Back to Journals » International Journal of Women's Health » Volume 15
Incidence, Bacterial Profile and Predictors of Surgical Site Infection After Cesarean Section in Ethiopia, A Prospective Cohort Study
Authors Mezemir R, Olayemi O, Dessie Y
Received 12 June 2023
Accepted for publication 26 September 2023
Published 13 October 2023 Volume 2023:15 Pages 1547—1560
DOI https://doi.org/10.2147/IJWH.S425632
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Rahel Mezemir,1,2 Oladapo Olayemi,3 Yadeta Dessie4
1Pan African University, Life and Earth Sciences Institute (Including Health and Agriculture), Ibadan, Nigeria; 2St. Paul’s Hospital Millennium Medical College, School of Nursing, Addis Ababa, Ethiopia; 3Department of Obstetrics and Gynecology, College of Medicine, Pan African University Life and Earth Sciences Institutes, University of Ibadan, Ibadan, Nigeria; 4College of Health and Medical Sciences Haramaya University, Harar, Ethiopia
Correspondence: Rahel Mezemir, Tel +251 911553348, Email [email protected]; [email protected]
Background: Surgical site infections (SSI) after cesarean section are common in Ethiopia and result in maternal morbidity, mortality, hospitalization, and medical costs. This study aimed to determine the incidence, bacterial profile, and associated factors of surgical site infection after cesarean section (CS) in public and private referral hospitals.
Methods: A prospective observational cohort study was conducted on 741 pregnant women who underwent CS from July to September 2022. Women who had CS were followed up for at least 30 days. Infected wound specimens from those who had SSIs were collected and bacteriologically analyzed. The data were analyzed with SPSS version 25. The logistic regression model assessed the relationship between the independent variable and the outcome with 95% confidence interval.
Results: The incidence of post-cesarean surgical site infection was 11.6% (95% Cl: 9.4, 13.6). Staphylococcus aureus was the most common bacteria in CS wounds 10 (21.2%). Two to three antenatal care visits (ANC) (AOR: 3.11, 95% CI: 1.69, 5.75), delayed antenatal booking (AOR: 6.99, 95% CI: 2.09, 23.32), membrane rupture (AOR: 2.10, 95% CI: 1.04, 4.24), multiple vaginal examinations (AOR = 4.21, 95% CI: 1.35, 6.92) and public hospitals (AOR: 11.1, 95% CI: 1.48, 45, 14) were associated with increased risk of SSI after CS, in contrary shorter hospital stays (AOR = 0.37, 95% CI: 0.15, 0.91) and transversal incisions (AOR = 0.38, 95% CI: 0.15, 0.91) were associated with lower risk SSI after CS.
Conclusion: The incidence of SSI after CS was high. Delayed antenatal booking, two to three antenatal visits, multiple vaginal exams, membrane rupture, vertical incision, longer postoperative hospital stays, and procedures in public hospitals were associated with increased risk of SSI after CS. Therefore, intervention programs should focus on post-discharge surveillance and identification of risk to reduce and prevent SSI after CS rate.
