Back to Journals » Clinical Epidemiology » Volume 12
Incidence Rate of Advanced Chronic Kidney Disease Among Privately Insured Adults with Neurodevelopmental Disabilities
Authors Whitney DG, Schmidt M, Bell S, Morgenstern H, Hirth RA
Received 13 December 2019
Accepted for publication 15 February 2020
Published 27 February 2020 Volume 2020:12 Pages 235—243
DOI https://doi.org/10.2147/CLEP.S242264
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Henrik Sørensen
Daniel G Whitney,1,2 Mary Schmidt,1 Sarah Bell,1 Hal Morgenstern,3– 5 Richard A Hirth2,6,7
1Department of Physical Medicine and Rehabilitation, University of Michigan, Ann Arbor, MI, USA; 2Institute for Healthcare Policy and Innovation, University of Michigan, Ann Arbor, MI, USA; 3Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, MI, USA; 4Department of Environmental Health Sciences, School of Public Health, University of Michigan, Ann Arbor, MI, USA; 5Department of Urology, School of Medicine, University of Michigan, Ann Arbor, MI, USA; 6Department of Health Management and Policy, University of Michigan School of Public Health, Ann Arbor, MI, USA; 7Department of Internal Medicine, University of Michigan, Ann Arbor, MI, USA
Correspondence: Daniel G Whitney Tel +1 734-936-7175
Email [email protected]
Purpose: Due to complex medical profiles, adults with neurodevelopmental disabilities (NDDs) may have a heightened risk for early development of chronic kidney disease (CKD) and accelerated CKD progression to advanced stages and kidney failure. The purpose of this study was to estimate the incidence rate of advanced CKD for adults with NDDs and compare the incidence rate to adults without NDDs.
Patients and Methods: Data were used from the Optum Clinformatics® Data Mart to conduct this retrospective cohort study. The calendar year 2013 was used to identify eligible participants: individuals ≥ 18 years of age and without advanced CKD. Participants were followed from 01/01/2014 to advanced CKD, loss to follow-up, death, or end of the study period (12/31/2017), whichever came first. Diagnostic, procedure, and diagnosis-related group codes identified NDDs (intellectual disabilities, cerebral palsy, autism spectrum disorders), incident cases of advanced CKD (CKD stages 4+), diabetes, cardiovascular diseases, and hypertension present in the year 2013. Crude incidence rates (IR) of advanced CKD and IR ratios (IRR), comparing adults with vs without NDDs (with 95% CI) were estimated. Then, Cox regression estimated the hazard ratio (HR and 95% CI) for advanced CKD, comparing adults with NDDs to adults without NDDs while adjusting for covariates.
Results: Adults with NDDs (n=33,561) had greater crude IR of advanced CKD (IRR=1.32; 95% CI=1.24– 1.42) compared to adults without NDDs (n=6.5M). The elevated rate of advanced CKD among adults with NDDs increased after adjusting for demographics (HR=2.19; 95% CI=2.04– 2.34) and remained elevated with further adjustment for hypertension and diabetes (HR=2.01; 95% CI=1.87– 2.15) plus cardiovascular disease (HR=1.84; 95% CI=1.72– 1.97). Stratified analyses showed that the risk of advanced CKD was greater for all NDD subgroups.
Conclusion: Study findings suggest that adults with NDDs have a greater risk of advanced CKD than do adults without NDDs, and that difference is not explained by covariates used in our analysis.
Keywords: chronic kidney disease, neurodevelopmental disabilities, intellectual disabilities, cerebral palsy, autism spectrum disorders
Introduction
Chronic kidney disease (CKD) is a public health issue and affects approximately 46 million individuals in the United States.1 As CKD progresses to renal failure and end-stage renal disease (ESRD), which is the permanent state of kidney loss-of-function, treatment strategies become increasingly burdensome for the patient and their caregivers, and are associated with exceedingly high medical costs.2 Unfortunately, nearly half of adults with low kidney function and not on dialysis are not aware they have CKD3 as CKD symptomatology is nearly absent in the early stages. Therefore, identifying populations at risk for CKD is needed in order to develop target-specific strategies to prevent, treat, and manage CKD.
