Back to Journals » International Journal of General Medicine » Volume 14
Incidence, Predictors and Clinical Impact of Ventricular Electrical Storm in Arrhythmogenic Cardiomyopathy Patients with an Implantable Cardioverter–Defibrillator: A Single-Center Report with Medium-Term Follow-Up
Authors Zhai L, Hu Y, Li X, Zhang X, Gu Z, Zhao Z, Yang X
Received 23 October 2021
Accepted for publication 8 December 2021
Published 20 December 2021 Volume 2021:14 Pages 10055—10063
DOI https://doi.org/10.2147/IJGM.S345872
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Lin Zhai, Yiran Hu, Xiang Li, Xuan Zhang, Zhe Gu, Zhenyan Zhao, Xu Yang
Department of Cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
Correspondence: Xu Yang
Department of Cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
Tel +86 10-88322133
Email [email protected]
Background: Implantable cardioverter–defibrillator (ICD) is the most effective strategy for prevention of ventricular tachyarrhythmia in patients with arrhythmogenic cardiomyopathy (ACM). However, some patients receive ventricular electrical storm (VES), characterized by multiple episodes of sustained ventricular tachyarrhythmia. The purpose of this study was to determine the incidence, predictors and prognostic implications of VES in ACM patients with an ICD.
Methods: A total of 88 patients with definite ACM who received an ICD and followed up continuously were included in this study. VES was defined as the occurrence of ≥ 3 separate episodes of sustained ventricular arrhythmias within a 24-hour period.
Results: During a median follow-up time of 4.0 years (range 1.6– 6.9), VES occurred in 19/88 patients (21.6%). The interval between the ICD implantation and the first VES ranged from 1 month to 128 months. The median number of ventricular tachyarrhythmia events per VES was 7.5 (range 3– 32). Multivariate analysis showed that VES was associated with a high body mass index (BMI) [adjusted hazard ratio (HR) 1.21, 95% confidence interval (CI) 1.00– 1.45, P=0.048)] and extensive T-wave inversion (TWI) (HR 23.39, 95% CI 1.74– 314.58, P=0.017). Kaplan–Meier method showed that patients with VES did not have a worse cardiac mortality compared to those without such an event.
Conclusion: There is a relatively high incidence of VES in ACM patients. The presence of high BMI and extensive TWI were strong predictors of VES occurrence in ACM patients with ICD. VES does not independently confer increased cardiac mortality.
Keywords: ventricular electrical storm, arrhythmogenic cardiomyopathy, implantable cardioverter–defibrillator, predictors, mortality
Introduction
Arrhythmogenic cardiomyopathy (ACM) is an arrhythmogenic heart muscle disorder not explained by ischemic, hypertensive, or valvular heart disease.1 Recently, expert consensus statement declared that the ACM phenotype can overlap with other cardiomyopathies, particularly dilated cardiomyopathy, in which the arrhythmia presentation may be associated with moderate-to-severe ventricular dilatation and/or impaired systolic function.2 For these patients with high risk of sudden cardiac death (SCD), prior studies have shown that an implantable cardioverter–defibrillator (ICD) is the most effective strategy for primary or secondary prevention.3
Ventricular electrical storm (VES), characterized by short-term recurrent ventricular tachycardia and fibrillation (VT/VF), is a major clinical problem in ACM patients with ICD.4 It represents severe electrical instability of the heart muscle, increasing mortality and hospitalization rates.5 However, features of VES in ACM patients have been evaluated only in a small series of patients and still remain unclear.6–8 Thus, the aim of this study was to analyze the incidence, predictors, and clinical impact of VES in larger ICD recipients with ACM during medium-term follow-up.
Methods
Study Population and Design
We reviewed 88 ACM patients who successfully underwent first ICD implantation for the prevention of SCD in Fuwai Hospital (Beijing, China) from January 2005 to January 2020. All enrolled patients had continuous records of outpatient clinic follow-up, data of ICD interrogation or remote monitoring. The study was performed with written informed consent from all patients and approval from the ethics committee of Fuwai Hospital and in accordance with the Declaration of Helsinki.
