Back to Journals » Infection and Drug Resistance » Volume 16
Increase in Multidrug Resistant Neisseria gonorrhoeae FC428-Like Isolates Harboring the Mosaic penA 60.001 Gene, in Nanjing, China (2017-2020)
Authors Zhao Y, Le W, Genco CA, Rice PA, Su X
Received 21 February 2023
Accepted for publication 8 June 2023
Published 23 June 2023 Volume 2023:16 Pages 4053—4064
DOI https://doi.org/10.2147/IDR.S408896
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Yuanyuan Zhao,1 Wenjing Le,1 Caroline A Genco,2 Peter A Rice,3 Xiaohong Su1
1Sexually Transmitted Disease Clinic, Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, Nanjing, People’s Republic of China; 2Department of Immunology, Tufts University School of Medicine, Boston, MA, USA; 3Division of Infectious Diseases and Immunology, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, MA, USA
Correspondence: Xiaohong Su, Sexually Transmitted Disease Clinic, Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, No. 12, Jiangwangmiao Road, Nanjing, 210042, Jiangsu Province, People’s Republic of China, Email [email protected]
Background: Since the first Chinese report of the ceftriaxone-resistant Neisseria gonorrhoeae FC428 clone in 2016, additional FC428-like, penA 60.001 isolates have been identified in China.
Objective: To document the rise in penA 60.001 isolates in Nanjing, China, and characterize their molecular and epidemiological features.
Methods: N. gonorrhoeae minimum inhibitory concentrations (MICs, mg/L) for ceftriaxone, cefixime, penicillin, tetracycline, ciprofloxacin, azithromycin, spectinomycin, gentamicin and zoliflodacin were determined by agar dilution. MICs for ertapenem were measured by E-test. N. gonorrhoeae antimicrobial sequence typing (NG-STAR) of seven loci (penA, mtrR, porB, ponA, gyrA, parC and 23S rRNA) was analyzed together with N. gonorrhoeae multiantigen sequence typing (NG-MAST) and multilocus sequence typing (MLST). Phylogenetic analysis was also performed using whole genomic sequencing (WGS).
Results: Fourteen FC428-related penA 60.001 N. gonorrhoeae infections were identified out of 677 infections from 2017 to 2020, in Nanjing, representing an incremental yearly rise in the percentage of the city’s N. gonorrhoeae isolates that were FC428-related. Seven FC428-related N. gonorrhoeae infections were acquired in Nanjing, proper; four others in eastern Chinese cities and three from unknown locations. All FC428-related isolates were resistant to ceftriaxone, cefixime, ciprofloxacin, tetracycline and penicillin but susceptible to spectinomycin, gentamicin, ertapenem and zoliflodacin; three strains were resistant to azithromycin. penA 60.001 isolates displayed closely related MLST types and NG-STAR types but relatively distant NG-MAST types. WGS showed a phylogenetic analysis that intermingled with other international isolates.
Conclusion: penA 60.001 N. gonorrhoeae isolates emerged in Nanjing, China, beginning in 2017, and have continued to rise.
Keywords: Neisseria gonorrhoeae, penA 60.001, ceftriaxone-resistance, FC428, WGS phylogenetic analysis
Introduction
Gonorrhea is the second most common bacterial sexually transmitted infection (Chlamydia is first), with a worldwide estimated incidence of new gonococcal infections in 2020 of 82.4 million cases.1 If untreated, gonorrhea in women can lead to severe complications, such as endometritis and salpingitis (pelvic inflammatory disease [PID]) that may result in substantial morbidity including infertility and ectopic pregnancy; in men epididymitis can also result in infertility.2 Furthermore, Neisseria gonorrhoeae has developed antimicrobial resistance to almost all antibiotic classes used for treatment that now requires intensive surveillance of gonococcal isolates for antibiotic susceptibility.3
Presently, ceftriaxone, an extended-spectrum cephalosporin (ESC) represents first-line treatment for gonorrhea. However, individual strains of N. gonorrhoeae with high-level resistance to ESCs have emerged sporadically outside of China; in Japan (eg, N. gonorrhoeae strains H041, GU140106 and most recently FC428),4–6 Australia (strain A8806),7 France (strain F89),8 Italy (strain G2891).9 Notably, the FC428 clone remains prominent; first described in Japan in 2015;6 since then, FC428-related sub-clones or FC428-like isolates (with a close phylogenomic relationship to FC428) have been identified in Australia (strains A7846 and A7536),10 Canada (47,707),11 Denmark (GK124),12 France (F90)13 and Ireland (IR72),14 thereby confirming international spread of FC428-related sub-clones that possess ESC resistance. A common feature of FC428 and FC428-like strains is possession of an ESC-resistant genetic determinant; the mosaic penA 60.001 gene.6,10–14 At present, the surveillance of FC428/FC428-like isolates is a focus in tracking emergence and spread of resistant N. gonorrhoeae strains. Notably, infections with FC428/FC428-related sub-clones have been associated with travel to the Asia-Pacific region, now including its identification in several distinct regions in China.15–21 Here, we report 14 cases of N. gonorrhoeae that possessed the FC428-associated penA 60.001 gene, isolated from 2017 to 2020 in Nanjing, China. We compared their molecular types, also including whole genome sequences (WGSs), with other FC428-like isolates and identified their contribution, overall, to the reported emergence of FC428-like N. gonorrhoeae isolates in China.