Due to complex medical profiles and unmet healthcare needs, adults with neurodevelopmental disabilities (NDDs) may be at high risk for early development of CKD and rapid disease progression to advanced CKD. Three common NDDs, accounting for approximately 8 million individuals in the US, are intellectual disabilities, cerebral palsy, and autism spectrum disorders.4 These NDDs often co-occur with one another. While the cause and etiology of NDDs vary, the greater likelihood of having complex and unmet healthcare needs, as well as the lack of clinical knowledge about their healthful aging process, are commonalities linking all types of individuals with NDDs. Children with NDDs have low fitness levels,5,6 excess body fat,7–10 poor psychosocial development,4 and orthopedic issues.7,11,12 Having complex healthcare needs throughout growth and development could have long-term and lasting implications on adult health status. Indeed, results from several studies have shown that the pediatric to adult transition for individuals with NDDs is accompanied by early development of high-burden chronic diseases.13–16 Specifically, adults with NDDs are at greater risk for early development of cardiometabolic diseases compared to the general population, which are strong predictors of CKD onset17,18 and rate of CKD progression19 in non-NDD populations.
Other than a few cross-sectional studies with mixed findings of elevated CKD prevalence among adults with vs without NDDs,16,20 very little knowledge exists regarding the incidence of CKD for NDD populations or relative risk of CKD compared to individuals without NDDs. By not knowing the extent to which NDD contributes to the progression of advanced CKD, clinicians and healthcare providers may miss important windows to prevent CKD onset or slow CKD progression to later and advanced stages that are irreversible, costly, and fatal. Therefore, identifying advanced CKD incidence among NDD populations is urgently needed, as this knowledge could inform early decision-making processes for the patient and clinician focused on preventing or mitigating the burden of CKD in populations with already compromised health status. This notion is supported by a recent needs-based assessment by experts in the field concluding that adult-focused medical care is a universal need that is currently lacking for these NDD populations.21 Accordingly, the purpose of this study was to estimate the incidence rate of advanced CKD among adults with NDDs, as compared to adults without NDDs. We hypothesized that adults with NDDs have an increased advanced CKD risk when compared to individuals without NDDs.
Materials and Methods
Data Source
Data from 2013 to 2017 were extracted from the Optum Clinformatics® Data Mart Database (OptumInsightTM, Eden Prairie, MN, USA). This database has been described previously.22 Briefly, this is a nationwide de-identified single private payer administrative claims database in the United States, which houses insurance-related data from individuals who have Medicare Advantage or commercial health plans. Individuals covered by Medicare may opt to enroll in a private Medicare Advantage health plan in addition to the traditional Medicare program. Medicare Advantage health plans may offer additional coverage that is not readily available in the traditional Medicare program.22 In order to enroll in a private payer health plan, including commercial or Medicare Advantage, individuals of any income, age, or disability status either pay for the insurance coverage or the insurance coverage is paid through their employer. To limit patient identifiers, research teams using the Optum Clinformatics® Data Mart Database are allowed either the Date of Death or Socioeconomic Status table. Since the current study is part of a larger study that examined mortality, the Date of Death table was selected and some socioeconomic status information (i.e., income, education) were not available. Data are de-identified and the University of Michigan Institutional Review Board approved this study as non-regulated. The investigators (DGW and SB) have a data use agreement to analyze this database.
Participant Selection
The calendar year 2013 was used to identify eligible participants: adults ≥18 years of age without advanced CKD; continuous enrollment in a health plan; with ≥1 service utilization (to limit detection bias due to failure of CKD detection among persons who were not seen by a physician). The requirement for continuous enrollment in the full calendar year 2013 was to ascertain baseline chronic disease comorbidity data, as described below. Participants were then followed from 01/01/2014 to whichever of the following came first: incident advanced CKD; loss to follow-up; death; end of the study period, 12/31/207.
