Back to Journals » Nutrition and Dietary Supplements » Volume 9
Increased eating control and energy levels associated with consumption of bitter orange (p-synephrine) extract: a randomized placebo-controlled study
Authors Kaats GR, Leckie RB, Mrvichin N, Stohs SJ
Received 11 March 2017
Accepted for publication 12 May 2017
Published 12 July 2017 Volume 2017:9 Pages 29—35
DOI https://doi.org/10.2147/NDS.S136756
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chandrika J Piyathilake
Gilbert R Kaats,1 Robert B Leckie,2 Nate Mrvichin,1 Sidney J Stohs3
1Integrative Health Technologies, Inc., 2R.B. Leckie Research Consultants, San Antonio, TX, 3Creighton University Medical Center, Omaha, NE, USA
Abstract: Using a placebo-controlled double-blinded 30-day protocol, 40 overweight adults were asked to consume a chocolate-flavored chew 15–30 min before their two largest meals of the day. The chews contained either a placebo or an “active” product (100 mg of a bitter orange extract, standardized to 51.5 mg p-synephrine). Subjects completed a 13-item Weight Control Support Scale (WCSS) containing eating control, energy level, and palatability subscales daily throughout the study. All 40 subjects completed the study. No adverse effects were reported in either the placebo or active groups. As compared to placebo, subjects consuming the active product reported statistically more (p≤0.001) positive responses on the WCSS as well as on each of the three subscales. This study suggests that, as compared to a placebo control, consuming a chew containing bitter orange extract (51.5 mg p-synephrine) 15–30 min before the two largest meals of the day resulted in a statistically significant greater and more positive response to eating/appetite control and a weight-control support scale.
Keywords: bitter orange extract, p-synephrine, Citrus aurantium, appetite suppression, energy, safety
Introduction
The most parsimonious recommendation for weight control is simply to eat less and move more. Dietary supplements that help facilitate these behaviors can make a significant contribution to weight management as long as the supplement is safe, efficacious, and palatable. The objective of this study was to evaluate the extent to which a daily chew product containing p-synephrine in the form of bitter orange (Citrus aurantium) extract could support these three weight-control criteria.
Numerous studies in humans and animals have demonstrated the safety and efficacy of bitter orange (Citrus aurantium) extract and its primary protoalkaloid p-synephrine.1–3 Kaats et al4 have shown that consumption of 98 mg p-synephrine per day for 60 days by human subjects was without adverse effects on blood pressure, heart rate, blood chemistry, or blood cell counts as compared to placebo controls. Other studies have found that p-synephrine facilitated weight loss and improved weight management.3
Ratamess et al5 have shown that consuming 100 mg p-synephrine in the form of two chews 45 min before exercise significantly increased resistance exercise performance (total repetitions and volume load) without increasing blood lactate and ratings of perceived exertion, relative to control and placebo groups. The consumption of 100 mg of caffeine with 100 mg p-synephrine increased mean power and velocity of squat performance without increasing total repetitions and volume load. No adverse effects were reported by any group.
The adrenergic receptor-binding properties of p-synephrine have been assessed and reviewed.6 In general, p-synephrine exhibits little or no binding to α1, α2, β1, and β2-adrenergic receptors and, therefore, it does not and would not be expected to exert significant cardiovascular effects.3,4,6,7 p-Synephrine has been shown to exhibit modest binding to β3-adrenergic receptors,8–10 the activation of which is associated with enhanced lipolysis, glycemic control, thermogenesis, and appetite control.11
Previous studies have shown that p-synephrine acts as a non-stimulant thermogenic agent, increasing lipolysis and the metabolic rate.3,5,12 The majority of studies have been conducted with the patented bitter orange extract (Advantra Z®, Novel Ingredients, East Hanover, NJ) which is standardized to p-synephrine. No studies have focused on the ability of bitter orange extract and p-synephrine to suppress appetite and depress food intake. However, in a survey regarding the use of dietary supplements containing bitter orange, the primary reasons for use consisted of appetite suppression as well as weight loss and energy enhancement.13
This 30-day study was designed to examine the extent to which consuming a daily chew enhanced with 51.5 mg p-synephrine 15–30 min before the two largest meals of the day (103 mg p-synephrine/day) could support the aforementioned criteria.
