Back to Journals » International Journal of Women's Health » Volume 15
Incidence of Fetal Myocardial Hypertrophy in Mother with Diabetes Mellitus by Using Cardio-Spatiotemporal Image Correlation (STIC) M-Mode
Authors Sapanont K , Luangdansakul W, Pleankong M, Smanchat B , Bhamarapravatana K, Suwannarurk K
Received 1 March 2023
Accepted for publication 5 May 2023
Published 9 May 2023 Volume 2023:15 Pages 703—710
DOI https://doi.org/10.2147/IJWH.S410697
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Kobporn Sapanont,1 Wiyada Luangdansakul,1 Monyada Pleankong,1 Buppa Smanchat,1 Kornkarn Bhamarapravatana,2 Komsun Suwannarurk3
1Department of Obstetrics and Gynecology, Bhumibol Adulyadej Hospital, Royal Thai Air Force, Bangkok, Thailand; 2Department of Preclinical Sciences, Faculty of Medicine, Thammasat University, Pathum Thani, Thailand; 3Department of Obstetrics and Gynecology, Faculty of Medicine, Thammasat University, Pathum Thani, Thailand
Correspondence: Wiyada Luangdansakul, Department of Obstetrics and Gynecology, Bhumibol Adulyadej Hospital, Royal Thai Air Force, Bangkok, 10220, Thailand, Tel +66-2-5347314, Email [email protected]
Objective: The aim was to demonstrate the prevalence of fetal myocardial hypertrophy (FMH) in diabetes mellitus (DM) pregnant women using spatio-temporal image correlation (STIC) M-mode.
Material and Methods: This prospective descriptive study was conducted at Bhumibol Adulyadej Hospital (BAH) Royal Thai Air Force between April and December 2022. Participants were singleton DM pregnant women with gestational age (GA) between 18 and 40 weeks who had antenatal care and delivery at BAH. DM screening was randomized blood sugar obtained from all participants. All participants underwent fetal heart exams by four-dimension ultrasound with STIC M-mode.
Results: One hundred and forty-five participants were recruited and classified as pregestational (PDM) and gestational DM (GDM) at 31 and 114 cases, respectively. The mean age of participants was 31.7 years old. Fasting blood sugar (FBS) of PDM was significantly higher than GDM (105.1 vs 87.0 mg%). GDMA2 had more elevated FBS than GDMA1 (p < 0.001). PDM had significantly greater FBS and two-hour postprandial blood sugar (2hr-PP) than GDM (105.1/87.0 and 151.5/117.9 mg%, respectively). FBS and 2hr-PP of GDMA2 were more than GDMA1 with statistical significance. Good glycemic control of GDM was significantly better than PDM. GDMA1 had better glycemic control than GDMA2 with statistical significance. Four-fifth (115/145) of participants had FMH. FMH and estimated fetal weight among PDM and GDM were comparable. Both good and poor glycemic control had similar FMH. Neonatal outcomes of FMH or non-FMH infants were similar.
Conclusion: The prevalence of FMH in diabetic pregnant women was 79.3%. Glycemic control had no correlation to FMH.
Keywords: STIC-M, gestational diabetes mellitus, pregestational diabetes mellitus, interventricular septal thickness
Introduction
Diabetes in pregnancy is one of the most common complications of pregnancy with an incidence of 15.8% worldwide.1 The risk of congenital heart anomalies in the fetus was increased with pre-existing diabetes in pregnant women, namely transposition of the great arteries, persistent truncus arteriosus, and septal defect.2,3 Simone’s fetal echocardiography work on diabetic mothers found that it correlated with fetal cardiac structural abnormality and interfered with fetal cardiac function.2 Cardiac hypertrophy was found both in pregestational and gestational diabetes mellitus mothers.4
Impaired fetal cardiac function was found to be associated with adverse pregnancy outcomes and neonatal morbidity.5,6 Cardiac hypertrophy could spontaneously be resolved in the postpartum period. However, in-utero and cardiac dysfunction in the early neonatal period might persist with possibly long-lasting effects and predisposed cardiovascular disease in adulthood.7–9
Interventricular septal thickness (IVST) is one of the echocardiographic parameters for a fetal cardiac function that assesses cardiac hypertrophy.4 The gold standard for IVST measurement can be obtained with two-dimensional ultrasound using M mode and B mode.10
Spatio-temporal image correlation (STIC) was a four-dimensional ultrasound. In addition to two-dimensional ultrasound, this new 4D technique allowed us to obtain fetal cardiac volume and stored data for later use.11 The use of STIC decreased time patient ultrasound time and gave physicians more leeway time to select the best location for M-mode.