NDDs were identified using the International Classification of Diseases, Ninth Revision (ICD-9), Clinical Modification codes for intellectual disabilities (ICD-9: 317–319.x, 758.0–758.3x), cerebral palsy (ICD-9: 343.x, 333.71), or autism spectrum disorders (ICD-9: 299.x). The group of adults without NDDs included adults without any claims for these NDDs. Identifying individuals with pediatric-onset conditions using administrative claims data has shown 99% sensitivity and 79% positive predictive value.23
Outcome Measure
The outcome event was the occurrence of new advanced CKD from 01/01/2014 to 12/31/2017. Advanced CKD was defined in two steps. First, we used the methodology set forth by the United States Renal Data System for detecting ESRD within the Optum Clinformatics® Data Mart Database.1 Second, we added ICD-9 and ICD-10 codes (due to the change in reporting ICD-9 to ICD-10 codes on 10/01/2015) to identify new advanced CKD at stage 4 or later when first identified. Advanced CKD was identified as ICD codes for a diagnosis of CKD stages 4 (ICD-9: 585.4; ICD-10: N18.4) or 5 (ICD-9: 585.5; ICD-10: N18.5) or ESRD (ICD-9: 585.6; ICD-10: N18.6); procedure codes for dialysis in the outpatient setting; or a diagnosis-related group code for kidney transplant surgery (see Table 10.1 for procedure and diagnosis-related group codes in “CKD Analytical Methods” in reference[1]).24 Identifying advanced CKD using administrative claims data has shown up to 67% sensitivity, 95% specificity, and 76% to 97% positive predictive value.25,26
Covariates
Covariates in the administrative claims database include known and probable risk factors for CKD that may be associated with NDD in adults. Variables to denote sociodemographic status included age (as continuous), sex (women, men), race, and region of the country (West, Midwest, South, Northeast). Baseline comorbidities were identified using medical claims in the year 2013 (≥2 claims with first claim in 2013), and included hypertension (ICD-9: 401–405.x), diabetes (ICD9: 249.xx, 250.xx), ischemic heart disease (ICD-9: 410–414.x), heart failure (ICD-9: 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.x), and cerebrovascular disease (ICD-9: 430–437.x), as previously described.24 Information pertaining to the severity of autism spectrum disorders and cerebral palsy is not available in administrative claims.
Statistical Analysis
Baseline descriptive characteristics were summarized for adults with and without NDDs. Incidence rates (IR) and 95% confidence intervals (CI) of advanced CKD were estimated for adults with NDDs and for adults without NDDs, and by the NDD subgroups, as the number of advanced CKD events divided by the number of person-years (expressed as per 1000/year). The IR 95% CI was estimated as (1000/n) (y ± (1.96 x square root of y), where y is the number of outcome events and n is the amount of person-years. Incidence rate ratios (IRR) and 95% CI were estimated using the group without NDDs as the reference. The IRR 95% CI was estimated as exp[ln(IR) ± 1.96(SD(ln(IR)))]. Consistent with our previous work delineating adverse health outcomes for these NDD populations,27 the NDD subgroups included adults with intellectual disabilities (ID only), cerebral palsy (CP only), autism spectrum disorders (ASD only), and multiple NDDs (any combination of the three subtypes).
Cox regression was used to adjust for covariates when comparing IR, by estimating hazard ratios (HR and 95% CI) of advanced CKD incidence, comparing each exposure group with the reference group. Three groups of covariates were used to explain the difference in crude rates between groups, as previously described:24 model 1 – age, sex, and US region; model 2 – group 1 covariates plus baseline hypertension and diabetes; and model 3 – group 2 covariates plus baseline ischemic heart disease, heart failure, and cerebrovascular disease. We examined for potential clinically relevant interactions between exposure status (NDD vs no NDD) with age and sex, which was assessed by performing separate analyses for age or sex strata (NDD effects) and including product terms in the Cox models (interactions).
Cox regression did not adjust for race to limit bias due to missing race data (~14%). We, therefore, conducted two related sensitivity analyses to assess possible confounding and selection bias, as previously described.24 Sensitivity analysis #1 involved the restricted study population with complete data on race but not adjusting for race; sensitivity analysis #2 involved the same study population in #1 but adjusted for race. Results were compared from sensitivity analyses #1 and #2 to assess possible confounding by race. Results were also compared from sensitivity analysis #1 and the main analysis (full study population not adjusting for race) to assess possible selection bias from exclusion of adults without race data.
Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Baseline descriptive characteristics of adults with (n=33,561) and without (n=6,533,687) NDDs are presented in Table 1. Notably, adults with NDDs were 8.7 years younger on average and had a higher proportion of men (55.9% vs 45.5%). The race distribution, however, was similar for adults with and without NDDs.
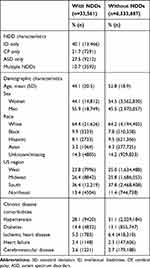 |
Table 1 Baseline Descriptive Characteristics of Adults With and Without Neurodevelopmental Disabilities (NDDs) |
Incidence Rate of Advanced CKD
The crude IR was 6.24 (95% CI=6.20–6.28) for adults without NDDs and 8.27 (95% CI=7.70–8.84) for adults with NDDs (IRR=1.32; 95% CI=1.24–1.42) (Table 2). For the NDD subgroups and compared to adults without NDDs, the crude IR and IRR was greater for adults with ID only and CP only, lower for adults with ASD only, and little difference was observed for the group with multiple NDDs.
 |
Table 2 Incidence Rate of Advanced Chronic Kidney Disease (CKD) Among Adults With and Without Neurodevelopmental Disabilities (NDDs) |
Cox Regression Analysis of Advanced CKD
Adjusted HRs of advanced CKD comparing adults with and without NDDs are presented in Table 3. Compared to adults without NDDs, the HR (95% CI) adjusting for demographic variables (model 1) was 2.19 (2.04–2.34) for all adults with NDDs, 2.31 (2.10–2.54) for those with ID only, 2.04 (1.79–2.31) for CP only, 1.80 (1.46–2.21) for ASD only, and 2.93 (2.26–3.81) for multiple NDDs. All the HRs decreased slightly when adjusting further for hypertension and diabetes (model 2) and additionally for cardiovascular disease (model 3), but remained elevated compared to adults without NDDs. In Model 3, the adjusted HR for all NDDs vs no NDDs was 1.84 (1.72, 1.97).
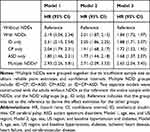 |
Table 3 Risk of Advanced Chronic Kidney Disease (CKD) Among Adults with Neurodevelopmental Disabilities (NDDs) Compared to Adults Without NDDs |
Interactions of NDD Status with Age and Sex
Table 4 shows the IR, IRR, and HR of advanced CKD by age category (18–39, 40–64, and ≥65 years) and sex. Adults with NDDs had an elevated IR, IRR, and HR compared to adults without NDDs for each age category (P for interaction <0.001) and sex (P for interaction 0.121). The absolute difference in IR for NDD vs no NDD increased with the older age categories, but the IRR and adjusted HR for NDD vs no NDD decreased with older age categories.
 |
Table 4 Incidence Rate and Hazard Ratio (HR) of Advanced Chronic Kidney Disease (CKD) Among Adults With and Without Neurodevelopmental Disabilities (NDDs) Stratified by Age and Sex |
Sensitivity Analysis
The advanced CKD IR and IRR for adults with complete race data (n=5,632,610) is presented in Table 5. The advanced CKD HR for adults with complete race data is presented in Table 6. A comparison of HR estimates from sensitivity analysis #1 (i.e., a sample with complete data on race but not adjusting for race) and #2 (i.e., a sample with complete data on race and adjusting for race) show similar results, suggesting that race is not a confounder in the main analysis. A comparison of HR estimates from sensitivity analysis #1 with results from the main analysis shows similar results, suggesting no evidence of selection bias.
 |
Table 5 Incidence Rate of Advanced Chronic Kidney Disease (CKD) Among Adults With and Without Neurodevelopmental Disabilities (NDDs) with Complete Data on Race |
 |
Table 6 Risk of Advanced Chronic Kidney Disease (CKD) Among Adults with Neurodevelopmental Disabilities (NDDs) Compared to Adults Without NDDs with Complete Data on Race |
Discussion
The main finding of the current investigation is that privately insured adults in the United States with NDDs had higher IRs of advanced CKD in a recent 4-year period than did adults without NDDs. Although the relative rate in NDD vs no NDD decreased slightly when adjusting for demographic and clinical risk factors—suggesting those covariates partially explained the higher rate in adults with NDDs—the overall IR remained more than 80% higher in the entire NDD group.