Materials and Methods
Isolation of Neisseria gonorrhoeae
Gonococcal isolates were collected from male subjects with symptomatic urethritis (urethral discharge and/or dysuria) and their female sex partners who were patients attending the STD Clinic at the Institute of Dermatology, Chinese Academy of Medical Sciences, Nanjing, China. Urethral or cervical exudates were collected using cotton swabs and plated immediately onto Thayer-Martin medium (Zhuhai DL Biotech, China) and incubated in candle jars at 36°C for 24 to 48 h. Colonial morphology, Gram’s stain, and oxidase testing were used to identify N. gonorrhoeae, which was then sub-cultured onto GC medium (chocolate agar base) (Difco, Detroit, MI) supplemented with 1% Isovitalex (Oxoid, USA). Gonococcal colonies were suspended in tryptone-based soy broth and stored frozen (−80°C).
Antimicrobial Susceptibility Testing
Mean inhibitory concentrations (MICs) for penicillin, tetracycline, ciprofloxacin, azithromycin, cefixime, ceftriaxone, spectinomycin, gentamicin and zoliflodacin were determined by agar dilution, according to the Clinical and Laboratory Standards Institute (CLSI) guidelines. For ertapenem, the E-test method was used.22,23 Using CLSI24 and the European Committee on Antimicrobial Susceptibility Testing (EUCAST)25 criteria for antimicrobial resistance (AMR), the following MIC breakpoints were used to ascertain resistance: ≥0.25 mg/L, ceftriaxone and cefixime; ≥1 mg/L, azithromycin and ciprofloxacin; ≥128 mg/L, spectinomycin and ≥2 mg/L, penicillin and tetracycline. Breakpoints for gentamicin, ertapenem and zoliflodacin have not been established formally by CLSI but we used MICs ≥32 mg/L for gentamicin and ≥0.5 mg/L for zoliflodacin, which have been used previously.26,27 Using Haemophilus influenzae criteria defined by CLSI, an MIC ≤ 0.5 mg/L for ertapenem was considered susceptible.28 β-lactamase production was determined by paper acidometric testing.29
Molecular Epidemiologic Typing
We employed molecular epidemiologic typing: NG-MAST (http://www.ng-mast.net); MLST (http://pubmlst.org/neisseria/) and NG-STAR (https://ngstar.canada.ca), which were analyzed as described previously;30–32 for NG-MAST; porB and tbpB, for MLST; abcZ, adk, aroE, fumC, gdh, pdh and pgm and for NG-STAR; penA, mtrR, porB, ponA, gyrA, parC and 23S rRNA.