Definitions
ACM was standardly diagnosed according to the 2010 arrhythmogenic right ventricular cardiomyopathy (ARVC) Task Force Criteria when 2 major, or 1 major plus 2 minor, or 4 minor criteria from different categories.9 VES was consensually defined as the occurrence of ≥3 separate episodes of sustained ventricular arrhythmias within a 24-hour period.10 VT was defined as the regular (monomorphic) or irregular (polymorphic) ventricular arrhythmia with a mean cycle length of more than 240 ms. VF or ventricular flutter was defined as ventricular arrhythmia, with a mean cycle length of 240 ms or less, which was considered potentially fatal in the absence of an ICD. Non-sustained ventricular tachycardia (NSVT) was defined as 3 or more consecutive ventricular premature beats lasting <30 seconds without hemodynamic compromise. Recent syncope was defined as cardiac syncope <6 months before diagnosis. Extensive T-wave inversion (TWI) was defined as inverted T waves in ≥3 precordial leads.11 Frequent premature ventricular contractions (PVCs) were defined as the presence of over 1000 PVCs recorded by 24-hour Holter monitoring. Appropriate ICD therapy was an ICD shock, low-energy cardioversion (CV), or anti-tachycardia pacing (ATP) delivered in response to a ventricular tachyarrhythmia. Inappropriate ICD therapy was defined as those triggered by a rapid ventricular rate due to supraventricular tachyarrhythmia, sinus tachycardia, or a device malfunction.
ICD Implantation and Programming
The indication for ICD implantation was based on the guidelines.3 ICD pacing lead was implanted transvenously. The passive atrial lead was located at the right auricle, and the active fixation ventricular lead was screwed into the septum. Defibrillation thresholds were not routinely tested during the operation. Decisions regarding ICD types, implantation, and programming were made at the discretion of the treating electrophysiologist. Programmed ATP or discharge or both was activated as soon as the operation was done successfully.
Data Collection and Follow-Up
Baseline clinical data during hospitalization, including demographic characteristics, laboratory data, medications, were obtained from Fuwai Electronic Medical Record System. Patients were continually given the recommended anti-arrhythmia drugs when discharged. After that, patients were required for examination at our hospital, typically at 6/12-month interval or in case of ICD discharges. At each visit, devices were interrogated. Classification of the arrhythmia stored by an ICD was confirmed by at least two experienced electrophysiologists. When detailed ICD tracings were incomplete, we relied on the previous interpretations made by the outpatient clinic electrophysiologists. All enrolled patients were followed up to July 2020.
Statistical Analysis
SPSS 22.0 statistical software (SPSS, Inc, IBM, Armonk, New York) was used for statistical analysis. The mean and the standard deviation were used as the descriptive statistics for continuous variables, and numbers and percentage for categorical variables. Group comparisons were carried out through the Student's t-test or Mann–Whitney U-test for continuous variables and chi-square test or Fisher’s exact test for categorical variables. Univariate and multivariate Cox regression analysis were performed to define significant predictors of the occurrence of VES. The factors with P values <0.05 in the univariate analysis were entered into a multivariate Cox regression model with a forward stepwise method to identify the independent predictors of VES. To compare the actual survival, time to first VES, and time to the first appropriate ICD therapy, Kaplan–Meier analysis with a Log rank test was used. A two-sided P-value<0.05 was considered statistically significant.
Results
Clinical Characteristics
Between January 2005 and January 2020, a total of 88 patients with ACM who received ICD implantation were included in this study. Baseline characteristics are summarized in Table 1. Briefly, 25 (30.4%) were female, and a family history of SCD was present in 9 (10.2%) patients. Seventy-one (80.7%) patients experienced sustained VT/VF and 44 (50.0%) patients had NSVT. ACM patients who experience VES had significantly higher proportion of complete right bundle branch block (cRBBB) and more extensive TWI than patients without VES. Overall, the two groups were approximately balanced with respect to baseline characteristics.
 |
Table 1 Baseline Characteristics of the ACM Patients with ICD According to VES |
Characteristics and Distribution of VES
All ACM patients with ICD were followed up to July 2020. During a median follow-up time of 4.0 years (interquartile range 1.6–6.9), 19 (21.6%) of them once experienced at least one episode of VES. As shown in Figure 1, the temporal trend in first VES occurrence in ACM patients tended to follow a U-shaped curve. Most of the patients (52.6%) experienced fiirst VES during the first year, while 4 (21.1%) patients after 5 years. The interval between the ICD implantation and the first VES ranged 1 month to 128 months. Among patients with VES, 10 (52.6%) experienced only one episode each, 4 (21.1%) patients suffered from two, and 3 (15.8%) experienced three episodes, respectively. There was only 2 (10.5%) patients experienced at least 3 VES episodes during the follow-up intervals. Syncope was observed in 4 (21.1%) patients during VES occurrence. The median number of VT/VF events per VES was 7.5 (range 3–32). The VES was treated by anti-tachycardia pacing (ATP) only in six episodes experienced by 6 (31.6%) patients, while the other 13 (68.4%) patients suffered at least one episode of VES that triggered cardioversion with low energy (CV) or shock therapies. The description of those ACM patients suffered VES is shown in Table 2.