These new findings are of great public health concern. The burden on the patient (e.g., dialysis ~4 hrs per day, 3 days per week once the patient reaches ESRD) and societal costs of advanced CKD are extremely high.1 In the current study, we found that the entire sample of adults with NDDs, ID only, and CP only had higher crude IR of advanced CKD compared to adults without NDDs, while ASD only and multiple NDD subgroups had similar or lower crude IR. However, since individuals with NDDs develop chronic conditions younger than expected and the NDD population in our claims-based sample is younger than the non-NDD population, age adjustment is needed. After adjusting for age and other relevant demographic variables, the rate of advanced CKD was greater and increased from the unadjusted analysis (i.e., IRR) for all NDD groups. The demographic adjusted analysis also revealed that the ASD-only group and multiple NDD group are indeed at a greater relative risk of advanced CKD with 80% and 193% higher rates, respectively, compared to adults without NDDs. Although it is important to note that adults with autism spectrum disorders in this study were considerably younger (mean age [SD] = 36.5 [17.3]) than adults with intellectual disabilities (50.5 [18.4]) and cerebral palsy (52.2 [19.5]). The large difference in age may be attributed to poor identification and under-diagnosis of the condition previously. There is an increasing trend in prevalence and diagnosis of autism spectrum disorders28 that may be in response to better awareness and clinical assessment and changes in clinical diagnostic modalities. The younger age may affect interpretations for ASD only and the multiple NDD groups, as average age was relatively low for the multiple NDD group (36.5 [17.3]). Further, model diagnostics confirmed that age influenced the findings for these groups.
When we further adjusted for hypertension and diabetes, and then cardiovascular disease, which are known risk factors for CKD, the elevated rate of advanced CKD reduced for all NDD groups, but still remained elevated for the entire sample of adults with NDD and for each NDD subgroup. These findings suggest that although adults with NDDs have a greater risk of advanced CKD compared to adults without NDDs beyond the presence of these chronic conditions, prevalent cardiometabolic diseases (i.e., hypertension, diabetes, and cardiovascular disease) do account for a considerable portion of the elevated advanced CKD risk among adults with NDDs. Further work is required to identify how much of the risk of advanced CKD can be attenuated by preventing or better managing cardiometabolic diseases among adults with NDDs.
When we stratified analyses by age to reflect the different stages of adulthood, we found that adults with NDDs had a greater rate of advanced CKD compared to adults without NDDs across all age strata. The magnitude of the difference in the absolute rate increased with older age categories, but the magnitude of relative rate was greater for younger than middle-aged and older adults, and even greater for middle-aged than older adults. As we found little evidence of an interaction between sex and NDD status, findings may suggest that both men and women with NDDs have greater rates of advanced CKD across the age spectrum. When planning treatment strategies to prevent CKD onset or slow CKD progression to advanced stages for adults with NDDs, earlier age for intervention may be required.
The greater advanced CKD rate among adults with NDDs is likely driven, in part, by their unhealthful aging process and greater chronic disease risk,13–16 which is associated with CKD. In addition, because of their complex medical profiles, adults with NDDs are typically taking several medications, which is associated with greater emergency room and hospital utilization29 likely due to unknown adverse drug reactions. For example, chronic pain is highly prevalent for individuals with NDDs.30,31 Common pain medications, including nonsteroidal anti-inflammatory drugs, are recognized as nephrotoxic agents. As the kidneys are involved in filtering the blood, a complex physiological environment due to various classes of medications, concomitant with unique drug–drug interactions, could lead to unintended renal toxicity and organ damage. Furthermore, individuals with NDDs may be at greater risk for acute kidney injury, which is a robust risk factor for mortality for children with cerebral palsy32 and is associated with a large decline in kidney function for both adutls33 and children34 without NDDs. Further work in the field is needed to determine the contribution of kidney-related problems experienced in childhood to CKD risk in the adult years for the population with NDDs.