Whole Genome Sequencing (WGS) and Phylogenetic Analysis
Genomic DNA was extracted using QIAxtractor DX Kits (QIAGEN, Hilden, Germany). Whole genomes of 13/14 strains were successfully sequenced using the HiSeq (Illumina, San Diego, CA, USA) and PacBio RSII SMRT (Pacific Biosciences, Menlo Park, CA, USA) platforms at Genewiz Inc. (Suzhou, China). The complete genomic sequences of the thirteen isolates were uploaded to NCBI (BioProject ID: PRJNA553852, PRJNA553854 and PRJNA916595). Phylogenetic analysis was performed as previously described.16 Sequences were aligned with the FC428 genome (BioProject ID: PRJDB5915) and single nucleotide polymorphisms (SNPs) identified using MUMmer software. Repetitive regions and SNPs in putative recombinogenic regions were removed using SyRI software. All available whole-genome sequences or short-read sequences of FC428-related clones were similarly analyzed. Iqtree was used to analyze the phylogenetic relationship (maximum-likelihood method, bootstrap 1000) and to visualize the phylogenetic tree.
Statistical Analyses
SPSS 23 was used to perform statistical analyses. Comparisons of the rates of isolation of FC428-like isolates over time were performed using a logistic regression test of trend; P ≤ 0.05 (2-sided) was considered significant.
Results
Between 2017 and 2020, we identified 14 N. gonorrhoeae FC428-like isolates with high-level resistance to ceftriaxone and cefixime in Nanjing, which represented an incremental yearly rise in the percentage of the city’s N. gonorrhoeae isolates that were FC428-like: 0.44% in 2017 (1/229); 0.49% (1/204) in 2018; 4.73% in 2019 (7/148) and 5.21% in 2020 (5/96), (χ2=12.467, P < 0.001) (Figure 1). Characteristics of subjects infected with FC428-like N. gonorrhoeae are shown in Table 1. Thirteen isolates were from men and one from a woman (sexual contact of a man with gonococcal infection with positive nucleic amplification test result for N. gonorrhoeae); none had traveled outside of China. All subjects had uncomplicated gonococcal infection and reported 1 or 2 sex partners in the recent month; partners were from Nanjing, Shanghai, Suzhou, Taizhou and Yancheng, indicating the presence of ESC-resistant FC428-like N. gonorrhoeae in (certain) eastern Chinese cities. Ten men provided sexual histories: five indicated coitus with regular partners only, five with non-regular partners. 9/14 took antibiotics (levofloxacin or cephalosporin) before attending the clinic. Microscopic examination of gram stained urethral (or cervical) smears revealed >10 polymorphonuclear neutrophils (PMNs)/per high-power field and gram-negative intracellular diplococci in all 14. All subjects were treated with spectinomycin: two that were coinfected with Chlamydia trachomatis were also treated with doxycycline (0.1g, twice a day) for ten days. Twelve had total resolution of symptoms and signs; 2 had residual urethral symptoms but no discharge. Two subjects had tests-of-cure; both of which were negative (Table 1).
 |
Table 1 Characteristics of 14 Subjects with Uncomplicated Gonococcal Infection, Nanjing, China |
Antimicrobial susceptibility testing of the 14 isolates showed similar resistance profiles (Table 2); all were resistant to ceftriaxone (MICs ≥0.5 mg/L), cefixime (MICs ≥2 mg/L), ciprofloxacin (MICs ≥16 mg/L), tetracycline (MICs ≥1 mg/L) and penicillin (MICs ≥2 mg/L) but were susceptible to spectinomycin, gentamicin, ertapenem and zoliflodacin. Three strains (NJ1914215, NJ203279 and NJ209649) were resistant to azithromycin (MIC, 8 mg/L); the remaining isolates were sensitive (0.25 mg/L).
