increase after SEB induction (p = 0.011).
Discussion
During AD exacerbations, S. aureus may act as a conventional antigen or as a superantigen to activate T cells. As a powerful toxin belonging to the toxin family, SEB is referred to as a superantigen.15,16 This enterotoxin is reported to have a relationship with several disease events, one of which contributes to the development of numerous allergy disorders such as AD.17 Superantigen also induces immunoglobulin (Ig) E, which can aggravate allergic skin inflammation. Unlike conventional antigens, superantigens do not require processing and can bind directly to the surface of the major histocompatibility complex (MHC) class II molecule with T cell receptor (TCR) Vß-chain specificity, resulting in polyclonal T-cell stimulation. MHC class II molecules may be involved in the activation of TLR signaling.18 Liu et al have reported that MHC class II molecules are required for full activation of TLR-triggered innate responses.19
The majority of the innate immune response depends on PRRs’ ability to identify nonself molecules. TLRs are one of the most important classes of PRRs, especially those that recognize bacterial pattern.10 As membrane-associated receptors, TLRs are expressed at the cell surface, such as on monocytes, where they can survey the extracellular environment for pathogen clues and then tracking intracellular stimuli. TLR2 has been identified as a crucial receptor for S. aureus identification due to its ability to recognize lipoproteins, which are abundantly expressed in the cell wall of Gram-positive bacteria.14 Niebuhr et al reported that keratinocytes from AD patients showed a diminished response to TLR2 agonists, as seen by lower production of IL-6, IL-8, CCL20, and matrix metalloproteinase-9 (MMP-9).20 The production of Th2 cytokines and the inhibition of TLR expression are significant contributors to the increased incidence of skin infections. Both the lesional skin and non-lesional skin of AD patients have intensive bacterial colonization, such as by S. aureus, which is known to stimulate TLR2.21 Song et al reported that interactions between TLR2 activation and the increase of FcεRI expression occurred through the p38 pathway. This finding in patients with severe extrinsic AD might provide insight into how bacterial infection can exacerbate clinical AD symptoms.22
This study showed that the expression of TLR2 in AD monocyte was significantly higher than in monocyte from control subjects. This result is in accordance with a study by Tsybikov et al in Russia that reported CD14+ HLA-DR+ TLR2+ expression on peripheral blood monocytes of AD patients during exacerbations was significantly higher than healthy controls, and there was a strong statistical correlation between TLR2 expression on monocytes and AD clinical severity (measured by the objective SCORAD).23 This was considered to be an effect of increased colonization of S. aureus, which is found in 90% of patients with active AD. S. aureus provides potent TLR2 ligands that are involved in the pathogenesis and flare-ups of AD.
TLR2 and TLR6 can form a heterodimer to recognize diacyl lipopeptides such as lipoteichoic acid, which are present on the cell walls of Gram-positive bacteria, or macrophage-activating lipopeptide (MALP). After ligand recognition, the TLR6 receptor dimerizes with TLR2. Ligand-mediated dimerization is crucial for recruiting the adaptor proteins, which are necessary for signal transmission inside the cell.14 The TLR2/TLR6 heterodimer, similar to the majority of the TLRs, generally activates the MyD88-dependent intracellular signaling pathway, which leads to the nuclear translocation of NF-κB, causing the release of pro-inflammatory cytokines.10 Our study showed that there was no significant increase in TLR6 expression on peripheral blood monocytes of AD patients compared to healthy controls before induction of SEB. This result was also shown in a study by Yu et al, in which the levels of TLR6 mRNA were not altered in both groups of AD patients and healthy controls.24 It was speculated that the amount of TLR6 expression in monocytes is indeed small. To the authors’ knowledge, there have been no studies on the expression of TLR6 on peripheral blood monocytes induced by SEB in AD patients.