Keywords: incidence, surgical site infection, cesarean section, bacterial, Addis Ababa
Introduction
Surgical site infection after cesarean section (SSI after CS) is the most frequent complication, which happens after a cesarean section is done for a delivering mother.1 It occurs within 30 days after a surgical procedure and involves either superficial, deep, or organ/space tissues. It can significantly lower the quality of life, lengthen hospital stays, and place a financial burden on the healthcare system.2–4
Worldwide, the incidence of surgical site infection after cesarean section reported in the literature ranges from 3% to 20% depending on the patient population, antibiotic prophylaxis, and infection surveillance techniques used.5 The country-specific reports deal with 5.3% in Egypt,6 1.7% in rural China,7 2% in Ireland,8 9.9% in Kosovo,9 2.1% in Kuwait 2.110 and 9.7% in Ethiopia.11 Despite advances in surgical procedures, options for sterilizing surgical instruments, improved surgical techniques, and improved infection prevention programs, surgical site infections are a major cause of nosocomial infections, and the incidence of infections is increasing worldwide.4 In sub-Saharan Africa, up to 20% of women giving birth by cesarean section develop SSI, and cesarean delivery is the most important known variable associated with a higher risk of postpartum infection than vaginal delivery.2 Studies show that the risk of infection ranges from 1% to 25% and 5 to 20 times that of vaginal delivery.12 Findings from studies conducted in different settings suggest that a number of factors contribute to the occurrence of SSI, including comorbidities, age, duration of operation, anemia, frequency of manual vaginal examinations, HIV infection, rupture of membrane, and inappropriate antibiotic prophylaxis. Therefore, identifying the risk factors that increase the number of SSI can help design interventions that take into account the context in which these operations are performed.5,6,13,14
In Ethiopia, the burden of SSI after CS has been noted as particularly increased due to weaker infection control infrastructures, limited resources, lack of adherence to infection control policies and guidelines, inadequately trained personnel, overcrowding, and understaffing.15
Clinical practice recommendations for SSI prevention include, but not limited to, preoperative bathing, surgical site preparation, surgical hand preparation, appropriate use of antibiotics to reduce the risk of infection, and applied infection prevention technique.16,17
Several studies on surgical site infections after cesarean section have been conducted in Ethiopia. However, most studies used a cross-sectional design to determine factors associated with surgical site infection after cesarean section.11,18–20 In other cases, according to a study from Kuwait 99% of SSI patients had their diagnoses made after being discharged from the hospital.10 Although a study was conducted in the gynecology departments of referral hospitals in Addis Ababa, it was only conducted in public hospitals, which can have a fairly homogeneous pool of women, bacterial profile was not isolated, ANC variables such as number of ANC, and time of ANC follow-up were not considered and used a small sample size.21 Unlike the previous studies, the current study filled the aforementioned gaps by including an adequate sample size, considering both government and private referral hospitals, and using laboratory techniques to identify bacterial pathogens. Therefore, our study aimed to assess the incidence, bacteriological profile, and associated factors of surgical site infection after CS.
Methods and Materials
Study Setting, Study Design Study Period, and Study Subject
A prospective cohort study was conducted at a referral hospital in Addis Ababa from July to September 2022. Addis Ababa is the capital of Ethiopia. It has a total area of 527 km2 and is inland has 51 hospitals in the city.22 The study was conducted in seven randomly selected hospitals, three private and four public hospitals. Hemen Maternal and Child Health (MCH) is one of the private hospitals that offer services in the specialty of maternal and child health and has 50 inpatient and 200 outpatient wards with a total of 39 beds. Betsegah MCH is similar to Hemen MCH and offers mother and child care has 24 beds, 5 gynecologists, 30 nurses, and 5 midwives. Myung Sung Christian Medical Center is also a private general hospital with 13 functional beds and 16 midwives. St. Paul’s Hospital Millennium Medical College Hospital (SPHMMC) is a public hospital. The gynecology department has 120 residents, 20 midwives, 90 midwives, and 60 beds.Zewditu Memorial Hospital is a public hospital that has 60 beds, 86 midwives, and eight gynecologists and obstetricians. Abebech Gobena and Gandhi Memorial Hospital are both MCH public hospitals. Abebech Gobena Hospital has 33 beds, 68 midwives, 11 gynecologists and obstetrician. Gandhi Memorial Hospital has 13 beds, 60 midwives, and 5 gynecologists and obstetricians.23 All eligible women were followed up for SSI for 30 days. Bacteriological samples were collected from subjects who had SSI within 30 days of follow-up. Women who had abdominal surgery in the month before their current surgery, women who were living outside of Addis Ababa, and women who were taking antibiotics prior to hospital admission were excluded from the study.