 |
Table 2 Antimicrobial Susceptibilities of Mosaic penA 60.001 Multidrug Resistant N. Gonorrhoeae Strains Isolated in Nanjing, China |
Sequence typing (ST) analysis showed that the 14 isolates displayed closely related MLST and NG-STAR types but relatively distant NG-MAST types (Table 3). Three strains (NJ189125, NJ195417 and NJ1911400) belonged to ST3435, identical to FC428; the NG-MAST of NJ1711654 (ST17114) differed in porB by a single base pair (tbpB was identical). However, the remaining 10 isolates belonged to 6 different MG-MAST types and displayed relatively large differences in the number of SNPs in porB and tbpB compared to FC428: ST19387 (22 SNPs in porB [1 isolate]); ST19389 (23 SNPs in porB [1isolate]); ST19489 (24 SNPs in porB [2 isolates]); ST20048 (25 SNPs in porB and 53 SNPs in tbpB [3 isolates]); ST20051 (16 SNPs in porB and 81 SNPs in tbpB [1 isolate]); ST22091 (29 SNPs in porB [1 isolate]) and ST21683 (25 SNPs in porB and 81 SNPs in tbpB [1 isolate]). Nonetheless, the 14 isolates belonged to only 3 MLST types: 10 were MLST1903, identical to FC428; three were MLST 7363 (1 SNP in abcZ and 1 SNP in fumC) and one was MLST 11710 (7 SNPs in aroE and 1 SNP in fumC). NG-STAR types were identified as: ST 233 (5 isolates); ST 2238 (3 isolates); ST 1961 and ST 1143 (2 isolates each) and ST 3523 and ST 2239 (1 isolate each). A few SNPs were identified in porB (ST 1143, ST 2239 and ST 3523), parC (ST 2238), 23S rRNA (ST 2238) and gyrA (ST 1961) alleles. NG-STAR typing identified mosaic penA 60.001 in all 14 strains. Notably, 3 strains (NJ1914215, NJ203279 and NJ209649) possessed a C2611T mutation in 23S rRNA and were resistant to azithromycin.
 |
Table 3 Molecular Characteristics of Mosaic penA 60.001 Multidrug Resistant N. Gonorrhoeae Strains Isolated in Nanjing, China |
Whole genomic sequencing (WGS) and resultant phylogenetic analysis demonstrated that 13 FC428-related clones were derived from five clades (A-E in Figure 2 [sequencing of NJ203279, the 14th isolate, was unsuccessful]). NJ1711654, NJ189125, NJ195417 were in clade A (similar to BJ16148 isolated in Beijing)15 and possessed 100–200 SNPs compared to FC428. NJ196610, NJ197542 and NJ1911400 belonged to a newly identified clade in our collection (clade B) and possessed 283–438 SNPs compared to FC428. NJ208430, NJ208756 and NJ1913940, also a new clade in our collection (clade C), possessed 297–566 SNPs compared to FC428. NJ1914215, NJ206649, NJ1914646 (clade D) represented isolates similar to seven additional isolates in the phylogenetic tree from Hangzhou.19 Genomic sequences of NJ20204705 and FC428 differed by >1000 SNPs; NJ20204705 was more closely related to G97687 and G7944, which are English isolates.33 However, NJ20204705 was susceptible to azithromycin (MIC 0.25ug/mL) while G97687 and G7944 were highly resistant to azithromycin (and ESCs).
Discussion
Sustained spread of N. gonorrhoeae FC428 and FC428-like isolates has been demonstrated by their continued emergence, suggesting a possible fitness advantage in humans. However, experiments with N. gonorrhoeae FC428 (in vitro and in vivo in mouse models) have shown ambiguous changes in fitness,34 distinct from ceftriaxone-resistant gonococcal strains H041 and F89, for example, that harbor mosaic penA alleles and where fitness is reduced,35 possibly explaining why these strains have not been transmitted widely.35 Resistance of N. gonorrhoeae FC428 to ESCs, first reported in Japan in 2015,6 and identified subsequently in Europe,10,12–14,36,37 North America,11,38 Southeast Asia (Vietnam,39 Singapore40 but not Thailand41–43), is caused by the mosaic penA-60.001 allele. In addition to the 14 isolates reported here, thirty-five FC428-like isolates have also been reported from 7 eastern Chinese cities (Figure 3),15–19,21,44,45 surpassing the 31 isolates that have been reported from other parts of the world.10–14,36–40,46 We report that FC428/FC428-like gonococcal isolates identified in Nanjing increased markedly from 2017 to 2020, exceeding the numbers of isolates reported from any other Chinese city (Figure 3).