Methods
Forty healthy subjects were enrolled in this 30-day study and were randomly assigned to one of the four study groups, as shown in the flow diagram in Figure 1, with 10 subjects assigned to each of the four study groups. All subjects signed an informed consent form. All subjects reported no gastrointestinal, cardiovascular, liver, kidney, or thyroid diseases. Subjects were to have refrained from taking any weight management products for at least the previous month. Of the 40 subjects, nine were males (average age of 48.4±12.9 [SD]) and 31 were females (average age 51.4±9.8 [SD]). A crossover component of the study facilitated assessment of product effectiveness at 15 days, as compared to at 30 days (Figure 1). The nature of the study was described to the participants based on the weight support scale questions noted in Figure 2. This study was approved by the Solutions Independent Review Board.
  | Figure 2 Weight Control Support Scale. |
The study sponsor packaged the placebo and active bottles of chews into two bulk packages labeled “A” or “B” with no indication as to which of the two boxes were active agent or placebo. The two bulk packages were provided to a study trustee who, using a random numbers program, assigned subject numbers to each of the product bottles which were then given to the study’s principal investigator (PI). Bottles were subsequently assigned randomly to subjects as they enrolled. Thus, study monitors, the PI, the trustee, and the subjects were blinded to the active and placebo allocation. Once study data were analyzed by the PI using the “A” and “B” identifiers, the dataset was provided to the trustee and the study sponsor provided the trustee with the active/placebo coding for a final analysis of the study outcome.
Subjects were given 30 chocolate-flavored chews containing either a placebo or 51.5 mg p-synephrine in the form of 100 mg bitter orange extract (Advantra Z). The chews were supplied by Novel Ingredients, and had been designed to contain 50 mg p-synephrine. An independent laboratory determined and confirmed that each chew contained an average of 51.5 mg p-synephrine. The placebo and active chews differed only by the presence of the bitter orange extract in the active chews. No caffeine or other active ingredients were present in the chews. After 15 days, the subjects reported to the research center clinic and were again given 30 chews containing either a placebo or 51.5 mg p-synephrine. The experimental dose of p-synephrine was determined based on previous studies.3
Subjects were instructed to slowly consume one chew before the largest two meals each day after holding the chew in their mouths for several minutes before swallowing. At the end of each study day, subjects recorded the number of chews they consumed that day and expressed their agreement or disagreement with the 13-items on the Weight Control Support Scale (WCSS) shown in Figure 2. The WCSS was created by the authors for this study based on a review of the literature cited earlier. Thus each subject was asked to complete 380 ratings over the study period. Items 1–7 were designed to assess eating and appetite control; items 8–11 to assess energy levels; and items 12 and 13 to assess palatability. As a measure of safety, subjects were asked to maintain a daily record of any adverse effects or discomfort they attributed to the chews.
Statistical methods
The statistical tools used were an analysis of variance (ANOVA) and a two-tailed t-test. Both were performed using the built-in functions of Microsoft Excel. Because the study had multiple subgroups, the first step in the statistical analysis was to conduct an ANOVA on the four subgroups. An ANOVA was also conducted on each of the 13 questions separately.
The subgroup using the active product for 30 days was compared with the subgroup using a placebo for 30 days by using a t-test. In addition, we compared every other pair of subgroups using a t-test. (Four sub-groups can be paired in six ways.)
Finally, we looked at every daily questionnaire from subjects taking the active product at the time that they completed the questionnaire. That is, we took all the questionnaires from the group taking the active product, the first 15 days of questionnaires from the group taking the active product for the first 15 days, and the last 15 days of questionnaires from the group taking active product for the last 15 days. We compared these questionnaires head-to-head using a t-test with the comparable questionnaires produced by subjects taking a placebo at the time they completed the questionnaire.
Results
All 40 subjects completed the study and provided 91% of the requested daily ratings. There were no statistically significant differences in the number of daily ratings completed by each of the four study groups. With regard to safety, there were no adverse events reported by subjects in any of the four subgroups.