Sampling Size Determination and Sampling Technique
Samples of 787 post-CS women were prospectively recruited into the study and 741 followed up for 30 postoperative days. A double population formula was used to calculate the sample size, with a two-sided confidence level of 95% and an 80% power variable. The study used a two-stage cluster sampling technique, selecting a random sample from seven hospitals from 15 private and 14 public hospital referrals. A random sample of post-operative women was obtained from each hospital using a systematic random sampling technique. The maximum sample size was selected variable (hospital stays greater than 7 days) as in a study conducted in Harar city.16 The total number of CS surgery women per month in each hospital was estimated by taking the average of the total number of CS surgical patients flow for the past 12 months. For those women who agreed to participate and fulfill the inclusion criteria, a unique study ID number was given immediately after their operation.
Data Collection Procedure and Tools
The data was collected on SSI using Kobo Collect software and collected sociodemographic, procedural, and clinical data from various sources. Four maternity nurses, four surgical nurses, two supervisors, and one laboratory technician were recruited for data collection. Surgical and obstetric nurses examined wounds daily and monitored patients for signs of infection. Women were briefed on SSI symptoms and given printed materials. The data was collected during structured telephone interviews, and SSI diagnosis was based on signs and symptoms. If SSI was identified, women were referred to the nearest healthcare facility for further treatment. Women who could not be contacted after three unsuccessful phone calls were considered lost to follow-up. The criteria for defining an SSI set by the CDC-NHSN and WHO are applied to recruit those patients suspected of developing SSI.
Laboratory Data Collection
The standard protocol for collecting specimens in the laboratory was followed by taking post-surgical wound swabs or pus aspirates from the clinically infected surgical sites. Before collecting, two sterile cotton swabs were used to briefly clean the surgical site surroundings with 70% ethyl alcohol, and extra debris from the wound base was removed by irrigation with normal saline. To avoid superficial microflora, the deepest part of the wound was sampled. All collected samples were kept in test tubes containing 1.0 mL of normal saline and taken to the microbiology laboratory of Arsho Medical Laboratory for analysis within 3 hours after collection. An aseptic technique was applied at all stages to avoid cross-contamination.
Culturing and Identification of Bacterial Pathogen
Swabs were used to inoculate MacConkey agar, chocolate, and blood. A candle jar was used to incubate a chocolate plate alongside other plates for 24–48 hours at 35–37 C. The morphological appearances of the cultures on selective and differential media were observed and characterized using a standard procedure. Biochemistry and motility tests were also performed.24,25
Variables of the Study
Dependent Variable
SSI after cesarean section (present/absent).
Independent Variables Study
The independent variables were divided into four categories: sociodemographic factors (age, religion, education), medical factors (Body Mass Index (BMI), Mid–upper Arm Circumference (MUAC), HIV infection, American Society of Anesthesiologists (ASA), gestational hypertension, gestational diabetes), pregnancy and obstetric factors (parity, antenatal screening (ANC), ANC booking, frequency of ANC visits, gestational age, premature rupture of membranes (PROM), duration of membrane rupture, number of vaginal examinations, chorioamnionitis) and procedural factors (type of cesarean section, amount maternal blood loss, duration of operation, the skill of gynecologist, duration of antibiotic prophylaxis, Timing of antibiotic prophylaxis, type of incision, type of anesthesia, previous cesarean section, type of skin closure, postoperative determination of hemoglobin, type of the operation and where the procedure performed (public and private) wound class).
Operational Definition
Mothers: For this study, mothers were referred to women of reproductive age 15–49 years according to WHO.26
Wound Infection: For this study, wound infection was restricted to only infections arising from surgical incision after cesarean section (CDC).27
Body Mass Index (BMI) is a medical screening tool that measures a person’s weight in kilograms (or pounds) divided by the square of height in meters. For this specific study use CDC guidelines for adult classification of BMI:<18.5 = underweight,18.5 to 24.9= healthy weight,25.0 to 29.9 = overweight, and 30 and higher =obesity.28
Mid-upper arm circumference (MUAC) is a measurement that enables medical professionals to quickly assess whether a patient is acutely malnourished. For this study <22 =malnutrition, ≥22 = Normal.28
Quality Assurance
Data quality was maintained through various methods such as using the Kobo Collect software, building a Kobo server (standardized data collection tools), 3-day training sessions for data collectors, monitoring the process of data collection, and pre-testing the questionnaire before the actual survey. In addition, the microbiology laboratory has strictly followed the quality assurance process. This means that Gram stain reagents and biochemical tests were checked with standard strains of Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) at 37°C for 24–48 hours to verify sterility.
