Back to Journals » Journal of Pain Research » Volume 13
Incidence of Adrenal Insufficiency and Cushing’s Syndrome After Long-Term Epidural Steroid Injections Over Six Months or Longer: A Preliminary Study
Authors Park J, Kwak J, Chung S, Hong HJ, Chon JY, Moon HS
Received 17 March 2020
Accepted for publication 27 May 2020
Published 24 June 2020 Volume 2020:13 Pages 1505—1514
DOI https://doi.org/10.2147/JPR.S252278
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Robert B. Raffa
JungHyun Park, Jueun Kwak, Sukyung Chung, Hyo Ju Hong, Jin Young Chon, Ho Sik Moon
Department of Anesthesiology and Pain Medicine, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
Correspondence: Ho Sik Moon
Department of Anesthesiology and Pain Medicine, Eunpyeong St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 1021, Tongil-ro, Eunpyeong-gu, Seoul 03312, Republic of Korea
Tel +822-2030-3864
Fax +822-2030-3861
Email [email protected]
Purpose: Endocrinological complications of an epidural steroid injection (ESI) are rare but dangerous. Nevertheless, despite the associated risks, repeated long-term ESIs are indispensable in some clinical situations. However, only a few reports to date have assessed the safety of this procedure. In this study, we evaluate the incidence of adrenal insufficiency (AI) and Cushing’s syndrome after long-term ESIs.
Methods: This clinical observational study enrolled herniated nucleus pulposus or spinal stenosis patients who had received ESIs over a period of six months or longer. The adrenocorticotropic hormone (ACTH) stimulation test was performed to confirm AI and the late-night salivary cortisol (LNSC) test was performed to diagnose Cushing’s syndrome. To evaluate the hypothalamus pituitary adrenal axis suppression, salivary cortisol (SC) levels were measured on days 0, 1, 7, 21, 28, 35, and 42.
Results: This study included 17 patients. Among these, two patients (11.8%) developed AI, but no cases of Cushing’s syndrome were reported. There was no predictor for the development of AI. The SC levels tended to increase with time after an ESI (β = 0.003). The slope of the mixed effect model between the AI and non-AI groups showed a significant difference (p value = 0.015). However, the differences in mean SC levels at each time point between the two groups were not significant (adjusted p value = 0.053).
Conclusion: Long-term ESI use may be associated with AI development. An unexpected adrenal crisis due to AI could be life threatening. Therefore, regular monitoring of adrenal function in patients who have received long-term ESIs may be prudent.
Keywords: epidural, glucocorticoid, salivary cortisol, adrenal insufficiency, long-term
Introduction
An epidural steroid injection (ESI) is frequently performed for patients with herniated nucleus pulposus (HNP) or spinal stenosis because of its efficacy and simplicity in comparison with spine surgery.1 Complications of ESIs are uncommon and are classified into generic, pharmacologic, and site-specific complications.2,3 In this study, we focused on adrenal insufficiency (AI), Cushing’s syndrome, and hypothalamus pituitary adrenal (HPA) axis suppression because these could be associated with repeated exposure to excess iatrogenic glucocorticoids.
Due to a lack of adequate information regarding the pharmacokinetics and pharmacodynamics of epidural steroid, there is an absence of a universal consensus among providers in regard to steroid dosage, the number of procedures, and the interval between procedures for ESIs.4 Many doctors perform an ESI empirically or follow the rules used in other steroid injections such as intraarticular steroid injection.5,6 Considering the risk-to-benefit ratio of an ESI in comparison to spine surgery, decisions regarding the number of ESIs and how long ESIs can be conducted safely will always remain challenging to clinicians.
HPA axis suppression occurs in most of the patients who receive ESIs.7,8 In severe cases, it can cause secondary AI, which increases mortality and morbidity and impairs patients’ quality of life. Although long-term exogenous glucocorticoid intake is an uncommon cause of AI, it can induce suppression of the HPA axis, which is mediated by downregulation of endogenous adrenocorticotropic hormone (ACTH) release.9 The common symptoms of AI are fatigue, loss of appetite, weight loss, nausea, vomiting, abdominal pain, and muscle and joint pain, which are nonspecific and therefore do not facilitate easy diagnosis. Moreover, specific symptoms such as hyperpigmentation, salt craving, and postural hypotension are uncommon in exogenous glucocorticoid-induced AI because the mineralocorticoid axis is intact.9 Therefore, an early diagnosis of exogenous glucocorticoid-induced AI is challenging for physicians.