 |
Table 2 Characteristics of VES Patients with ACM |
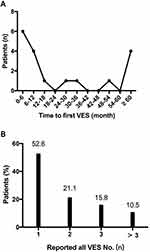 |
Figure 1 Temporal trend in the occurrence of VES. (A) the distribution of time to first VES; (B) the distribution of total VES numbers in each patient. VES, ventricular electrical storm. |
Predictors of VES
As shown in Table 3, body mass index (BMI) [hazard ratio (HR): 1.18, 95% confidence interval (CI): 1.02–1.36, P=0.028], cRBBB (HR: 3.22, 95% CI: 1.29–8.05, P=0.012) and extensive TWI (HR 8.89, 95% CI: 1.18–66.80, P=0.007] was significantly associated with VES occurrence in the univariate Cox regression analysis. After the adjustment of the common risk factors, multiple Cox regression analysis revealed two factors as independent predictors for VES: BMI (HR: 1.21, 95% CI: 1.00–1.45, P=0.048) and extensive TWI (HR: 23.39, 95% CI: 1.74–314.58, P=0.017).
 |
Table 3 Predictors of VES in ACM Patients |
Clinical Outcomes After VES
During follow-up period, 4 (21.1%) patients with VES died from cardiac death. As shown in the Kaplan–Meier curve in Figure 2, one patient died within 1-year follow-up, and the other three patients died during the interval between 3 and 12 years. The rates of cardiac death were not significantly different between ACM patients with and without VES (21.1% vs 7.2%; P=0.183). Moreover, no VES was attributed as the direct cause of death in ACM patients. Besides, Figure 3 shows the comparisons between the cumulative probability of actual survival and cumulative rate free of VES or appropriate ICD therapies. The divergence of the actual survival line and the other two lines reflects the survival benefit that patients might take from appropriate ICD therapy in termination of isolated VT/VF or multiple consecutive ventricular arrhythmia.
 |
Figure 2 Kaplan-Meier survival curve of survival probability after the occurrence of VES. VES, ventricular electrical storm. |
Discussion
The main results of our study are as follows: 1) almost 22% of ICD recipients with ACM will present VES during a median follow-up time of 4.0 years; 2) VES could occur during any time of the follow-up, and nearly half of the patients suffered from recurrent VES; 3) a high BMI and presenting with extensive TWI at baseline are independently predictors of the VES occurrence during follow-up; and 4) patients who once experienced an episode of VES was not associated with a poor cardiac prognosis.
Incidence and Time of Occurrence of VES in ACM Patients
ACM is an inherited heart muscle disorder and classically manifested as ventricular arrhythmias.2 Structural alterations associated with ACM predispose to reentrant VT, and patients may experience a high risk of VES occurrence.4 Previous studies have shown that 10–30% of the patients with secondary prevention ICDs can sustain VES. Bänsch et al studied 106 consecutive patients with dilated cardiomyopathy and ICDs and demonstrated that 28.3% of the patients experienced VES during a mean follow-up of 33 ± 23 months.12 A prospective study of 42 patients with ARVC based in American, demonstrated at least one episode of VES might occur among 12% of the patients during a mean follow-up of 42 ± 26 months.13 Moreover, prior study with a small sample by our center found that 31% of ARVC patients suffered at least one episode at a median follow-up of 49 months.14 Awareness of frequent left ventricular involvement in ARVC promoted the use of ACM to define this disease.2 However, there is a lack of data concerning the occurrence of electrical storm during follow-up in such patients. To our knowledge, our study specifically evaluated the incidence and time of occurrence of VES in ACM patients. According our results, the prevalence of VES among ACM patients was 21.6% (19/88), similar to those reported previously. The discrepancy in the prevalence of VES between our study and the previous one reported by our center might be contributed to some of the ACM patients with only left ventricular involvement. Besides, distinct genetic background of the enrolled patients may be responsible for the variations.