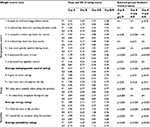
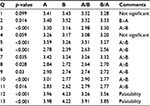
The subgroups were designated as follows: “A” is the subgroup that took active product (p-synephrine) for 30 days; “B” is the subgroup that took placebo for 30 days; “A/B” is the subgroup that took active product for 15 days followed by 15 days of taking a placebo; and “B/A” is the subgroup that took placebo for 15 days followed by 15 days of taking the active product.
Table 1 shows the initial ANOVA for the four subgroups. This table shows that the differences in the four means are significant and warrant further analysis.
Table 2 shows a breakdown of the results by question. This table shows that subgroup A, taking only active product for 30 days, reported significantly higher positive scores on the WCSS (p<0.001) than any other group. Furthermore, the scores on the three WCSS subscales – appetite control, energy, and palatability – were significantly higher (p<0.001).
Table 3 provides a summary of ANOVA results broken down question by question. Of the 13 questions, 11 showed a significant p-value, with the majority of those showing p<0.001. Of the 11 significant questions (p<0.05), three questions showed the placebo with a better result than the active chew. Of these, two questions dealt not with efficacy but with palatability, which indicates that, in future work, steps should be taken to improve this metric. However, there was one efficacy question (Q2) which showed a preference for the placebo. We believe this is an anomaly of the sort that is reasonably expected with the small sample size associated with a single question.
A direct comparison between all questionnaires completed by subjects taking active product (A) compared to all questionnaires completed by subjects taking a placebo (B) was made. Subjects taking active product (A) reported a significantly better (p=0.001) overall weight control score (WCSS) than subjects taking a placebo (B). Table 2 shows that the sub-scores dealing with eating/appetite control and energy were also significantly better (p<0.001) for those taking the active product (A).
Discussion
Based on subject self-reports, the results indicate that the consumption of chews containing 51.5 mg p-synephrine twice daily before the two largest meals of the day enhanced eating/appetite control (p<0.001) and also increased energy level (p<0.001) Table 1.
With respect to energy production, a single dose of p-synephrine (51.5 mg) in human subjects exhibited a 65-kcal increase in resting metabolic rate (RMR) relative to the placebo group 75 min after oral ingestion.7 The addition of the bioflavonoids naringin and hesperidin to the p-synephrine further increased the RMR – an increase that was statistically significant with respect to the placebo control.
Calapai et al14 conducted one of the earliest and most detailed studies on the effects of a bitter orange extract on food intake. Rats were orally given bitter orange extracts with two concentrations of p-synephrine (4% and 6%). Dose and time-dependent decreases in food intake were demonstrated, clearly indicating the appetite-suppression effects of these extracts. At a dose of 20 mg/kg of a 6% extract, food intake by these animals decreased by approximately 40% after 7 days.
A study was conducted involving the tissue lipid-lowering effects of an infusion of C. aurantium (bitter orange) and Rauwolfia vomitoria in genetically diabetic mice.15 The product was administered orally for 6 weeks. Significant weight loss was observed in the treated group, relative to the untreated control group. The most significant effect was observed over the first few days of treatment where treated animals consumed less food and drank less water. Increased lipid mobilization was also observed in the treated animals. The p-synephrine content of the product was not reported.
Arbo et al16 treated mice orally for 28 days with a range of doses of a bitter orange extract containing 7.5% p-synephrine. A dose-dependent reduction in weight gain was observed. The authors assessed various antioxidant markers and demonstrated significant antioxidant effects, but did not measure effects on food intake.
The effect of an immature Citrus peel extract on body weight gain and food intake was examined in high-fat-diet-induced obese mice.17 The extract was administered orally at a dose of 150 mg/kg per day for 70 days. Body weight gain, adipose tissue weight, serum total cholesterol, and triglycerides decreased significantly relative to animals fed the normal diet as well as the high-fat diet. A statistically significant decrease in food intake was observed relative to the normal diet, while a small but statistically insignificant decrease in food intake was observed in the treated animals relative to the high-fat diet. No information was provided with regard to chemical composition of the Citrus peel extract and it is, therefore, not known what may have contributed to the observed effects.
Although the above studies demonstrate a decrease in body weight gain and food intake, the mechanism of action of p-synephrine and bitter orange extract involved in the appetite suppression is not well understood. Appetite suppression may be due to several possible mechanisms.