Diagnosis of AI involves several steps.9 The first step is “clinical suspicion of AI with symptoms and signs,” followed by the “ACTH stimulation test,” which involves measurement of serum cortisol levels at baseline and after injection of 250 μg of cosyntropin (at 30 and 60 min). If the peak cortisol level is below 18~20 μg/dL, it suggests AI. In addition, baseline levels of plasma ACTH, serum cortisol, renin, aldosterone, dehydroepiandrosterone (DHEA), sodium, potassium, serum 17-hydroxyprogesterone, antibody against 21-hydroxylase and the adrenal cortex, adrenal imaging data, pituitary gland MRI findings, and other data are required for differential diagnosis and confirmation of disease cause.10
Salivary cortisol (SC) measurement is a reliable method for assaying biologically active free cortisol.8,11 Although AI cannot be diagnosed by SC measurement alone, it is a useful tool to evaluate the HPA axis.12 However, late-night salivary cortisol (LNSC) measurement, usually performed between 2300 and 2400, is a very sensitive and specific diagnostic method for Cushing’s syndrome.12–15
The hypothesis of this preliminary study was that the longer the duration of ESIs or the greater the dosage of the injected steroid, the longer the period of HPA suppression. The primary objective of the study was to evaluate the risk of AI in long-term ESIs. The secondary objective was to evaluate the incidence of iatrogenic Cushing’s syndrome.
Materials and Methods
Study Design and Setting
This preliminary observational study included 20 patients who had received multiple ESIs over six months or longer at a university hospital from November 2014 through February 2015. They participated in the study for 6 weeks after the last ESI. The Institutional Review Board of the Catholic University of Korea approved the protocols for this study (SC13OISI0092). This study was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent to participate in the study.
Participants
Inclusion criteria were as follows: adults aged over 50 years; men and postmenopausal women; patients who had received multiple ESIs over six months or longer, a period that was defined from the date of their first ESI to their enrollment date, based on their medical record. Whether the ESIs had been performed regularly was not considered important. We considered patients who had received multiple ESIs, defined as ESIs that were performed more than three times a year. Patients diagnosed with lumbar HNP or spinal stenosis on the basis of symptoms (pain nature, pain location, neurogenic intermittent claudication, pain aggravating or relieving factors, etc.), signs (straight leg raising test, sensory, motor, deep tendon reflex, etc.), and magnetic resonance imaging (MRI) findings. Exclusion factors were as follows: patients absolutely contraindicated for an ESI (bleeding tendency and infection at the procedure site); patients who could not take corticosteroids (allergy to steroid agents, pregnancy, all endocrinological diseases); patients with chronic medical illnesses (hypertension, heart failure, renal disease, liver disease, etc.) or sleep disturbances; patients taking herbal medicine or contraceptives; patients who had experienced severe stress including emotional stress, interpersonal relationships deterioration, and so forth during the preceding month; and patients who had consumed alcohol for 12 hours or food one hour before sampling. When the participants were enrolled, they received an ESI and visited the hospital on the 42nd day.
All participants received a single epidural injection of 20 mg triamcinolone acetonide using transforaminal approach under C-arm guidance, in order to observe their response to the epidural steroid. Since intravascular injection of triamcinolone acetonide can affect drug pharmacokinetics, contrast medium was used to rule out intravascular injection. To monitor their sequential response to a single-dose epidural steroid, none of the participants received additional ESIs during the 42-day follow-up period.
Variables and Bias
The primary outcomes of this study were the results of the ACTH stimulation test to diagnose AI and the early morning SC concentration to evaluate HPA suppression. The secondary outcomes were LNSC concentration to assess the iatrogenic Cushing’s syndrome, symptoms and signs, numeric rating scale (NRS) scores (0, no pain; 10, the worst pain imaginable), fasting blood sugar (FBS) levels, serum ACTH levels, etc. Among these, SC concentration could be a potential confounder because of its circadian rhythm and age, seasonal, and gender differences.16 Therefore, this variable was obtained at the same time (0700~0800 h or 2300~2400 h), similar age (above 50 years old), and same season (winter in Korea). In addition, postmenopausal women were selected to minimize the effect of sex hormones. The diagnostic criteria for diseases in this study were as follows: AI, peak serum cortisol concentration <18 μg/dL after parenteral administration of 250 μg cosyntropin;9 iatrogenic Cushing’s syndrome, LNSC > 0.34 μg/dL (negative predictive value 100%);17 HPA suppression, normalization of early morning SC concentration on day 42 (reference ranges: 0.1 μg/dL ~ 1.0 μg/dL).18,19
Other variables were demographic data such as diagnosis, age, gender, and body mass index (BMI), total ESI treatment duration in months, the total dosage of steroid administered during the entire ESI period, the total number of ESIs, and SC normality on day 0 (D0) and day 42 (D42). The duration of the ESI treatments in months was defined as the period from the date of the patient’s first ESI to their enrollment date, which was based on their medical record, the total number of ESIs was defined as the number of performed ESIs during that period from the date of their first ESI to their enrollment date. The time criteria for all the variables applied in this study were within the patient’s lifetime, which was confirmed during their screening session.