This study aimed to detect fetal myocardial hypertrophy by using spatiotemporal image correlation M-mode (STIC-M) in diabetic pregnant women.
Materials and Method
The protocol of this investigation was approved by Bhumibol Adulyadej Institutional Review Board (BAIRB) (registration number 16/65). The clinical trial registration number was TCTR2022031006. A prospective descriptive study was conducted at Bhumibol Adulyadej Hospital (BAH) Royal Thai Air Force between April and December 2022. Informed consent was obtained from all the participants.
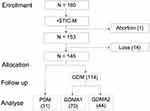
A total of 145 singleton diabetes mellitus pregnant women at gestational age (GA) between 18 and 40 weeks who had antenatal care and delivery at BAH were included. Exclusion criteria were evidence of congenital fetal anomaly of any organ, fetal arrhythmia, chromosomal abnormalities, and possibly affected fetal circulation and function such as fetal growth restriction, and low amniotic fluid.
DM screening was offered to all pregnant women at BAH. Random blood sugar was used as a screening test at 200 mg/dL cutoff in the first trimester or the mother’s first visit. A 75-g glucose tolerance test (OGTT) was used as a diagnostic test for gestational diabetes mellitus (GDM) with a cut-off of ≥92 mg/dL fasting blood sugar, ≥180 mg/dL after 1 hour, ≥153 mg/dL after 2-hours. GDM was divided into diet control treatment (GDMA1) and insulin therapy (GDMA2). Pregestational diabetes mellitus (PDM) was diagnosed with a fasting blood glucose ≥126 mL/dL or HbA1c ≥6.5. Well glycemic control had to be ≤95 mg/dL fasting blood glucose, ≤140 mg/dL 1-hour postprandial, and ≤120 mg/dL 2-hours postprandial (2hr-PP).
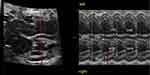
Singleton pregnant women diagnosed with DM were recruited in the study at gestational age 18–40 weeks. All participants received fetal echocardiogram examinations by four-dimension ultrasound with STIC (Figure 1), using a Voluson E8 (General Electric Healthcare, Zipf, Austria) and Voluson E10 (General Electric Healthcare, Zipf, Austria) equipped with a volumetric convex probe (GE RAB4-8L). The acquisition angle was 20 to 40 degrees with an acquisition time of 7.5 to 15 seconds.12 All investigations were performed by one examiner. Images were acquired with a single automatic sweep in a transverse four-chamber view. The region of interest (ROI) was selected to capture the entire fetal heart. The fetal cardiac volumes were obtained and stored in DICOM. The offline volume was displayed in multiplane with 4D view software version 10.0 (General Electric Medical System, Zipf, Austria) (Figure 2). The interventricular septum was selected as a reference point in planes A, B, and C. Plane A was selected and then measured with STIC-M at the thickest point of the interventricular septum. Image from STIC-M was measured for Biventricular outer diameter (BVOD), Left ventricular internal diameter in diastole (LVID), Right ventricular internal diameter in diastole (RVID), Left ventricular wall thickness (LVWT), Right ventricular wall thickness (RVWT), Interventricular septum thickness (IVST), Left ventricular internal diameter in systole (LVIS), Right ventricular internal diameter in systole (RVIS), Left ventricular shortening fraction (LVSF) was calculated from (LVID – LVIS)/LVID. Right ventricular shortening fraction (RVSF) was calculated from (RVID – RVIS)/RVID (Figure 3). All fetal cardiac measurements were compared to the existing nomogram for STIC-M in Thailand.13 Fetal myocardial hypertrophy (FMH) was considered when the measured values exceed 95 percentiles. Estimate fetal weight was calculated with the Hadlock formula from the biparietal diameter (BPD), head circumference (HC), abdominal circumference (AC), and femur length (FL).
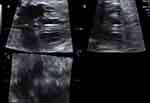 |
Figure 2 Transverse four-chamber view: gray-scale spatio-temporal image correlation (STIC) volume displayed in the multiplanar mode. Notes: (A), plane A; (B), plane B; (C), plane C. |
Pregnancy outcome gestational age at delivery, birthweight, preterm delivery, neonatal intensive care unit (NICU) admission, and neonatal death were collected from hospital delivery records.