Data Analysis
The Kobo collection was used to collect data. The collected data was exported to Epi-data for analysis. Data were checked and cleaned with Epi-data version 3.1 and exported to the SPSS software version 25. Descriptive statistics such as frequencies and summary statistics (mean, standard deviation, and percentage) describe the study population in terms of sociodemographic, bacterial profile, and other relevant variables. For continuous variables, normality was tested using visual inspection and Shapiro–Wilk statistical tests. Assumed normality if the p-value was > 0.05. Categorical data was compared using the chi-square test. SSI after the CS rate was calculated by performing descriptive statistics and subsequent bivariate and multivariate logistic regression. Factors with a significance level of ≤ 0.25 were included in the bivariate logistic regression analysis. In multivariate logistic regression analysis, the presence and degree of association between dependent and independent variables were calculated using odds ratios with 95% confidence intervals (CI) and p-value. Statistical significance was considered at p-values ≤ 0.05. The presence of collinearity between the explanatory variables was assured by the inflation variance coefficient at the threshold of 1048 and no collinearity was found.
Ethical Consideration
The Institute for Advanced Medical Research and Training (IAMRAT), College of Medicine, University of Ibadan, Ibadan, Nigeria approved the study under registration numbers I/UCH EC NHREC/05/01/2008a and UI/UCH. The Ethics Committee has assigned the number UI/EC/20/0501. The study was also approved by Saint Paul’s Hospital Millennium Medical College and Addis Ababa Health Bureau Ethics Committee. Written informed consent was obtained from each study participant prior to beginning data collection techniques. Written consent was obtained after discussing study objectives, data collection processes, benefits and risks of participating in the study, and the volunteer nature of the study. The study conforms to the standards of the Declaration of Helsinki.29
Results
Socio-Demographic and Medical Characteristics
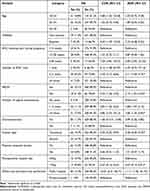
The final analysis included 741 women with a response rate of 90.2% (Figure 1). The median age of the women was 28 years and varied between 25 and 30 years. Almost all women were married, 715 (96.5%), and more than half, 438 (59.1%) were housewives. About 493 (66.6%) of the women were Orthodox Christians and 505 (68.2%) of the women had secondary or tertiary education. The median body mass index (BMI) for the women was 24.4 kg/m2, between 21.7 kg/m2 and 27.3 kg/m2. Most women had a BMI between 18–24 3 (51%). The mean MUAC was 24.4 cm, ranging from 22 cm to 26 cm. Regarding health status, most women had ASA class II, 328 (54.1%), about 157 (21.2%) of them had gestational hypertension, 21 (2.8%) were HIV positive, and 43 (58%) of the women had gestational diabetics (Table 1).
 |
Table 1 Socio-Demographic and Medical Characteristics of Women SSI After CS at Public and Private Hospitals in Addis Ababa, Ethiopia, from July to September 2022 (N= 741) |
 |
Figure 1 Flow diagram of the overall recruitment and follow-up process of women after CS surgery in government and private hospitals in Addis Ababa, Ethiopia. |
Pregnancy and Obstetrics-Related Characteristics of Women
Four hundred and forty-nine (60.6%) women were multipara with a median of 2 (IQR: 1–3). Almost all of the women, 705 (95.1%) had a history of ANC follow-up. Of these, 400 (54%) women initiated ANC bookings between 12 and 28 weeks, and approximately 572 (77.2%) women had at least four ANC visits. Most women, 399 (53.8%) had a cesarean section at the time of terms. About 84 (11.3%) of female membrane rupture (PROM) before surgery. Of these, 31 (36.5%) women had membrane rupture lasting longer than 12 hours, and approximately 455 (61.4%) women had labor duration ≤ 24 hours. Approximately two hundred and nine (39.3%) of women had four or more vaginal examinations (Table 2).