Data Sources and Measurement
The data were collected before the single-dose epidural steroid was injection (D0) and on day 1 (D1), day 7 (D7), day 21 (D21), day 28 (D28), day 35 (D35), day 41 (D41), and D42 after the ESI. Before the ESI (D0), demographic data, NRS score, FBS level, and SC concentration were measured. On D1, D7, D21, D28, D35, D41, and D42 after the ESI, salivary samples were collected. The sampling time was determined in a previous pilot study, ie, HPA axis function was suppressed after the ESI until D21.8 Saliva was obtained from the participants by using a commercially available cotton sampling device, Salivette (Salimetrics, State College, PA, USA). Participants were instructed to rinse their mouth thoroughly with water 10 minutes before sample collection. They stored their saliva sample in a freezer compartment. Salivary samples were frozen at or below −20°C within 4 hours after collection.19 On D42, the participants revisited the hospital with their saliva samples in a cooler. All saliva samples except the D41 sample were obtained at early morning (0700~0800), but the D41 sample was collected at late night (2300~2400) for diagnosis of Cushing’s syndrome. SC concentration was measured by ELISA using the VersaMax ELISA Microplate Reader (Molecular Devices, Sunnyvale, CA, USA). On D42, ACTH testing was performed in addition to the pretests of serum ACTH concentrations, NRS levels, and FBS levels.
Statistical Methods
R language version 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria) and T&F program ver. 2.9 (YooJin BioSoft, Seoul, Korea) were used for all statistical analyses. Data were expressed as mean ± standard deviation (SD) for continuous variables. Student’s t test or Mann–Whitney test were performed to compare mean differences between the AI and non-AI groups. For categorical variables, data were expressed as sample number and percentage, N (%). Fisher’s exact test was used to test the association between complications and other categorical variables. For multiple test correction, FDR (false discovery rate) was adopted. Univariate binary logistic regression analysis was performed to analyze the effect of each clinical measurement on AI. A linear mixed effect model was generated to analyze the fixed effects of time and other baseline variables on the SC, where time and each variable were used as fixed-effect covariates with the random effect of intercept and slope of time for subjects. The slope of SC derived from the mixed effect model was compared between the AI and non-AI groups using Mann–Whitney U-test. Lattice plots were generated to compare the time trend of responses for each patient.
Results
Participants and Descriptive Data
Twenty participants were examined for eligibility, and all participants were included in the study. Among these, three participants were excluded because one was lost to follow-up and the others had missing data for their salivary samples (Figure 1). The demographic and clinical data of the participants are analyzed in Table 1. Before the enrollment, all participants had not been receiving ESIs at fixed time intervals, rather they were receiving them irregularly in 2–6 week intervals, depending on the patients’ pain intensity. When the subject’s pain intensity was below NRS 3/10, ESIs were not performed (Table 2).
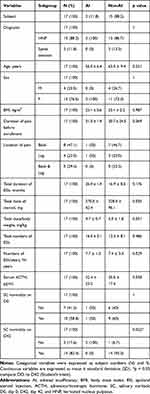 |
Table 1 Demographic and Clinical Characteristics of Variables |
 |
Table 2 Results of ACTH Stimulation Test, LNSC, Symptoms of AI, Cushing’s Syndrome, Total Duration of ESIs in Months, and Total Number of ESIs |
 |
Figure 1 Flow diagram of the study. |
Incidence of AI and Iatrogenic Cushing’s Syndrome
In this study, AI after long-term ESIs was observed in two out of 17 patients (11.8%), but none of the subjects showed iatrogenic Cushing’s syndrome (Table 2). There was no difference in diagnosis, age, gender, BMI, duration of ESIs treatment, total dose of steroid, total dose/body weight, total number of ESIs, serum ACTH level on D0, and SC normality on D0, but there was a statistically significant difference (p value = 0.022) in SC normality on D42 between AI and non-AI participants (Table 1). SC normality on D42 did not appear to be a regressor but was a result of AI development.