The advanced CKD incident rates found in this study may be under-reporting the magnitude of the issue for the adult populations with NDDs, especially for the NDD subgroups. The current study used a private insurance database that likely represents healthier and higher functioning segments of the NDD populations.31 We have previously shown that while 18- to 64-year-old adults with NDDs had higher prevalence of CKD at stages I–V and several other high-burden conditions as compared to adults without NDDs of the same age range (4.3% vs 1.9%), the prevalence of these high-burden conditions was, in general, higher for adults with NDDs that had public insurance as compared to adults with NDDs that had private insurance.31 In support, this study observed that the prevalence of multiple NDDs among those with any NDD, which complicates the health needs of the individual, was only 10.7%, which is lower than expected based on other population-based studies.35,36
The limitations of this study must be noted. First, administrative claims data to detect medically diagnosed conditions is limited. It is not known if “detection bias” differs by the presence of NDDs. For example, adults with NDDs use healthcare services more than adults without NDDs, which allows for additional opportunities for medical conditions (e.g., advanced CKD) to be identified and reported on a claim. However, in general, adults with physical disabilities, including NDDs, are less likely to be adequately screened for chronic medical conditions or given a comprehensive medical examination.37 This could prevent opportunities for medical conditions (e.g., advanced CKD) to be identified and therefore reported on a claim. Further, using claims data to identify incidence of advanced CKD and associated risk factors is limited between groups, as there is either no information or inconsistently reported information on weight status (e.g., body mass index), blood pressure values, and many other health conditions and socioeconomic indicators that may influence the association between NDDs and advanced CKD. Second, as this sample may reflect a healthier segment of the greater NDD population, it is possible that the incidence of advanced CKD is a larger public health issue than what the current study was able to capture. Third, we were not able to reliably determine the severity of NDDs. Fourth, we did not have access to data regarding medications (e.g., NSAIDs, psychotropics). A recent study has shown that adults >50 years of age with intellectual disabilities have an elevated prevalence of polypharmacy, which is a risk factor for mortality for this population.38 Further work in the field is needed to examine the extent that medications, and specifically medications that induce nephrotoxicity, associate with the elevated CKD burden for these NDD populations.
Conclusion
Adults with NDDs that have private insurance have higher IRs of advanced CKD as compared to the general population of adults. Although demographic and clinical factors partially explained the difference in rates, appreciable differences remained after adjustment for those factors. Future studies are needed to identify risk factors for CKD progression in adults with NDDs and to develop intervention strategies to prevent CKD onset, progression, and end-stage renal disease.
Accessibility of Protocol, Raw Data, and Programming Code
As part of the Date Use Agreement, authors are not allowed to provide raw data. Upon reasonable request, the corresponding author will provide statistical programming code used to generate results.
Acknowledgments
This research was developed in part under grants from the University of Michigan Office of Health Equity and Inclusion Diversity Fund and American Academy of Cerebral Palsy and Developmental Medicine. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. United States Renal Data System. 2018 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda (MD): National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018.
2. Ojo A. Addressing the global burden of chronic kidney disease through clinical and translational research. Trans Am Clin Climatol Assoc. 2014;125:229–243.
3. Centers for Disease Control and Prevention. Chronic Kidney Disease in the United States, 2019. Atlanta (GA): US Department of Health and Human Services, Centers for Disease Control and Prevention; 2019.
4. Whitney DG, Shapiro DN, Warschausky SA, Hurvitz EA, Peterson MD. The contribution of neurologic disorders to the national prevalence of depression and anxiety problems among children and adolescents. Ann Epidemiol. 2019;29:81–84 e82. doi:10.1016/j.annepidem.2018.11.003
5. Tyler K, MacDonald M, Menear K. Physical activity and physical fitness of school-aged children and youth with autism spectrum disorders. Autism Res Treat. 2014;2014:312163.