For the time of occurrence of VES, previous studies have demonstrated that VES in patients with ICD can occur early during the post-implanting period or later during long-term follow-up.4 Data from our study showed that over half of the patients experienced their fiirst VES during the first year of ICD implantation and another 21.1% patients experienced after 5 years. Although ICD is an effective strategy for secondary prevention of SCD, electrophysiologist should pay more attention to the early stage of ICD implantation in ACM patients.3 For this period, the compliance to the antiarrhythmic drugs or the restriction of competitive activities may decrease the number of VT/VF during VES.15
Precipitating Factors for VES in ACM Patients
Previous studies have demonstrated a number of well-known predictors are significantly associated with VES. A meta-analysis had showed that lower ejection fraction, monomorphic ventricular tachycardia as triggering arrhythmia, and class I anti-arrhythmic drugs therapy were all associated with VES.16 Of the two independent predictors of VES identified in our study, a high BMI means overweight or obesity, which was regarded as risk factor for the development of cardiovascular disease. Prior studies have indicated that BMI was associated with ventricular arrhythmias. For example, Samanta et al demonstrated that BMI was a significant predictor for the combined primary outcome of spontaneously occurring ventricular arrhythmias and mortality in patients with cardiomyopathy and ICDs.17 In a study involving 476 patients with left ventricular dysfunction, obesity was an independent risk factor for ventricular tachy-arrhythmias.18 In terms of extensive TWI in baseline ECG, it is considered a major diagnostic abnormality in arrhythmogenic cardiomyopathy, reflecting ventricular repolarization abnormalities.2,19 The prevalence of extensive TWI varies from 19% to 67% and the presence of TWI in lateral and/or inferior leads suggests LV involvement in patients with ARVC.20 Consistent with these former studies, our findings showed that a high BMI and the presence of extensive TWI on baseline ECG were independent predictors of VES occurrence in ACM patients. According to our results, we may suggest that optimal antiarrhythmic drugs, in-hospital and post-discharge cardiac rehabilitation are recommended for those patients with high BMI and (or) extentive TWI.21
Prognostic Implications of VES in ACM Patients
The association between VES and poor outcomes has been thoroughly demonstrated. A meta-analysis including 13 studies has demonstrated a 3.15-fold increased risk of death in patients with VES.16 However, some other studies showed that VES was not associated with increased risk of cardiac death.22,23 According to our results, an increased cardiac mortality in ACM patients with VES was greater than without VES, but the difference was not statistically significant. The results may attribute to the enrolled patients: most of them are not elderly and have a normal left ventricular function. Nevertheless, patients with VES are prone to psychological disorders and cardiac insufficiency. Antiarrhythmic drugs, such as amiodarone and beta-blocker, are the backbone of VES management. Besides, successful radiofrequency ablation has been shown to reduce the frequency of VES occurrence and mortality in patients with ACM.4 Data from 18-year experience in our center showed that ventricular tachycardia-free survival rate of the first, second and last ablation procedure was 56.7%, 73.2% and 78.1%, respectively.24 It would be interesting to analyze whether radiofrequency ablation in ACM patients with VES after ICD implantation would reduce the rate of VES occurrence and improve survival. Further studies are needed to validate this assumption.
Limitations
There are several limitations in our study. Firstly, it was based on the retrospective, single-center experience and limited number of patients, and the definition of ACM was based on 2010 ARVC Task Force criteria of diagnosis. Secondly, this present study was related mainly to secondary prevention for very high-risk patients; thus, some of the baseline features may be distinct. Finally, the endpoints were not including all-cause death in this study.
Conclusions
In this single-center with medium-term follow-up study, we demonstrate a relatively high incidence of VES in ACM patients with ICDs and the VES could occur during any time of the follow-up. The presence of high BMI and extensive TWI are predictors of VES occurrence in ACM patients with ICD. Patients who once experienced an episode of VES was not associated with an increased cardiac mortality. Whether radiofrequency ablation improves outcomes in these patients would require further studies.
Funding
CAMS Innovation Fund for Medical Sciences (No. 2021-I2M-C&T-B-025).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Corrado D, Link MS, Calkins H, Jarcho JA. Arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2017;376(1):1489–1490. doi:10.1056/NEJMra1509267