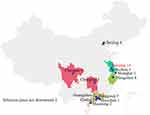 |
Figure 3 Distribution of FC428-like isolates reported from Chinese cities. |
In nearly six years, from 2015 to 2021, nine cases of ceftriaxone-resistant N. gonorrhoeae were reported from the United Kingdom (UK); all were associated international travel, including two that possessed documented whole genomic similarity to N. gonorrhoeae strain FC428, also exhibiting multilocus sequence type ST-1903; N. gonorrhoeae multi-antigen sequence type ST-1614 (porB 1053, tbpB 33) and antimicrobial resistance sequence type ST233.36 In just 6 months, between December 2021 and June 2022, 9 mosaic penA 60.001 N. gonorrhoeae isolates were obtained from patients presenting to sexual health service across the UK.47 MLST types for the 9 isolates were ST16406 (1 isolate) and ST8123 (8 isolates), differing from ST1903, the most prevalent Nanjing MLST type (11 of 14 isolates). ST8123 was the most common ST type among isolates collected in Shenzhen, China, between 2014 and 2018.48 The rapid emergence of mosaic penA 60.001 N. gonorrhoeae in two separate areas of the world suggests that mosaic penA 60.001 strains are spreading rapidly throughout the world.
Effective antimicrobial treatment of gonorrhea is essential to interrupt transmission of FC428/FC428-like isolates. Although differences in antimicrobial susceptibilities of the 14 isolates in our study were evident, MICs of ceftriaxone for the 14 isolates were similar to those for FC428.20 Three isolates showed a moderate level of resistance to azithromycin, which has also been reported in FC428-like isolates from several Chinese cities including Hangzhou, Changsha and Shenzhen,18,19,21 indicating that FC428-related sub-clones have evolved further and spread in China.
FC428-like genotypes (including NG-MAST, MLST and NG-STAR) have evolved to possess additional gene mutations during their spread. The 14 strains displayed relatively distant NG-MAST types; only one other FC428-like isolate, ST3435, has been reported to harbor an NG-MAST type that differed from the FC428 “signature” NG-MAST type. SNPs in porB resulted in different NG-MAST types. MLST1903, which is identical to FC428, was the predominant MLST type;19 however, three isolates were typed as MLST 7363 (identical to SC18-68 isolated in Chengdu, China17) and one was typed as MLST 11710 (not yet reported). NG-STAR types were similar to ST 233 (same as the FC428 type). NG-STAR ST1961, ST 2238, ST 2239 and ST 3523 were all new types. NG-STAR ST1143 was identical to FC428-like isolates from Changsha.18
Phylogenetic analysis showed that 13/14 of the isolates reported in this study were closely related to other FC428-like isolates (Figure 2); these subdivided into 5 clades, which were linked to other clades from Beijing,15 Hangzhou19 and England.33
Conclusion
In summary, we describe fourteen recent ceftriaxone-resistant FC428-related penA 60.001 N. gonorrhoeae infections in Nanjing, China, which represents a significant rise in this infection locally. Other Chinese cities have seen similar cases; however, reporting from nearby SE Asian countries has been sporadic or absent. In the West, isolated infections and even outbreaks have been caused by this organism. The rapid emergence of FC428-related penA 60.001 N. gonorrhoeae infections in separate areas of the world suggests that this infection is rapidly gaining a foothold worldwide.
Ethics Approval and Informed Consent
Our study complies with the Declaration of Helsinki. The Ethics Committee of the Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College (approval number: 2016-KY 022), and the Institutional Review Board (IRB) at Tufts University School of Medicine (approval number: 12219) approved the study; all enrolled participants provided written informed consent. Participants themselves and a parent or legal guardian of participants under 18 years of age provided written informed consents.
Data Sharing Statement
The data of complete genomic sequences of the thirteen isolates were available from NCBI (BioProject ID: PRJNA553852, PRJNA553854 and PRJNA916595).
Funding
This work was supported by grants from the Chinese Academy of Medical Sciences Initiative for Innovative Medicine (2016-I2M-3-021) and the US National Institutes of Health (AI116969).
Disclosure
No potential conflict of interest was reported by the authors.
References
1. WHO. Gonorrhoea: latest antimicrobial global surveillance results and guidance for vaccine development published; 2021. Available from: https://wwwwhoint/news/item/22-11-2021-gonorrhoea-antimicrobial-resistance-results-and-guidance-vaccine-development.