Analysis of the Factors Associated with the Incidence of AI
The authors analyzed the association of predictors with the incidence of AI using univariate binary logistic regression analysis (Table 3). Factors evaluated for their predictive value were diagnosis, age, gender, BMI, duration of ESIs, total dose of steroid, total number of ESIs, serum ACTH level, and serum cortisol level on D42, and FBS on D0. However, there was no associated predictor.
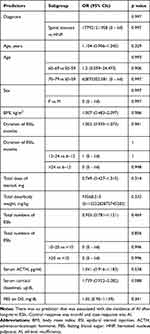 |
Table 3 Results of Univariate Binary Logistic Regression Analysis Using Incidence of AI as Response |
Analyses of the Trend of Repeated SC Measurements According to the Existence of Complications
Time was the only factor that affected SC concentration with a fixed effect with a result that adjusted with random effect and baseline covariates (p value = 0.002), ie, only the SC level increased according to time (β = 0.003) (Table 4). The slope of the mixed effect model represented the SC increment rate, which was measured for each subject. There was significant difference in the slope of the mixed effect model between the AI and non-AI groups (Table 5, Figure 2). When the SC trend of each subject was observed with a lattice plot, the AI patients’ slope of the mixed effect model was nearly zero (Figure 3). However, in the analyses of the difference in mean SC levels that were measured at each time point between the AI and non-AI groups, there was no statistically significant intergroup difference (adjusted p value = 0.053) (Table 6, Figure 4).
 |
Table 4 Fixed Effect of Time and Covariates |
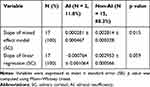 |
Table 5 Comparison of the Slope of SC Concentration Between AI and Non-AI Groups |
 |
Table 6 Comparison of the Mean SC at Each Time Between the AI and Non-AI Groups |
Discussion
Among seventeen patients who had received long-term (17.9 ± 8.4 months) ESIs, two patients developed AI. No subject developed iatrogenic Cushing syndrome. Among the patients who developed AI, the SC concentration did not recover until 42 days after an ESI in comparison with other patients who had normal adrenal function. There was a significant difference in the slope of the mixed effect model between the AI and non-AI groups (p value = 0.015). However, there was no statistically significant difference in the mean SC at each time point between both groups (adjusted p value = 0.053).
In the largest cohort study on ESIs, El-Yahchouchi reported that the incidence of ESI-related complications was 2.6% of 16,638 procedures.20 Although they did not analyze the relationship between the incidence of ESIs complications and long-term epidural steroid use, most complications were minor adverse effects such as flushing, agitation, sleeplessness, and headache.20 In this study, the incidence of AI in the cases of long-term ESIs was much higher than we expected (11.8%). The prevalence of secondary AI is rare, ranging between 150 and 280 cases seen per million people (0.00015–0.00028%).21 However, in the results of univariate binary logistic regression analysis using the incidence of AI as response (Table 3), there was no predictor that was associated with the incidence of AI. This could be a false-positive or false-negative result because of the small sample size, but we should consider the individual differences. Generally, suppression of the HPA axis is known to be associated with the duration of steroid therapy, the dosage, and the serum half-life of the steroid used; however there is an individual variability due to differences in drug metabolism or sensitivity.22
HPA axis suppression by exogenous steroid intake is associated with negative feedback loops for the corticotropin-releasing hormone (CRH) and ACTH. This pathologic state causes failure of pituitary ACTH and adrenal cortisol release.22 The symptoms of iatrogenic AI were associated with glucocorticoid deficiency, such as fatigue, lack of energy, weight loss, anorexia, myalgia, joint pain, fever, normochromic anemia, lymphocytosis, eosinophilia, slightly increased TSH, hypoglycemia, low blood pressure, postural hypotension, and hyponatremia; however, chronic AI has relatively nonspecific symptoms like fatigue and loss of energy.23 Because of these nonspecific symptoms, patients who have received long-term ESIs would be exposed to the danger of an unexpected adrenal crisis. Thus, regular monitoring of adrenal function is required in the long-term ESI patients.