 |
Table 2 Pregnancy and Obstetrics of Women SSI After CS at Public and Private Hospitals in Addis Ababa, Ethiopia, from July to September 2022 (N= 741) |
Procedural-Related Characteristics of Women
Prophylaxis antibiotics were administered to all cesarean delivery patients within 30 minutes before surgery. The most commonly used antibiotic was ampicillin, which was administered to 637 (86%) women. The technique of subcuticular skin closure was used in 722 (97.4%) women and 488 (65.9%) women were emergency delivery. Almost all women received spinal anesthesia (96.8%). The majority of the women had estimated blood loss of 500 mL or less (96.11%). Most women were discharged from the hospital within the first three days following CS, with a range of hospital stays from two to four days. The average time for the development of post-CS SSI during the follow-up study was nine days, ranging between 8 and 10 days. As for the person who performed the cesarean section operation, most of the procedures were performed by residents in the second or third year of the obstetrics and gynecology training program. The median procedure time was 40 minutes (range 30 to 50 minutes) and most women had clean contaminated wounds 710 (95.8%). A considerable number of gynecologists 633 (85.4%) used soap and water for surgical hand scrubbing, and 632 (85.4%) used povidone-iodine for skin preparation (Table 3). Regarding to indication for operation, two hundred and nine women were fetal distress (28.2%) (Table 4).
 |
Table 3 Procedural-Related Characteristics of Women SSI After CS at Public and Private Hospitals in Addis Ababa, Ethiopia, from July to September 2022 (N= 741) |
 |
Table 4 Indication for CS Operation of Women SSI After CS at Public and Private Hospitals in Addis Ababa, Ethiopia, from July to September 2022 (N= 741) |
Incidence of SSI After Cesarean Section
Of 741 study women, 86 had developed SSI after CS, making an incidence rate of 11.6% (95%Cl: 9.4, 13.6). Among these, 75 women were traced through follow-up and readmission. The remaining 10 of them were identified before discharge. Almost all (94.1%) cases were superficial wounds, and the highest incidence of post-CS infection was observed in public referral hospitals 85 (11.4%).
Identified Bacterial Isolates
Wound swabs or pus aspirates were taken from individuals who developed a post-CS infection (n = 62). Among these, 75.8% (47/62) had positive for a culture test while the remaining 24.2% were negative. Forty-seven bacterial isolates were identified with 70.2% being Gram-negative, 25.5% being Gram-positive, and 4.2% showed no growth. The most commonly isolated bacteria were S. aureus, accounting for 22 (37.3%), followed by E. coli (27.1%), Klebsiella pneumoniae (22.0%), Pseudomonas aeruginosa (5.1%), and no growth in 4.2% of cases (Figure 2).
 |
Figure 2 The proportion and type of bacteria isolated from women who had post-cesarean surgical site infection (n =47). |
Factors Associated with Surgical Site Infection Following Cesarean Section
In binary logistic regression analysis, variables with p < 0.25 have been included in multiple binary logistic regression models. Fourteen of the thirty independent variables met this criteria and were selected for multivariate analysis. In the multivariate logistic regression, where the procedure was performed, ANC booking, frequency of ANC visits, number of vaginal examinations, PROMs, type of incision, and postoperative hospital stays were significant predictors of surgical site infection after CS.