In a rat model involving diet-induced obesity, the daily oral administration of a combination of bitter orange (C. aurantium) extract and Rhodiola rosea extract for 10 days resulted in a significant decrease in food intake, a 30% reduction in visceral white adipose tissue, a 15% increase in hypothalamic nor-epinephrine, and a 150% increase in frontal cortex dopamine compared with the pair-fed control group.18 This study not only demonstrated beneficial effects on food intake, lipid mobilization, and weight management but also provided information on the potential mechanism of action.
Kim et al19 showed that p-synephrine exhibits antidepressant-like effects, and proceeded to characterize these neurological effects in various murine model systems. The role of neurotransmitters in appetite suppression is well known,20,21 and may play a role in the appetite-suppressant effects of p-synephrine. The appetite suppression may also be due in part to the antioxidant and/or anti-inflammatory effects of bitter orange extract and p-synephrine.16,22
Because bitter orange extracts have traditionally been used as appetite suppressants and weight-loss products, several studies23,24 have examined the effects of bitter orange extract and p-synephrine on various anabolic and catabolic pathways in perfused rats. Bitter orange extract and p-synephrine both increased glycolysis, gluconeogenesis, and oxygen uptake – actions that are compatible with the weight-management effects of these ingredients.
The limitations of the study relate to the fact that the results are based upon perceptions and a questionnaire, with no comparison to direct outcomes. Furthermore, small differences in taste of the two products were noted although the participants did not know which chew was the placebo or the active test product and were provided no information that might identify the products. Future longer term studies should compare appetite suppression, energy, and tolerance directly with weight loss/weight management or other appropriate endpoint as sports performance.
Conclusion
Using a randomized double-blinded placebo-controlled protocol, this study provides evidence that, as compared to a placebo group, consumption of a chocolate-flavored chew containing bitter orange extract (51.5 mg p-synephrine) 15–30 min before the two largest meals of the day (103 mg p-synephrine per day) for 15 or 30 days was associated with statistically significant increases in self-reported appetite control and energy levels without any measurable adverse effects. These results are consistent with previous studies in humans and animals that have reported appetite suppression, increased energy, and decreases in food intake associated with bitter orange extract, a non-stimulant dietary supplement containing p-synephrine.
Acknowledgment
This study was funded by a grant from Novel Ingredients, Inc. to Integrative Health Technologies, Inc., an independent clinical research organization.
Disclosure
SJS has served as a consultant for Novel Ingredients. At no time did Novel Ingredients have access to the study data or analyses. The authors are solely responsible for the content and writing of this manuscript. The other authors report no conflicts of interest in this work.
References
Stohs SJ, Preuss HG. The safety of bitter orange (Citrus aurantium) and its primary protoalkaloid p-synephrine. HerbalGram. 2011;89:34–39. | ||
Stohs SJ, Preuss HG, Shara M. The safety of Citrus aurantium (bitter orange) and its primary protoalkaloid p-synephrine. Phytother Res. 2011;25(10):1421–1428. | ||
Stohs SJ, Preuss HG, Shara M. A review of the human clinical studies involving Citrus aurantium (bitter orange) extract and it primary protoalkaloid p-synephrine. Int J Med Sci. 2012;9(7):527–538. | ||
Kaats GR, Miller H, Preuss HG, Stohs SJ. A 60 day double-blind, placebo-controlled safety study involving Citrus aurantium (bitter orange) extract. Food Chem Toxicol. 2013;55:358–362. | ||