Staphylococcal enterotoxin B induced the maturation of monocyte-derived dendritic cells. SEB-activated dendritic cells were able to polarize naive T cells into the Th2 subset, as assessed by the qualitative analysis of cytokine secretion by T cells.25 The role of TLR2 signaling in Th1/Th2 commitment of T cells is debatable, and it might depend on the nature, dose, and timing of exposure to ligands on the one hand and the host’s genetic background on the other.26 This study showed that in the AD group, when we stimulated monocyte cultures with SEB, the expression of TLR2 and TLR6 in AD monocyte cultures increased significantly compared with unstimulated cultures. A study by Niebuhr et al found that SEB stimulation of monocytes resulted in a functional upregulation of TLR2.26 Mandron et al also investigated the analysis of TLR2 expression on dendritic cells responding to SEB. RT-PCR analyses revealed that TLR2 mRNA was expressed in monocyte-derived populations of immature dendritic cells.27 The significant increase in TLR2 and TLR6 after SEB exposure in AD patients may be due to TLR which is a homologous protein on the APC membrane and belonging to the PRR group, which serves as a functional receptor in activating leukocytes to fight pathogens.12 TLR signals cause ligands to attach the cell surface, causing cytoplasmic signaling molecules, specifically the MyD88 adapter protein, to react and activate IRAK and TRAF6 molecules, stimulating cytokine production and phagocytic activity.28 The malfunction of the TLR response in AD patients can be associated with deficits in the signaling components. Some studies point out that alterations in the MyD88 pathway are needed for the development of a SEB-induced AD-like phenotype and it has been demonstrated that SEB increases the expression of TLR2, improving the host’s reaction to other microbial products.12,29
The pathophysiologic situation at the site of skin inflammation and the contributions of monocytes and their TLRs to the pathophysiology of AD are still unclear and need further investigation based on the severity of AD.
Conclusion
TLR2 and TLR6 are believed to contribute to the pathogenesis of AD. Our results suggest that significantly increased expression of TLR2 and TLR6 on peripheral blood monocytes induced by SEB during exacerbation in AD patients. Thus, based on this study, it may be possible to inhibit the colonization of S. aureus or the production of TLR2/TLR6 in monocytes as a management therapy for AD. However, further research is needed to delineate the pathogenesis of AD in the context of TLR.
Acknowledgments
The authors wish to express their sincere gratitude to Yuli from the Integrated Research and Testing Laboratory and Farid from the Clinical Pathology Laboratory, Gadjah Mada University, Yogyakarta, Indonesia for providing technical support.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Simpson LE, Leung DYM, Eichenfield LF, Dan Boguniewicz M. Atopic dermatitis. In: Dalam KS, Amagai M, Bruckner AL, et al., editors. Fitzpatrick’s Dermatology in General Medicine: Dermatitis. Edisi Ke- 9. New York: McGraw-Hill; 2019:363–384.
2. Simon D, Wollenberg A, Renz H, Dan simon HU. Atopic dermatitis: Collegium Internationale Allergologicum (CIA) update 2019. Int Arch Allergy Immunol. 2019;178(3):207–218. doi:10.1159/000497383
3. Wisneiwski JA, Agrawal R, Minnicozzi S, et al. Sensitization to food and inhalant allergens in relation to age and wheeze among children with atopic dermatitis. Clin Exp Allergy. 2013;43(10):1160–1170. doi:10.1111/cea.12169
4. Bauer Stephen M. Atopic Eczema: genetic associations and potential links to developmental exposures. Int J Toxicol. 2017;36(3):1–12.
5. Al-Shobaili HA, Ahmed AA, Alnomair N, Alobead ZA, Dan Rasheed Z. Molecular genetic of atopic dermatitis: an update. Int J Health Sci. 2016;10(1):1–25. doi:10.12816/0031218
6. Zaniboni MC, Orfali RL, Samorano LP, Dan Aoki V. Skin barrier in atopic dermatitis: beyond filaggrin. An Bras Dermatol. 2016;91(4):472–478. doi:10.1590/abd1806-4841.20164412
7. Muluk NB, Altın F, Cingi C. Role of superantigens in allergic inflammation: their relationship to allergic rhinitis, chronic rhinosinusitis, asthma, and atopic dermatitis. Am J Rhinol Allergy. 2018;32(6):1–16.
8. Koymans KJ, Goldmann O, Christofer AQ, et al. The TLR2 antagonist staphylococcal superantigen-like protein 3 acts as a virulence factor to promote bacterial pathogenicity in vivo. J Innate Immun. 2017;9:561–573. doi:10.1159/000479100
9. Askarian F, Wagner T, Johannessen M, Nizet V. Staphylococcus aureus modulation of innate immune responses through Toll-like (TLR), (NOD)-like (NLR) and C-type lectin (CLR) receptors. Fems Microbiol Rev. 2018;42(5):656–671. doi:10.1093/femsre/fuy025
10. Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5(461):1–8. doi:10.3389/fimmu.2014.00461
11. Tokuyama M, Mabuchi T. New treatment addressing the pathogenesis of psoriasis. Int J Mol Sci. 2020;21(20):1–16. doi:10.3390/ijms21207488
12. Fournier B. The function of TLR2 during staphylococcal diseases. Front Cell Infect Microbiol. 2013;2(167):1–8. doi:10.3389/fcimb.2012.00167
13. Patra MC, Choi S. Recent progress in the development of Toll-like receptor (TLR) antagonis. Expert Opin Ther Pat. 2016;26(6):719–730. doi:10.1080/13543776.2016.1185415
14. Heine H, Riekenberg S. Toll-like receptor recognition of lipoglycans, glycolipids and lipopeptides. Microbial Glycobiol. 2009;31:623–635.