Accordingly, women who underwent operation procedures in public hospitals were 11.1 times more likely to develop an infection after a cesarean section than in private ones (AOR: 11.1, 95% CI: 1.48, 45, 14). The odds of women who started ANC followed -up at ≥ 28 weeks were 6.99 times more likely to develop post-CS infection than women who started ANC followed up before 12 weeks (AOR: 6.99, 95% CI: 2.09, 23.32). Likewise, women who had two to three ANC frequency visits were 3.11 times more likely to develop an infection after a cesarean section than women who had four or more ANC visits (AOR: 3.11, 95% CI: 1.69, 5.75). The odds of developing an infection after a cesarean section with a history of PROM was two-fold higher than those without PROM (AOR: 2.10, 95% CI: 1.04, 4.24). The odds of developing an infection after cesarean delivery was three-fold higher in women who had at least four vaginal examinations (PV) than women who had not (AOR = 4. 21, 95% CI%: 1.35, 6.92). In addition, the likelihood of infection after a cesarean section increased with the length of hospital stay. Women who stayed in the hospital for less than seven days were 63% less likely to develop an infection after cesarean than women who stayed in the hospital for more than seven days (AOR = 0.37, 95% CI: 0.15, 0.91). Similarly, women with transverse incisions were 62% less likely to develop infections after cesarean delivery than women with vertical incisions (AOR = 0.38, 95% CI: 0.20, 0.72)).(Table 5).
Discussion
This study was conducted to estimate SSI after cesarean section by identifying the incidence, predictors, bacterial profile, and associated factors in four public hospitals and three private referral hospitals in Addis Ababa. Of a total of 741 women who were studied, 86 developed SSI with an incidence of 11.6% (95% CI: 9.4, 13.6). The procedure performed in public hospitals, ANC booking start during pregnancy, frequency of antenatal visits, number of vaginal examinations, type of incision, premature rupture membrane, and postoperative hospital stay were independent predictors of surgical site infection.
This study showed relatively higher incidence of post-cesarean infection (11.6%) than studies conducted in other regions of Ethiopia. Reported in Felegehiwot referral hospitals (7.8%),30 Debretabor General Hospital (8%)14 Nekemte referral hospitals (8.9%),5 and Zewditu Memorial Hospital (8.4%).18 This incidence is also higher than study conducted in other African countries such as 5.34% in Egypt,6 7.3% in sub-Saharan Africa,31 Asian countries, 5.8% in Pakistan,17 and 1.7% in China7 and others developed countries, the United States of America (5%),21 and Ankara (6.2%).22 Moreover, in agreement study conducted in Ayder referral hospital (11.7%)19 and Sierra Leone (11%).32 The Possible explanations for the difference might be the study setting, the study design, the type of operation, the patient’s characteristics, and patient follow-up to evaluate wound complications and perioperative infection prevention and control practices. In addition, some studies rely solely on medical records, which may underestimate the incidence of SSI due to unrecorded outcomes, such as the study conducted at Nekemte Hospital and Felegehiwot Referral Hospital.
In this study, women with an ANC booking ≥28 weeks and two to three ANC visits had a seven and three times greater risk of developing infection after cesarean section than women with ANC booking < 12 weeks and more than four ANC visits, respectively. Similar study from Debre-Markos Referral Hospital13 and Egypt.6 A possible reason for this could be that booking ANC <12 reduces complications such as prolonged membrane rupture, chorioamnionitis, and obstructed labor. In addition, regular ANC monitoring provides women and their families with relevant information and advice for a healthy pregnancy, safe delivery, and postnatal recovery. Evidence from World Health Organization (WHO) also supports this finding, the minimum number of ANC visits increased from four to eight experiences in 2016, with the first contact made within the first 12 weeks of pregnancy.26 Further, the results of a cross-sectional study conducted at a facility in central Tigray, Ethiopia, showed that the number and timing of ANC (Early Book) visits is good timing to rise awareness of pregnancy problems, Signs, and symptoms that could lead to early access to the relevant pregnancy complications.33
Likewise, in this study, women who underwent vertical incisions had an increased rate of SSI. This study is consistent with studies conducted in India,34 Nigeria,35 and Ethiopia.11 The reason may be due to the type of skin incision used in cesarean section, which runs parallel to the course of the transected segmental nerves, thereby minimizing postoperative muscle strain and paralysis, which can result in decreased muscle activity. It gets to the puncture site, resulting in delayed wound healing, increased risk of wound infection, and increased risk of infection. This study found that multiple vaginal examinations during labor were significantly associated with the development of post-CS infection. This finding is consistent with a study from Ireland8 and Debre-Markos Referral Hospital in Ethiopia.13 The reason may be that repeated vaginal examinations can transmit the endogenous vaginal flora that causes SSI to the upper genital tract.