2. Costa-Lourenço A, Barros Dos Santos KT, Moreira BM, Fracalanzza SEL, Bonelli RR. Antimicrobial resistance in Neisseria gonorrhoeae: history, molecular mechanisms and epidemiological aspects of an emerging global threat. Braz J Microbiol. 2017;48(4):617–628. doi:10.1016/j.bjm.2017.06.001
3. Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev. 2014;27(3):587–613. doi:10.1128/cmr.00010-14
4. Ohnishi M, Golparian D, Shimuta K, et al. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother. 2011;55(7):3538–3545. doi:10.1128/aac.00325-11
5. Lindberg R, Fredlund H, Nicholas R, Unemo M. Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime and ceftriaxone: association with genetic polymorphisms in penA, mtrR, porB1b, and ponA. Antimicrob Agents Chemother. 2007;51(6):2117–2122. doi:10.1128/aac.01604-06
6. Nakayama S, Shimuta K, Furubayashi K, Kawahata T, Unemo M, Ohnishi M. New Ceftriaxone- and Multidrug-Resistant Neisseria gonorrhoeae Strain with a Novel Mosaic penA Gene Isolated in Japan. Antimicrob Agents Chemother. 2016;60(7):4339–4341. doi:10.1128/aac.00504-16
7. Lahra MM, Ryder N, Whiley DM. A new multidrug-resistant strain of Neisseria gonorrhoeae in Australia. N Engl J Med. 2014;371(19):1850–1851. doi:10.1056/NEJMc1408109
8. Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother. 2012;56(3):1273–1280. doi:10.1128/aac.05760-11
9. Anselmo A, Ciammaruconi A, Carannante A, et al. Draft genome sequence of Neisseria gonorrhoeae sequence type 1407, a multidrug-resistant clinical isolate. Genome Announc. 2015;3(4):e00903–00915. doi:10.1128/genomeA.00903-15
10. Lahra MM, Martin I, Demczuk W, et al. Cooperative recognition of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain. Emerg Infect Dis. 2018;24(4):735–740. doi:10.3201/eid2404.171873
11. Lefebvre B, Martin I, Demczuk W, et al. Ceftriaxone-Resistant Neisseria gonorrhoeae, Canada, 2017. Emerg Infect Dis. 2018;24(2):381–383. doi:10.3201/eid2402.171756
12. Terkelsen D, Tolstrup J, Johnsen CH, et al. Multidrug-resistant Neisseria gonorrhoeae infection with ceftriaxone resistance and intermediate resistance to azithromycin, Denmark, 2017. Euro Surveill. 2017;22(42):17–00659. doi:10.2807/1560-7917.Es.2017.22.42.17-00659
13. Poncin T, Fouere S, Braille A, et al. Multidrug-resistant Neisseria gonorrhoeae failing treatment with ceftriaxone and doxycycline in France, November 2017. Euro Surveill. 2018;23(21):1800264. doi:10.2807/1560-7917.Es.2018.23.21.1800264
14. Golparian D, Rose L, Lynam A, et al. Multidrug-resistant Neisseria gonorrhoeae isolate, belonging to the internationally spreading Japanese FC428 clone, with ceftriaxone resistance and intermediate resistance to azithromycin, Ireland, August 2018. Euro Surveill. 2018;23(47):1800617. doi:10.2807/1560-7917.Es.2018.23.47.1800617
15. Chen SC, Han Y, Yuan LF, Zhu XY, Yin YP. Identification of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain FC428, China. Emerg Infect Dis. 2019;25(7):1427–1429. doi:10.3201/eid2507.190172
16. Chen SC, Yuan LF, Zhu XY, van der Veen S, Yin YP. Sustained transmission of the ceftriaxone-resistant Neisseria gonorrhoeae FC428 clone in China. J Antimicrob Chemother. 2020;75(9):2499–2502. doi:10.1093/jac/dkaa196
17. Wang H, Wang Y, Yong G, et al. Emergence and genomic characterization of the ceftriaxone-resistant Neisseria gonorrhoeae FC428 clone in Chengdu, China. J Antimicrob Chemother. 2020;75(9):2495–2498. doi:10.1093/jac/dkaa123
18. Yuan Q, Li Y, Xiu L, et al. Identification of multidrug-resistant Neisseria gonorrhoeae isolates with combined resistance to both ceftriaxone and azithromycin, China, 2017-2018. Emerg Microbes Infect. 2019;8(1):1546–1549. doi:10.1080/22221751.2019.1681242