2. Towbin JA, McKenna WJ, Abrams DJ, et al. 2019 hrs expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm. 2019;16(11):e301–e372. doi:10.1016/j.hrthm.2019.05.007
3. Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 aha/acc/hrs guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018;138:e210–e271. doi:10.1161/CIR.0000000000000548
4. Kowlgi GN, Cha Y-M. Management of ventricular electrical storm: a contemporary appraisal. Europace. 2020;22(12):1768–1780. doi:10.1093/europace/euaa232
5. Noda T, Kurita T, Nitta T, et al. Significant impact of electrical storm on mortality in patients with structural heart disease and an implantable cardiac defibrillator. Int J Cardiol. 2018;255:85–91. doi:10.1016/j.ijcard.2017.11.077
6. Zhang N, Song Y, Hua W, et al. Left ventricular involvement assessed by LGE-CMR in predicting the risk of adverse outcomes of arrhythmogenic cardiomyopathy with ICDs. Int J Cardiol. 2021;337:79–85. doi:10.1016/j.ijcard.2021.04.015
7. Yin K, Ding L, Li Y, et al. Long-term follow-up of arrhythmogenic right ventricular cardiomyopathy patients with an implantable cardioverter-defibrillator for prevention of sudden cardiac death. Clin Cardiol. 2017;40(4):216–221. doi:10.1002/clc.22648
8. Mussigbrodt A, Dinov B, Bertagnoli L, et al. Precordial QRS amplitude ratio predicts long-term outcome after catheter ablation of electrical storm due to ventricular tachycardias in patients with arrhythmogenic right ventricular cardiomyopathy. J Electrocardiol. 2015;48:86–92. doi:10.1016/j.jelectrocard.2014.10.013
9. Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi:10.1161/CIRCULATIONAHA.108.840827
10. Israel CW, Barold SS. Electrical storm in patients with an implanted defibrillator: a matter of definition. Ann Noninvasive Electrocardiol. 2007;12:375–382. doi:10.1111/j.1542-474X.2007.00187.x
11. Peters S, Trummel M. Diagnosis of arrhythmogenic right ventricular dysplasia-cardiomyopathy: value of standard ECG revisited. Ann Noninvasive Electrocardiol. 2003;8:238–245. doi:10.1046/j.1542-474X.2003.08312.x
12. Bansch D, Bocker D, Brunn J, et al. Clusters of ventricular tachycardias signify impaired survival in patients with idiopathic dilated cardiomyopathy and implantable cardioverter defibrillators. J Am Coll Cardiol. 2000;36(2):566–573. doi:10.1016/S0735-1097(00)00726-9
13. Roguin A, Bomma CS, Nasir K, et al. Implantable cardioverter-defibrillators in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol. 2004;43(10):1843–1852. doi:10.1016/j.jacc.2004.01.030
14. Yin K, Ding L, Hua W, et al. Electrical storm in icd recipients with arrhythmogenic right ventricular cardiomyopathy. Pacing Clin Electrophysiol. 2017;40(6):683–692. doi:10.1111/pace.13070
15. Santangeli P, Muser D, Maeda S, et al. Comparative effectiveness of antiarrhythmic drugs and catheter ablation for the prevention of recurrent ventricular tachycardia in patients with implantable cardioverter-defibrillators: a systematic review and meta-analysis of randomized controlled trials. Heart Rhythm. 2016;13(7):1552–1559. doi:10.1016/j.hrthm.2016.03.004
16. Guerra F, Shkoza M, Scappini L, et al. Role of electrical storm as a mortality and morbidity risk factor and its clinical predictors: a meta-analysis. Europace. 2014;16(3):347–353. doi:10.1093/europace/eut304
17. Samanta R, Narayan A, Pouliopoulos J, et al. Influence of body mass index on recurrence of ventricular arrhythmia, mortality in defibrillator recipients with ischaemic cardiomyopathy. Heart Lung Circ. 2020;29(2):254–261. doi:10.1016/j.hlc.2018.12.018
18. Pietrasik G, Goldenberg I, McNitt S, et al. Obesity as a risk factor for sustained ventricular tachyarrhythmias in madit ii patients. J Cardiovasc Electrophysiol. 2007;18(2):181–184. doi:10.1111/j.1540-8167.2006.00680.x
19. Zagkli F, Georgakopoulou A, Chiladakis J. QRS fragmentation and T-wave inversion as factors of vulnerability to recurrent ventricular tachycardia. Future Cardiol. 2019;15:89–93. doi:10.2217/fca-2017-0104
20. Jain R, Dalal D, Daly A, et al. Electrocardiographic features of arrhythmogenic right ventricular dysplasia. Circulation. 2009;120(6):477–487. doi:10.1161/CIRCULATIONAHA.108.838821
21. Kato J, Koike A, Kuroki K, et al. Safety and efficacy of in-hospital cardiac rehabilitation following antiarrhythmic therapy for patients with electrical storm. J Cardiol. 2019;73(2):171–178. doi:10.1016/j.jjcc.2018.08.004
22. Brigadeau F, Kouakam C, Klug D, et al. Clinical predictors and prognostic significance of electrical storm in patients with implantable cardioverter defibrillators. Eur Heart J. 2006;27(6):700–707. doi:10.1093/eurheartj/ehi726
23. Guerra F, Flori M, Bonelli P, et al. Electrical storm and heart failure worsening in implantable cardiac defibrillator patients. Europace. 2015;17(2):247–254. doi:10.1093/europace/euu298
24. Liang E, Wu L, Fan S, et al. Catheter ablation of arrhythmogenic right ventricular cardiomyopathy ventricular tachycardia: 18-year experience in 284 patients. Europace. 2020;22(5):806–812. doi:10.1093/europace/euaa046
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