Statistical analyses were performed using Stata version 14 software (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP). Continuous data were statistically evaluated and expressed as mean±standard deviation (SD) or median (interquartile range; IQR). Categories data was presented by Chi-square, Fisher’s exact, and Mann–Whitney U-test with appropriate application.
Results
From 160 recruited participants, 15 dropped out resulting in a total of 145 participants. The average age was 31.2±5.7 in the GDM group and 33.7±4.1 in the PDM group. The mean BMI of the PDM was higher than that of the GDM group with no significance (29.8 vs 27.9 kg/m2, p-value= 0.138). However, the different BMI among the two GDM subgroups was statistically not significant (p = 0.122).
The fasting blood sugar of the PDM group was significantly higher than that of the GDM group (105.1 vs 87.0 mg%, p < 0.001). GDMA2 cases had higher fasting blood sugar than GDMA1 (94.4 vs 82.3 mg%, p < 0.001). The 2hr-PP of GDM was lesser than that of the PDM group with statistical significance (151.5 vs 117.9, p-value <0.001) as shown in Table 1. GDMA2 participants had significantly higher 2hr-PP levels than those with GDMA1 (137.1 vs 105.9 mg%, p < 0.001). The glycemic control of GDM cases was better than that of the PDM group (63.1% vs 22.5%, p < 0.001). Among groups of GDM cases, GDMA1 had significantly better glycemic control than GDMA2 (80% vs 36.3%, p < 0.001).
 |
Table 1 Maternal and Fetal Demographic Characters Between Gestational Diabetes Mellitus and Pregestational Diabetes Mellitus (n = 145) |
IVST was the most common predictor of FMH. Among 145 cases of GDM and PDM, there were 115 cases of FMH that were detected by the STIC technique of 4D ultrasonography. The prevalence of FMH in this study was 79.31%. However, the prevalence of FMH among pregnancy with diabetes mellitus in each group was statistically insignificant as shown in Table 1. The estimated fetal weight of the GDM and PDM groups was comparable. GDMA2 had significantly more incidences of large gestational age fetuses (LGA) than those found in GDMA1.
LVWT, RVWT, and IVST were ultrasonographic parameters of myocardial thickness. LVSF and RVSF were one of the factors that represented fetal cardiac function. In the comparison of well and poor glycemic control groups, the myocardial thickness was not statistically significant as shown in Table 2.
 |
Table 2 Relation Between Fetal Myocardial Hypertrophy and Glycemic Control of Diabetes Mellitus |
There was no difference in pregnancy outcome of gestational age at delivery, birthweight, preterm delivery, and NICU between fetal myocardial hypertrophy and normal fetal heart as shown in Table 3. No neonatal death was found in this study.
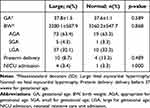 |
Table 3 Relation Between Neonatal Outcome and Fetal Myocardial Hypertrophy |
Discussion
The prevalence of FMH from this investigation was 79.3% by STIC-M technique. Cardiomyopathies were shown in 8–11% of cardiovascular abnormality problems found in pregnancy. Palmieri’s work using 2D M-mode to measure fetal myocardial thickness in 30.59 weeks GA mother showed 50.8% FMH prevalence.14 The higher prevalence found in this work might be because the advanced technology allowed the practitioner more time and opportunity to carefully measure fetal cardiac parameters.
Septal hypertrophy is the best representation of hypertrophic cardiomyopathy.
Even though the right ventricle and the posterior left ventricular wall are also affected, septal hypertrophy is the most prominent feature due to the high amount of insulin receptors located there.15 The prevalence of FMH in this study resulted from the value of septal hypertrophy.
Fetal echocardiography is crucial in mothers with diabetes mellitus.16 Mothers both with GDM and PDM are known to develop fetal hypertrophic cardiomyopathy.17 This investigation shared no significant difference in FMH measurement between mothers who maintained good glycemic control and those who could not (Table 2).