In this study, no Cushing’s syndrome patients were reported unexpectedly. Although ESIs could be associated with the development of iatrogenic Cushing’s syndrome, it is a very rare complication and its incidence has not been reported yet. In the patients receiving intraarticular corticosteroids, Cushing’s syndrome developed in 5% of children with juvenile idiopathic arthritis who had received triamcinolone acetonide and usually developed weeks after the last injection.24
It is not easy to diagnose AI or Cushing’s syndrome caused by exogenous glucocorticoids because the symptoms are nonspecific.5,9,22,25 The ACTH stimulation test is the most useful method to diagnose an AI.9,26 The ACTH stimulation test (250 μg of cosyntropin) for primary AI shows a diagnostic value of 95% specificity and 97% sensitivity.27 However, its sensitivity for secondary AI was not good: 57% (250 μg of cosyntropin) and 61% (1 μg of cosyntropin).27 In this study, the mixed effect model of SC that obtained measurements over time was built. Its slope was significantly related to the development of AI (p value < 0.05). Although the sample size was small (17 subjects), it was helpful to diagnose or predict AI. Moreover, it offered the advantage of non-invasiveness in comparison with the ACTH stimulation test.
There were several limitations in this study. The major potential limitation was the small sample size, especially since there were only two AI patients. Because of this limitation, we could not conduct sufficient statistical analyses. However, it should be considered that long-term ESIs itself is an unusual situation, ie, ESIs are usually applied three times a year restrictively,28 and AI is an infrequent complication from ESIs. The second limitation was selection bias. When the subjects were enrolled, a sufficient number of examinations to exclude other conditions that could affect the occurrence of AI or Cushing’s syndrome were not conducted. However, the authors took the patients’ medical history and performed their physical examinations carefully. In our hospital, we usually follow a standard treatment regimen for HNP or spinal stenosis, ie, ESIs are applied three times a year restrictively.28 Most of the patients in this study had refused spinal surgery for a long time, and were chronic pain patients. A chronic pain is known to be associated with hypocortisolism. Besides, we did not evaluate subjects’ adrenal function before the experimental ESI; therefore, we cannot confirm whether AI developed due to the ESIs that the patients received or not. However, these two patients did not show AI symptoms before the experimental ESI. On the basis of this preliminary study, authors are planning a larger cohort study to evaluate the incidence of AI in patients received multiple ESIs. In addition, a future research to make an AI estimation model using the linear mixed effect model of SC trend after ESIs could be considered and it will provide an additional diagnostic method for AI.
In conclusion, long-term ESIs may be associated with AI development. An unexpected adrenal crisis due to AI could be life threatening; therefore, regular monitoring of adrenal function for patients who have been receiving long-term ESIs may be prudent.
Acknowledgment
We would like to thank Editage for English language editing.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no competing interests.
References
1. Manchikanti L, Pampati V, Falco FJ, Hirsch JA. Growth of spinal interventional pain management techniques: analysis of utilization trends and Medicare expenditures 2000 to 2008. Spine (Phila Pa 1976). 2013;38(2):157–168. doi:10.1097/BRS.0b013e318267f463
2. McGrath JM, Schaefer MP, Malkamaki DM. Incidence and characteristics of complications from epidural steroid injections. Pain Med. 2011;12(5):726–731.
3. Bogduk N. Epidural steroid injections. In: Fishman S, Ballantyne J, Rathmell J, editors. Bonica’s Management of Pain.
4. Hooten WM, Nicholson WT, Gazelka HM, Reid JM, Moeschler SM, Lamer TJ. Serum triamcinolone levels following interlaminar epidural injection. Reg Anesth Pain Med. 2016;41(1):75–79. doi:10.1097/AAP.0000000000000333
5. Habib GS. Systemic effects of intra-articular corticosteroids. Clin Rheumatol. 2009;28(7):749–756.
6. Mader R, Lavi I, Luboshitzky R. Evaluation of the pituitary-adrenal axis function following single intraarticular injection of methylprednisolone. Arthritis Rheum. 2005;52(3):924–928. doi:10.1002/art.20884
7. Kay J, Findling JW, Raff H. Epidural triamcinolone suppresses the pituitary-adrenal axis in human subjects. Anesth Analg. 1994;79(3):501–505.
8. Chon JY, Moon HS. Salivary cortisol concentration changes after epidural steroid injection. Pain Physician. 2012;15(6):461–466.