Women with a history of PROM were twice as likely to develop an infection as women without a history of PROM. Many studies supported the findings and confirmed that SSI was significantly associated with PROM.13,19,20,36 A possible explanation could be that the natural barriers to infection during pregnancy include the cervical mucus plug, amniotic membrane, and amniotic fluid; however, this protective function is disrupted when these natural barriers are damaged as the amniotic fluid loses its sterility. It is believed that unsterile amniotic fluid can serve as a portal of entry for germs into the uterine and skin incision, and instead cause chorioamnionitis and associated inflammation.
The risk of infection after cesarean section increased with the length of hospital stay. This is in agreement with a study conducted in Switzerland,37 Malaysia,38 and Harar City.16 The reason could be hospital spaces are assumed to be contaminated due to patients, attendants, and health professionals. Cross-contamination occurs between patients, health professionals, equipment, the environment, and air. Further, patients with longer post-operative stays have more exposure to colonization and contamination with pathogenic microorganisms, potentially lowering the host’s resistance to infections. As a result, the risk of surgical site infection after CS was increased.37 In contrast a study conducted in Kosovo9 found that the length of hospital stay after surgery was not associated with post-CS infection. This discrepancy result might be due to differences in the quality of healthcare facilities, ward cleanliness, healthcare experience, and health status among these countries and socioeconomic groups.
This study also revealed the odds of developing surgical site infection after CS were 11 times higher among women who operated public hospitals than private hospitals. This study is in agreement with a study conducted in Bahir Dar hospitals, in Ethiopia.39 One possible explanation would be that public hospitals had a higher patient flow and performed (85.7%) more CS procedures than private hospitals (14.2%), which raised the incidence of surgical site infections and caused problems. Evidence supports this finding that the number of cesarean case flows is high, and the number of post-cesarean section infections is expected to increase.40 In addition, the present study showed that the majority (81.1%) of surgeries in public hospitals were performed by third- and fourth-year residence students, compared to just (18.2%) of the patients who were operated on by senior surgeons. Another reason could be that private hospitals are relatively clean and implement strict infection prevention strategies to increase patient satisfaction.
In this study the most common bacteria isolated from CS wounds was S. aureus 22 (37.3%); which agrees with a study from Indonesia at Cipto Mangunkusumo Hospital41 and Kosovo.9 Contrary to what was found at Kenyatta National Hospital, Escherichia coli was the most frequently isolated bacteria from CS wounds (27.2%).42 The discrepancy may be due to different aseptic techniques, geographic distributions of pathogens, patterns of bacterial resistance, different surgical procedures, and different identification methods used in bacterial isolation and the hospital setting.
Limitation of Study
The limitation of this study was authors did drug sensitivity tests; however, more data was needed to draw any conclusions. In addition, the study did not examine a significant association between variables related to health professionals, methods of sterilization of equipment, frequency, and number of operation rooms. The methodological strength of this study was in the presence of post-discharge follow-up of surgical patients, considering the large sample size and bacterial profile used.
Conclusion
In this study, the incidence of surgical site infection after cesarean section was high compared to previously published studies. Delayed ANC booking, two to three ANC visits, multiple vaginal examinations, PROM, vertical incision, longer postoperative hospital stays, and procedures in public hospitals were associated with increased risk of SSI after CS. Therefore, hospitals should think about creating and putting into place mechanisms like post-discharge surveillance, starting treatment early to prevent complications, and evaluating the impact of all preventive and corrective measures to make sure their rate is below the expected range. Moreover, planning to avoid unnecessarily prolonged hospital stays, education about the need for early ANC booking during pregnancy, increased ANC follow up time, compliance with infection prevention guidelines, considered a comprehensive evaluation of the membrane to avoid avoidable SSI and maternal morbidity. In addition, Staphylococcus aureus was the most common bacterial finding in cesarean section wounds. To prevent Staphylococcus bacterial infection, it is very important to wash hands regularly and cover the wounds with clean, dry bandages until they are fully healed.