STIC-M measurement of LVWT, RVWT, IVST, LVSF, and RVSF from well glycemic control and poor glycemic control group demonstrated no significant difference in FMH. Some studies that had a smaller number of diabetic pregnant women presented glycemic control associated with FMH described increased IVST in the poor control group (Table 4).18 However, systematic review and meta-analysis mentioned FMH in well-controlled diabetic pregnancy.4
 |
Table 4 Studies Comparison of GC with FMH |
Between PDM and GDM, FPG and 2hrPP were significantly different (p < 0.001) as well as GDMA1 and GDMA2. Glycemic control was statistically different and related to higher insulin resistance in PDM and GDMA2.17 Adverse perinatal outcomes are correlated to FMH. An extreme postnatal consequence of hypertrophic cardiomyopathy with maternal diabetes might lead to a no desirable intrauterine fetal outcome.17 There was no intrauterine fetal distress or neonatal death in this current work. Fourteen preterm births were found during our investigation with no severe consequences (Table 3). NICU admission was found in 5 cases (3.4%) of the total DM mother population.
Neonatal outcomes in this study had no difference in fetuses with FMH and fetuses with normal hearts (Table 3). Similarly, Mrudhula Tejaswi G et al reported only two cases of perinatal asphyxia and were unable to predict perinatal mortality. However, they found that severe septal hypertrophy was linked to neonatal problems such as hypoglycemia, hyperbilirubinemia, and a NICU stay of more than 7 days.18 Other neonatal problems were not recorded in this study.
The strength of the current study was the first application of 4D STIC-M to measure fetal myocardial thickness in diabetic pregnant women. Four-dimensional ultrasound with STIC-M had an advantage over the 2D model because it decreased the duration that the patient needed to spend during the examination, and increased time and flexibility for practitioners to obtain accurate fetal cardiac structure measurements. It took an average of 10 minutes with the patient to complete an investigation. In the hands of an experienced operator, it took another 10 minutes to analyze data offline.
An old-school opinion was that all DM pregnant mothers should undergo a routine 2D ultrasound sonography. However, this investigation with a more detailed 4D STIC-M reading found that there was no significant difference in all FMH measurements between DM mothers whether they had good glycemic control or not. Our recommendation surprisingly would be geared toward a diabetic awareness campaign geared to all reproductive-age female population. Any measure to encourage a DM-free condition is worth its weight in the goal toward good future neonatal health, including good myocardial health, for a healthy generation to come.
Confirmation of FMH with 4D STIC-M gave more detailed information resulting in a high percentage of prevalence. The limitation of this study was limited neonatal adverse outcome cases and lack of newborn echocardiography.
Conclusion
The prevalence of fetal myocardial hypertrophy in diabetic pregnant women investigated by 4D STIC-M was 79.3%. The myocardial thickness of LVWT, RVWT, IVST as well as LVSF and RVSF were not related to glycemic control. A diabetic awareness education program is recommended for the reproductive-age female population to have a better health outcome for children in the next generation.
Data Sharing Statement
All data are available and submitted upon reasonable request from the corresponding author.
Ethics Approval
This study was approved by the Bhumibol Adulyadej Institutional Review Board (BAIRB) (registration number 16/65). The present study was conducted in accordance with the Declaration of Helsinki. All information and data were encrypted and confidential. All participants provided informed consent. All samples were handled and processed strictly, as stipulated by an approved local review board protocol.
Acknowledgment
The present study was funded and supported by the Bhumibol Adulyadej Hospital research fund. We would like to show our gratitude to Dr. Ratiporn Chartchaiyarerk, Dr. Suthasinee Mataneedol, Dr. Teenat Kanjanasingh, and Dr. Nattapat Kanjanawilai for their comment that greatly improved the study as well as our colleagues from the Department of Obstetrics and Gynecology, Bhumibol Adulyadej Hospital, Royal Thai Air Force, who provided insight and experience that greatly assisted the research.
Disclosure
The authors declare no conflict of interest.
References
1. International Diabetes Federation. IDF diabetes atlas; 2019. Available from: https://www.diabetesatlas.org/data/en/region/8/wp.html.