9. Bancos I, Hahner S, Tomlinson J, Arlt W. Diagnosis and management of adrenal insufficiency. Lancet Diabetes Endocrinol. 2015;3(3):216–226. doi:10.1016/S2213-8587(14)70142-1
10. Restituto P, Galofre JC, Gil MJ, et al. Advantage of salivary cortisol measurements in the diagnosis of glucocorticoid related disorders. Clin Biochem. 2008;41(9):688–692. doi:10.1016/j.clinbiochem.2008.01.015
11. Gozansky WS, Lynn JS, Laudenslager ML, Kohrt WM. Salivary cortisol determined by enzyme immunoassay is preferable to serum total cortisol for assessment of dynamic hypothalamic–pituitary–adrenal axis activity. Clin Endocrinol (Oxf). 2005;63(3):336–341. doi:10.1111/j.1365-2265.2005.02349.x
12. Raff H. Utility of salivary cortisol measurements in Cushing’s syndrome and adrenal insufficiency. J Clin Endocrinol Metab. 2009;94(10):3647–3655. doi:10.1210/jc.2009-1166
13. Deutschbein T, Broecker-Preuss M, Flitsch J, et al. Salivary cortisol as a diagnostic tool for Cushing’s syndrome and adrenal insufficiency: improved screening by an automatic immunoassay. Eur J Endocrinol. 2012;166(4):613–618. doi:10.1530/EJE-11-0945
14. Raff H. Update on late-night salivary cortisol for the diagnosis of Cushing’s syndrome: methodological considerations. Endocrine. 2013;44(2):346–349. doi:10.1007/s12020-013-0013-0
15. Raff H. Salivary cortisol and the diagnosis of Cushing’s syndrome: a coming of age. Endocrine. 2012;41(3):353–354. doi:10.1007/s12020-012-9661-8
16. Tornhage CJ. Salivary cortisol for assessment of hypothalamic-pituitary-adrenal axis function. Neuroimmunomodulation. 2009;16(5):284–289. doi:10.1159/000216186
17. Alwani RA, Schmit Jongbloed LW, de Jong FH, van der Lely AJ, de Herder WW, Feelders RA. Differentiating between Cushing’s disease and pseudo-Cushing’s syndrome: comparison of four tests. Eur J Endocrinol. 2014;170(4):477–486. doi:10.1530/EJE-13-0702
18. Aardal E, Holm AC. Cortisol in saliva–reference ranges and relation to cortisol in serum. Eur J Clin Chem Clin Biochem. 1995;33(12):927–932. doi:10.1515/cclm.1995.33.12.927
19. Salimetrics. High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit. Available from: https://salimetrics.com/analyte/salivary-cortisol/.
20. El-Yahchouchi CA, Plastaras CT, Maus TP, et al. Adverse event rates associated with transforaminal and interlaminar epidural steroid injections: a multi-institutional study. Pain Med. 2016;17(2):239–249. doi:10.1111/pme.12896
21. Chabre O, Goichot B, Zenaty D, Bertherat J. Group 1. Epidemiology of primary and secondary adrenal insufficiency: prevalence and incidence, acute adrenal insufficiency, long-term morbidity and mortality. Ann Endocrinol (Paris). 2017;78(6):490–494. doi:10.1016/j.ando.2017.10.010
22. Jacobs JWG, Bijlsma JWJ. Glucocorticoid therapy. In: Firestoin GS, Budd RC, Gabriel SE, Mclnness IB, O’Dell JR, editors. Kelly and Firestein’s Textbook of Rheumatology.
23. Arlt W. Disorders of the adrenal cortex. In: Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, editors. Harrison’s Principles of Internal Medicine.
24. Gondwe JS, Davidson JE, Deeley S, Sills J, Cleary AG. Secondary Cushing’s syndrome in children with juvenile idiopathic arthritis following intra-articular triamcinolone acetonide administration. Rheumatology (Oxford). 2005;44(11):1457–1458. doi:10.1093/rheumatology/kei154
25. Bornstein SR. Predisposing factors for adrenal insufficiency. N Engl J Med. 2009;360(22):2328–2339. doi:10.1056/NEJMra0804635
26. Neary N, Nieman L. Adrenal insufficiency: etiology, diagnosis and treatment. Curr Opin Endocrinol Diabetes Obes. 2010;17(3):217–223. doi:10.1097/MED.0b013e328338f608
27. Dorin RI, Qualls CR, Crapo LM. Diagnosis of adrenal insufficiency. Ann Intern Med. 2003;139(3):194–204. doi:10.7326/0003-4819-139-3-200308050-00009
28. Kim EJ, Moon JY, Park KS, et al. Epidural steroid injection in Korean pain physicians: a national survey. Korean J Pain. 2014;27(1):35–42. doi:10.3344/kjp.2014.27.1.35
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