19. Yan J, Chen Y, Yang F, et al. High percentage of the ceftriaxone-resistant Neisseria gonorrhoeae FC428 clone among isolates from a single hospital in Hangzhou, China. J Antimicrob Chemother. 2021;76(4):936–939. doi:10.1093/jac/dkaa526
20. Lin X, Chen W, Xie Q, et al. Dissemination and genome analysis of high-level ceftriaxone-resistant penA 60.001 Neisseria gonorrhoeae strains from the Guangdong Gonococcal antibiotics susceptibility Programme (GD-GASP), 2016-2019. Emerg Microbes Infect. 2022;11(1):344–350. doi:10.1080/22221751.2021.2011618
21. Zhang L, Zhang C, Zeng Y, et al. Emergence and characterization of a ceftriaxone-resistant Neisseria gonorrhoeae FC428 clone evolving moderate-level resistance to azithromycin in Shenzhen, China. Infect Drug Resist. 2021;14:4271–4276. doi:10.2147/idr.S336212
22. Unemo M, Golparian D, Limnios A, et al. In vitro activity of ertapenem versus ceftriaxone against Neisseria gonorrhoeae isolates with highly diverse ceftriaxone MIC values and effects of ceftriaxone resistance determinants: ertapenem for treatment of gonorrhea? Antimicrob Agents Chemother. 2012;56(7):3603–3609. doi:10.1128/aac.00326-12
23. Li X, Le W, Lou X, Genco CA, Rice PA, Su X. In vitro activity of ertapenem against Neisseria gonorrhoeae clinical isolates with decreased susceptibility or resistance to extended-spectrum Cephalosporins in Nanjing, China (2013 to 2019). Antimicrob Agents Chemother. 2022;66(5):e0010922. doi:10.1128/aac.00109-22
24. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing.
25. Testing TECoAS. Breakpoint tables for interpretation of MICs and zone diameters. Version 50; 2015. Available from: http://www.eucast.org.
26. Mann LM, Kirkcaldy RD, Papp JR, Torrone EA. Susceptibility of Neisseria gonorrhoeae to Gentamicin-Gonococcal Isolate Surveillance Project, 2015-2016. Sex Transm Dis. 2018;45(2):96–98. doi:10.1097/olq.0000000000000693
27. Taylor SN, Marrazzo J, Batteiger BE, et al. Single-Dose Zoliflodacin (ETX0914) for treatment of urogenital gonorrhea. N Engl J Med. 2018;379(19):1835–1845. doi:10.1056/NEJMoa1706988
28. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: twenty-third informational supplement. CLSI document M100-S23. Clinical Lab Standards Institute. 2013;33:100–102.
29. Su X, Jiang F, Qimuge D. Surveillance of antimicrobial susceptibilities in Neisseria gonorrhoeae in Nanjing, China, 1999-2006. Sex Transm Dis. 2007;34(12):995–999. doi:10.1097/OLQ.0b013e3180ca8f24
30. Martin IM, Ison CA, Aanensen DM, Fenton KA, Spratt BG. Rapid sequence-based identification of gonococcal transmission clusters in a large metropolitan area. J Infect Dis. 2004;189(8):1497–1505. doi:10.1086/383047
31. Jolley KA, Maiden MC. BIGSdb: scalable analysis of bacterial genome variation at the population level. BMC Bioinform. 2010;11:595. doi:10.1186/1471-2105-11-595
32. Demczuk W, Sidhu S, Unemo M, et al. Neisseria gonorrhoeae sequence typing for antimicrobial resistance, a novel antimicrobial resistance multilocus typing scheme for tracking global dissemination of N. gonorrhoeae strains. J Clin Microbiol. 2017;55(5):1454–1468. doi:10.1128/jcm.00100-17
33. Eyre DW, Sanderson ND, Lord E, et al. Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018. Euro Surveill. 2018;23(27):1800323. doi:10.2807/1560-7917.Es.2018.23.27.1800323
34. Zhou K, Chen SC, Yang F, van der Veen S, Yin YP. Impact of the gonococcal FC428 penA allele 60.001 on ceftriaxone resistance and biological fitness. Emerg Microbes Infect. 2020;9(1):1219–1229. doi:10.1080/22221751.2020.1773325