2. Simeone RM, Devine OJ, Marcinkevage JA, et al. Diabetes and congenital heart defects: a systematic review, meta-analysis, and modeling project. Am J Prev Med. 2015;48:195–204. doi:10.1016/j.amepre.2014.09.002
3. Lisowski LA, Verheijen PM, Copel JA, et al. Congenital heart disease in pregnancies complicated by maternal diabetes mellitus. An international clinical collaboration, literature review, and meta-analysis. Herz. 2010;35:19–26. doi:10.1007/s00059-010-3244-3
4. Depla AL, De Wit L, Steenhuis TJ, et al. Effect of maternal diabetes on fetal heart function on echocardiography: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2021;57:539–550. doi:10.1002/uog.22163
5. Pilania R, Sikka P, Rohit MK, Suri V, Kumar P. Fetal cardiodynamics by echocardiography in insulin dependent maternal diabetes and its correlation with pregnancy outcome. J Clin Diagn Res. 2016;10:QC01–4. doi:10.7860/JCDR/2016/17993.8079
6. Bhorat I, Pillay M, Reddy T. Determination of the fetal myocardial performance index in women with gestational impaired glucose tolerance and to assess whether this parameter is a possible prognostic indicator of adverse fetal outcome. J Matern Fetal Neonatal Med. 2018;31:2019–2026. doi:10.1080/14767058.2017.1334047
7. Han SS, Wang G, Jin Y, et al. Investigating the mechanism of hyperglycemia-induced fetal cardiac hypertrophy. PLoS One. 2015;10:e0139141. doi:10.1371/journal.pone.0139141
8. Cade WT, Levy PT, Tinius RA, et al. Markers of maternal and infant metabolism are associated with ventricular dysfunction in infants of obese women with type 2 diabetes. Pediatr Res. 2017;82:768–775. doi:10.1038/pr.2017.140
9. Schierz IAM, Pinello G, Piro E, Giuffre M, La Placa S, Corsello G. Transitional hemodynamics in infants of diabetic mothers by targeted neonatal echocardiography, electrocardiography and peripheral flow study. J Matern Fetal Neonatal Med. 2018;31:1578–1585. doi:10.1080/14767058.2017.1320544
10. Elmekkawi SF, Mansour GM, Elsafty MS, Hassanin AS, Laban M, Elsayed HM. Prediction of fetal hypertrophic cardiomyopathy in diabetic pregnancies compared with postnatal outcome. Clin Med Insights Womens Health. 2015;8:39–43. doi:10.4137/CMWH.S32825
11. Araujo Júnior E, Rolo LC, Rocha LA, Nardozza LM, Moron AF. The value of 3D and 4D assessments of the fetal heart. Int J Womens Health. 2014;6:501–507. doi:10.2147/IJWH.S47074
12. Gonçalves LF, Lee W, Espinoza J, Romero R. Examination of the fetal heart by four-dimensional (4D) ultrasound with spatio-temporal image correlation (STIC). Ultrasound Obstet Gynecol. 2006;27:336–348. doi:10.1002/uog.2724
13. Luewan S, Yanase Y, Tongprasert F, Srisupundit K, Tongsong T. Fetal cardiac dimensions at 14-40 weeks’ gestation obtained using cardio-STIC-M. Ultrasound Obstet Gynecol. 2011;37:416–422. doi:10.1002/uog.8961
14. Palmieri CR, Simões MA, Silva JC, Santos AD, Silva MR, Ferreira B. Prevalence of hypertrophic cardiomyopathy in fetuses of mothers with gestational diabetes before initiating treatment. Rev Bras Ginecol Obstet. 2017;39:9–13. doi:10.1055/s-0037-1598602
15. Fouda UM, Abou ElKassem MM, Hefny SM, Fouda RM, Hashem AT. Role of fetal echocardiography in the evaluation of structure and function of fetal heart in diabetic pregnancies. J Matern Fetal Neonatal Med. 2013;26:571–575. doi:10.3109/14767058.2012.743521
16. Quaresima P, Fesslova V, Farina A, et al. How to do a fetal cardiac scan. Arch Gynecol Obstet. 2023;307:1269–1276. doi:10.1007/s00404-023-06951-8
17. Giancarol M, Robert R. Diabetes in Pregnancy. In: Lockwood DJ, Copel JA, Dugoff L, et al., editors. Creasy and Resnik’s Maternal Fetal Medicine Principles and Practice.
18. Mrudhula Tejaswi G, Samanth J, Vasudeva A, et al. Fetal echocardiography at term in diabetic pregnancies helps predict the adverse neonatal outcome - Results of a prospective observational study from South India. Indian Heart J. 2020;72:576–581. doi:10.1016/j.ihj.2020.09.017
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.