6. Garcia CC, Alcocer-Gamboa A, Ruiz MP, et al. Metabolic, cardiorespiratory, and neuromuscular fitness performance in children with cerebral palsy: a comparison with healthy youth. J Exerc Rehabil. 2016;12(2):124–131. doi:10.12965/jer.1632552.276
7. Whitney DG, Singh H, Miller F, et al. Cortical bone deficit and fat infiltration of bone marrow and skeletal muscle in ambulatory children with mild spastic cerebral palsy. Bone. 2017;94:90–97. doi:10.1016/j.bone.2016.10.005
8. Whitney DG, Singh H, Zhang C, Miller F, Modlesky CM. Greater visceral fat but no difference in measures of total body fat in ambulatory children with spastic cerebral palsy compared to typically developing children. J Clin Densitom. 2018. doi:10.1016/j.jocd.2018.09.006
9. Whitney DG, Miller F, Pohlig RT, Modlesky CM. BMI does not capture the high fat mass index and low fat-free mass index in children with cerebral palsy and proposed statistical models that improve this accuracy. Int J Obes (Lond). 2018;43(1):82–90.
10. Corvey K, Menear KS, Preskitt J, Goldfarb S, Menachemi N. Obesity, physical activity and sedentary behaviors in children with an autism spectrum disorder. Matern Child Health J. 2016;20(2):466–476. doi:10.1007/s10995-015-1844-5
11. Neumeyer AM, Cano Sokoloff N, McDonnell E, Macklin EA, McDougle CJ, Misra M. Bone accrual in males with autism spectrum disorder. J Pediatr. 2017;181:195–201 e196. doi:10.1016/j.jpeds.2016.10.080
12. da Silva VZ, de Franca Barros J, de Azevedo M, de Godoy JR, Arena R, Cipriano G
13. Croen LA, Zerbo O, Qian Y, et al. The health status of adults on the autism spectrum. Autism. 2015;19(7):814–823. doi:10.1177/1362361315577517
14. Morin D, Merineau-Cote J, Ouellette-Kuntz H, Tasse MJ, Kerr M. A comparison of the prevalence of chronic disease among people with and without intellectual disability. Am J Intellect Dev Disabil. 2012;117(6):455–463. doi:10.1352/1944-7558-117.6.455
15. Carey IM, Shah SM, Hosking FJ, et al. Health characteristics and consultation patterns of people with intellectual disability: a cross-sectional database study in English general practice. Br J Gen Pract. 2016;66(645):e264–e270. doi:10.3399/bjgp16X684301
16. Whitney DG, Kamdar NS, Ng S, Hurvitz EA, Peterson MD. Prevalence of high-burden medical conditions and health care resource utilization and costs among adults with cerebral palsy. Clin Epidemiol. 2019;11:469–481. doi:10.2147/CLEP.S205839
17. Tsai WC, Wu HY, Peng YS, et al. Risk factors for development and progression of chronic kidney disease: a systematic review and exploratory meta-analysis. Medicine (Baltimore). 2016;95(11):e3013. doi:10.1097/MD.0000000000003013
18. McMahon GM, Preis SR, Hwang SJ, Fox CS. Mid-adulthood risk factor profiles for CKD. J Am Soc Nephrol. 2014;25(11):2633–2641. doi:10.1681/ASN.2013070750
19. Arora P, Jalal K, Gupta A, Carter RL, Lohr JW. Progression of kidney disease in elderly stage 3 and 4 chronic kidney disease patients. Int Urol Nephrol. 2017;49(6):1033–1040. doi:10.1007/s11255-017-1543-9
20. de Winter CF, Echteld MA, Evenhuis HM. Chronic kidney disease in older people with intellectual disability: results of the HA-ID study. Res Dev Disabil. 2014;35(3):726–732. doi:10.1016/j.ridd.2013.11.005
21. Burke SL, Wagner E, Marolda H, Quintana JE, Maddux M. Gap analysis of service needs for adults with neurodevelopmental disorders. J Intellect Disabil. 2019;23(1):97–116. doi:10.1177/1744629517726209
22. Whitney D, Kamdar N, Hirth RA, Hurvitz EA, Peterson MD. Economic burden of paediatric-onset disabilities among young and middle-aged adults in the USA: a cohort study of privately insured beneficiaries. BMJ Open. 2019;9(9):e030490. doi:10.1136/bmjopen-2019-030490