15. Yoshikawa FSY, JFd L, Sato MN, Ramos YAL, Aoki V, Orfali RL. Exploring the role of S. aureus toxin in atopic dermatitis. Toxin. 2019;321(11):1–13.
16. Geoghegan JA, Irvine AD, Foster TJ. Staphylococcus aureus and atopic dermatitis: a complex and evolving relationship. Trends Microbiol. 2018;26(6):484–497. doi:10.1016/j.tim.2017.11.008
17. Fries BC, Varshney AK, Crowe JE., Boraschi D, Rappuoli R. Bacterial Toxins—Staphylococcal enterotoxin B. Microbiol Spectr. 2013;1(2):1–21. doi:10.1128/microbiolspec.AID-0002-2012
18. Liu X, Zhan Z, Li D, et al. Intracellular MHC class II molecules promote TLR-triggered innate immune response by maintaining activation of the kinase Btk. Nat Immunol. 2011;12(5):416–425. doi:10.1038/ni.2015
19. Niebuhr M, Heratizadeh A, Wichmann K, Satzger I, Werfel T. Intrinsic alterations of pro-inflammatory mediators in unstimulated and tlr-2 stimulated keratinocytes from atopic dermatitis patients. Exp Dermatol. 2011;20:468–472. doi:10.1111/j.1600-0625.2011.01277.x
20. Kim JE, Kim JS, Cho DH, Park HJ. Molecular mechanisms of cutaneous inflammatory disorder: atopic dermatitis. Int J Mol Sci. 2016;17:1234. doi:10.3390/ijms17081234
21. Song Z, Deng X, Chen W, et al. Toll-like receptor 2 agonist Pam3CSK4 up-regulates FcepsilonRI receptor expression on monocytes from patients with severe extrinsic atopic dermatitis. J Eur Acad Dermatol Venereol. 2015;29:2169–2176. doi:10.1111/jdv.13172
22. Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi:10.3109/08830185.2010.529976
23. Tsybikov NN, Petrisheva I, Fevelova EV, Kuznik BI, Dan Magen E. Expression of TLR2 and TLR4 on peripheral blood monocytes during exacerbation of atopic dermatitis. Allergy Asthma Proc. 2015;36(6):140–145. doi:10.2500/aap.2015.36.3901
24. Yu Y, Zhang Y, Zhang J, et al. Impaired toll-like receptor 2-mediated Th1 and Th17/22 cytokines secretion in human peripheral blood mononuclear cells from patients with atopic dermatitis. J Trans Med. 2015;13(384):1–10. doi:10.1186/s12967-015-0744-1
25. Mandron M, Aries M-F, Brehm RD, et al. Human dendritic cells conditioned with Staphylococcus aureus toxin B (SEB) promote TH-2 cell polarization. J Allergy Clin Immunol. 2006;117:1141–1147. doi:10.1016/j.jaci.2005.12.1360
26. Niebuhr M, Schorling K, Heratizadeh A, Werfel T. Staphylococcal α-toxin induces a functional upregulation of TLR2 on human peripheral blood monocytes. Exp Dermatol. 2015;24:381–400. doi:10.1111/exd.12674
27. Mandron M, Aries M-F, Boralevi F, et al. Age-related differences in sensitivity of peripheral blood monocytes to lipopolysaccharide and Staphylococcus aureus toxin B in atopic dermatitis. J Invest Dermatol. 2008;128:882–889. doi:10.1038/sj.jid.5701112
28. Kissner TL, Ruthel G, Cisney ED, Ulrich RG, Fernandez S, Saikh KU. Myd88-dependent pro-inflammatory cytokine response contributes to lethal toxicity of staphylococcal enterotoxin b in mice. Innate Immun. 2011;17:451–462. doi:10.1177/1753425910374092
29. Fassbender S, Opitz FV, Johnen S, Forster I, Weighardt H. Myd88 contributes to staphylococcal enterotoxin b-triggered atopic dermatitis-like skin inflammation in mice. J Investig Dermatol. 2017;137:1802–1804. doi:10.1016/j.jid.2017.04.015
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