35. Vincent LR, Kerr SR, Tan Y, et al. In vivo-selected compensatory mutations restore the fitness cost of mosaic penA alleles that confer ceftriaxone resistance in Neisseria gonorrhoeae. mBio. 2018;9(2):e01905–01917. doi:10.1128/mBio.01905-17
36. Eyre DW, Town K, Street T, et al. Detection in the United Kingdom of the Neisseria gonorrhoeae FC428 clone, with ceftriaxone resistance and intermediate resistance to azithromycin, October to December 2018. Euro Surveill. 2019;24(10):1900147. doi:10.2807/1560-7917.Es.2019.24.10.1900147
37. Poncin T, Merimeche M, Braille A, et al. Two cases of multidrug-resistant Neisseria gonorrhoeae related to travel in south-Eastern Asia, France, June 2019. Euro Surveill. 2019;24(36):1900528. doi:10.2807/1560-7917.Es.2019.24.36.1900528
38. Berenger BM, Demczuk W, Gratrix J, Pabbaraju K, Smyczek P, Martin I. Genetic characterization and enhanced surveillance of ceftriaxone-resistant Neisseria gonorrhoeae strain, Alberta, Canada, 2018. Emerg Infect Dis. 2019;25(9):1660–1667. doi:10.3201/eid2509.190407
39. Trinh TM, Nguyen TT, Le TV, et al. Neisseria gonorrhoeae FC428 Subclone, Vietnam, 2019-2020. Emerg Infect Dis. 2022;28(2):432–435. doi:10.3201/eid2802.211788
40. Chio MTW, Goh SS, Tan AL, Koh TH, Abdul Rahman NB. First case of ceftriaxone-resistant multidrug-resistant Neisseria gonorrhoeae in Singapore. Antimicrob Agents Chemother. 2019;63(5):e02624–02618. doi:10.1128/aac.02624-18
41. Nokchan N, Wongsurawat T, Jenjaroenpun P, Nitayanon P, Tribuddharat C. Whole-genome sequence analysis of high-level penicillin-resistant strains and antimicrobial susceptibility of Neisseria gonorrhoeae clinical isolates from Thailand. PLoS One. 2022;17(7):e0271657. doi:10.1371/journal.pone.0271657
42. Golparian D, Kittiyaowamarn R, Paopang P, et al. Genomic surveillance and antimicrobial resistance in Neisseria gonorrhoeae isolates in Bangkok, Thailand in 2018. J Antimicrob Chemother. 2022;77(8):2171–2182. doi:10.1093/jac/dkac158
43. Kueakulpattana N, Wannigama DL, Luk-In S, et al. Multidrug-resistant Neisseria gonorrhoeae infection in heterosexual men with reduced susceptibility to ceftriaxone, first report in Thailand. Sci Rep. 2021;11(1):21659. doi:10.1038/s41598-021-00675-y
44. Lin X, Qin X, Wu X, et al. Markedly increasing antibiotic resistance and dual treatment of Neisseria gonorrhoeae isolates in Guangdong, China, from 2013 to 2020. Antimicrob Agents Chemother. 2022;66(4):e0229421. doi:10.1128/aac.02294-21
45. Yang F, Zhang H, Chen Y, et al. Detection and analysis of two cases of the internationally spreading ceftriaxone-resistant Neisseria gonorrhoeae FC428 clone in China. J Antimicrob Chemother. 2019;74(12):3635–3636. doi:10.1093/jac/dkz384
46. Lee K, Nakayama SI, Osawa K, et al. Clonal expansion and spread of the ceftriaxone-resistant Neisseria gonorrhoeae strain FC428, identified in Japan in 2015, and closely related isolates. J Antimicrob Chemother. 2019;74(7):1812–1819. doi:10.1093/jac/dkz129
47. Day M, Pitt R, Mody N, et al. Detection of 10 cases of ceftriaxone-resistant Neisseria gonorrhoeae in the United Kingdom, December 2021 to June 2022. Euro Surveill. 2022;27(46):2200803. doi:10.2807/1560-7917.Es.2022.27.46.2200803
48. Li Y, Li Y, Xiu L, et al. Typing of Neisseria Gonorrhoeae isolates in Shenzhen, China from 2014-2018 reveals the shift of genotypes associated with antimicrobial resistance. Antimicrob Agents Chemother. 2021;65(5):e02311–02320. doi:10.1128/aac.02311-20
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