23. Reeves S, Garcia E, Kleyn M, et al. Identifying sickle cell disease cases using administrative claims. Acad Pediatr. 2014;14(5 Suppl):S61–S67. doi:10.1016/j.acap.2014.02.008
24. Whitney DG, Pruente J, Schmidt M. Risk of advanced chronic kidney disease among adults with spina bifida. Ann Epidemiol. 2020. doi:10.1016/j.annepidem.2020.01.003
25. Muntner P, Gutierrez OM, Zhao H, et al. Validation study of medicare claims to identify older US adults with CKD using the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Am J Kidney Dis. 2015;65(2):249–258. doi:10.1053/j.ajkd.2014.07.012
26. Winkelmayer WC, Schneeweiss S, Mogun H, Patrick AR, Avorn J, Solomon DH. Identification of individuals with CKD from medicare claims data: a validation study. Am J Kidney Dis. 2005;46(2):225–232. doi:10.1053/j.ajkd.2005.04.029
27. Whitney DG, Whibley D, Jepsen KJ. The effect of low-trauma fracture on one-year mortality rate among privately insured adults with and without neurodevelopmental disabilities. Bone. 2019;129:115060. doi:10.1016/j.bone.2019.115060
28. Van Naarden Braun K, Christensen D, Doernberg N, et al. Trends in the prevalence of autism spectrum disorder, cerebral palsy, hearing loss, intellectual disability, and vision impairment, metropolitan atlanta, 1991–2010. PLoS One. 2015;10(4):e0124120. doi:10.1371/journal.pone.0124120
29. Blaskowitz MG, Hernandez B, Scott PW. Predictors of emergency room and hospital utilization among adults with Intellectual and Developmental Disabilities (IDD). Intellect Dev Disabil. 2019;57(2):127–145. doi:10.1352/1934-9556-57.2.127
30. Whitney DG, Warschausky SA, Peterson MD. Mental health disorders and physical risk factors in children with cerebral palsy: a cross-sectional study. Dev Med Child Neurol. 2019;61(5):579–585. doi:10.1111/dmcn.2019.61.issue-5
31. Whitney DG. Prevalence of high-burden medical conditions among young and middle-aged adults with pediatric-onset medical conditions: findings from us private and public administrative claims data. Int J Health Policy Manag. 2019;8(11):629–635. doi:10.15171/ijhpm.2019.62
32. Prastiya IG, Risky VP, Mira I, Retno AS, Darto S, Erny P. Risk factor of mortality in indonesian children with cerebral palsy. J Med Invest. 2018;65(1.2):18–20. doi:10.2152/jmi.65.18
33. Asar O, Ritchie J, Kalra PA, Diggle PJ. Short-term and long-term effects of acute kidney injury in chronic kidney disease patients: a longitudinal analysis. Biom J. 2016;58(6):1552–1566. doi:10.1002/bimj.201500270
34. Benisty K, Morgan C, Hessey E, et al. Kidney and blood pressure abnormalities 6 years after acute kidney injury in critically ill children: a prospective cohort study. Pediatr Res. 2020. doi:10.1038/s41390-019-0737-5
35. Reid SM, Meehan EM, Arnup SJ, Reddihough DS. Intellectual disability in cerebral palsy: a population-based retrospective study. Dev Med Child Neurol. 2018;60(7):687–694. doi:10.1111/dmcn.2018.60.issue-7
36. Tonnsen BL, Boan AD, Bradley CC, Charles J, Cohen A, Carpenter LA. Prevalence of autism spectrum disorders among children with intellectual disability. Am J Intellect Dev Disabil. 2016;121(6):487–500. doi:10.1352/1944-7558-121.6.487
37. McColl MA, Forster D, Shortt SE, et al. Physician experiences providing primary care to people with disabilities. Healthc Policy. 2008;4(1):e129–e147.
38. Schoufour JD, Oppewal A, van der Maarl HJK, et al. Multimorbidity and polypharmacy are independently associated with mortality in older people with intellectual disabilities: a 5-year follow-up from the HA-ID study. Am J Intellect Dev Disabil. 2018;123(1):72–82. doi:10.1352/1944-7558-123.1.72
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