Ratamess NA, Bush JA, Kang J, et al. The effects of supplementation with p-synephrine alone and in combination with caffeine on resistance exercise performance. J Int Soc Sports Nutr. 2015;12:35. | ||
Stohs SJ, Preuss HG, Shara M. Review of the receptor-binding properties of p-synephrine as related to its pharmacological effects. Oxid Med Cell Longev. 2011;2011:482973. | ||
Stohs SJ, Preuss HG, Keith SC, Keith PL, Miller H, Kaats GR. Effects of p-synephrine alone and in combination with selected bioflavonoids on resting metabolism, blood pressure, heart rate and self-reported mood changes. Int J Med Sci. 2011;8(4):295–301. | ||
Carpéné C, GalitzkyJ, Fontana E, Algié C, Lafontan M, Berlan M. Selective activation of beta3-adrenoceptors by octopamine: comparative studies in mammalian fat cells. Naunyn Schmiedebergs Arch Pharmacol. 1999;359(4):310–321. | ||
Carpéné MA, Testar X, Carpéné C. High doses of synephrine and octopamine activate lipolysis in human adipocytes, indicating that amines from Citrus might influence adiposity. In: Hayat K, editor. Citrus. Ney York: Nova Science Publishers Inc; 2014:Chapter 8:141–168. | ||
Mercader J, Wanecq E, Chen J, Carpéné C. Isopropylnorsynephrine is a stronger lipolytic agent in human adipocytes than synephrine and other amines present in Citrus aurantium. J Physiol Biochem. 2011;67(3):443–452. | ||
Alemzadeh R, Karlstad MD, Tushaus K, Buchholz M. Diazoxide enhances basal metabolic rate and fat oxidation in obese Zucker rats. Metabolism. 2008;57(11):1597–1607. | ||
Stohs SJ, Badmaev V. A review of natural stimulant and non-stimulant thermogenic agents. Phytother Res. 2016;30(5):732–740. | ||
Klonz KC, Timbo BB, Street D. Consumption of dietary supplements containing Citrus aurantium (bitter orange)--2004 California behavioral risk factor surveillance survey (BRFSS). Ann Pharmacother. 2006;40(10):1747–1751. | ||
Calapai G, Firenzuoli F, Saitta A, et al. Antiobesity and cardiovascular toxic effects of Citrus aurantium extracts in the rat: a preliminary report. Fitother. 1999;70(6):586–592. | ||
Campbell JI, Mortensen A, Mølgaard P. Tissue lipid lowering-effect of a traditional Nigerian anti-diabetic infusion of Rauwolfia vomitoria foliage and Citrus aurantium fruit. J Ethnopharmacol. 2006;104(3):379–386. | ||
Arbo MD, Schmitt GC, Limberger MF, et al. Subchronic toxicity of Citrus aurantium L. (rutaceae) extract and p-synephrine in mice. Regul Toxicol Pharmacol. 2009;54(2):114–117. | ||
Kang SI, Shin HS, Kim HM, et al. Immature Citrus sunki peel extract exhibits antiobesity effects by β-oxidation and lipolysis in high-fat diet induced obese mice. Biol Pharm Bull. 2012;35(2):223–230. | ||
Verpeut JL, Walters AL, Bello NT. Citrus aurantium and Rhodiola rosea in combination reduce visceral white adipose tissue and increase hypothalamic norepinephrine in a rat model of diet-induced obesity. Nutr Res. 2013;33(6):503–512. | ||
Kim KW, Kim HD, Jung JS, et al. Characterization of antidepressant-like effects of p-synephrine stereoisomers. Naunyn Schmiedebergs Arch Pharmacol. 2001;364(1):21–26. | ||
Halford JC. Pharmacology of appetite suppression: implication for the treatment of obesity. Curr Drug Targets. 2001;2(4):353–370. | ||
Rodgers RJ, Holch P, Tallett AJ. Behavioral satiety sequence (BSS): separating wheat from chaff in the behavioral pharmacology of appetite. Pharmacol Biochem Behav. 2010;97(1):3–14. | ||
Kang SR, Han DY, Park KI, et al. Suppressive effect on lipopolysaccharide-induced proinflammatory mediators by Citrus aurantium L. in macrophage RAW 264.7 cells via NF-κB signal pathway. Evid Based Complement Alternat Med. 2011;2011:pii: 248592. | ||
Peixoto JS, Comar JF, Moreira CT, et al. Effects of Citrus aurantium (bitter orange) fruit extracts and p-synephrine on metabolic fluxes in the rat liver. Molecules. 2012;17(5):5854–5869. | ||
de Oliveira AL, Comar JF, de Sá-Nakanishi AB, Peralta RM, Bracht A. The action of p-synephrine on hepatic carbohydrate metabolism and respiration occurs via both Ca (2+)-mobilization and cAMP production. Mol Cell Biochem. 2014;388(1–2):135–147. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.