Abbreviations
AOR, Adjusted Odds Ratio; CI, Confidence Interval; CS, Caesarian Section; PCSSI, Post cesarean surgical site infection; OR, Odds Ratio; WHO, World Health Organization; ANC, antenatal care; BMI, body mass index; PROM, the premature rapture of the membrane; SSIs, surgical site infection; MUAC, mid-upper arm circumference current; HIV, human immunodeficiency virus; ASA, American Society of Anesthesiologists; S. aureus, Staphylococcus aureus; E. coli, Escherichia coli; AU, African Union Commission; PAULESI, Pan African University Life and Earth Science Institute; IQR, interquartile range.
Data Sharing Statement
The datasets used during this study are available from the principal investigator upon reasonable request.
Acknowledgments
We acknowledge the African Union Commission (AU) for funding this research. In addition, we would like to thank the academic and non-academic staff at the Pan African University Life and Earth Science Institute (PAULESI) at the University of Ibadan in Nigeria. Our gratitude also goes to data collectors and supervisors for their diligence in gathering vital information during data collection. Our heartfelt gratitude also goes to the participants who kindly shared their views and sentiments despite other commitments.
Author Contributions
All authors made up significantly to the work reported, whether in the conception, study design, execution, data acquisition, analysis, and interpretation, or all of these areas; participated in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article was submitted; and agree to be accountable for all aspects of the work.
Funding
The Pan-African University of Life and Earth Sciences Institute, Pan-African University, and the African Union provided support for this research. The sponsoring organization played no role in the study’s design, data collection, analysis, interpretation, or paper writing; this was the responsibility of the authors.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Sivan Z-E, Noah Z, Gali G, Raed S. Postcesarean Wound Infection: Prevalence, Impact, Prevention, and Management Challenges. Dovepress; 2017.
2. World Health Organization. Preventing surgical site infections: implementation approaches for evidence-based recommendations; 2018. Available from: https://www.who.int/publications/i/item/9789241514385.
3. World Health Organization. Protocol for surgical site infection surveillance with a focus on settings with limited resources. World health organization; 2018.
4. Bonet M, Souza JP, Abalos E, et al. The global maternal sepsis study and awareness campaign (GLOSS): study protocol. Reprod Health. 2018;15(1):16. doi:10.1186/s12978-017-0437-8
5. Ayala D, Tolossa T, Markos J, Yilma MT. Magnitude and factors associated with surgical site infection among mothers underwent cesarean delivery in Nekemte town public hospitals, western Ethiopia. PLoS One. 2021;16(4):e0250736. doi:10.1371/journal.pone.0250736
6. Gomaa K, Abdelraheim AR, El Gelany S, Khalifa EM, Yousef AM, Hassan H. Incidence, risk factors and management of post cesarean section surgical site infection (SSI) in a tertiary hospital in Egypt: a five year retrospective study. BMC Pregnancy Childbirth. 2021;21:1–9. doi:10.1186/s12884-021-04054-3
7. He X, Li D, Sun T, et al. Risk factors for surgical site infection after cesarean delivery in a rural area in China: a case-controlled study. Ann Med Surg. 2021;72:103110. doi:10.1016/j.amsu.2021.103110
8. Saeed KB, Corcoran P, O’Riordan M, Greene RA. Risk factors for surgical site infection after cesarean delivery: a case-control study. Am J Infect Control. 2019;47(2):164–169. doi:10.1016/j.ajic.2018.07.023
9. Zejnullahu VA, Isjanovska R, Sejfija Z, Zejnullahu VA. Surgical site infections after cesarean sections at the University Clinical Center of Kosovo: rates, microbiological profile and risk factors. BMC Infect Dis. 2019;19(1):752. doi:10.1186/s12879-019-4383-7
10. Alfouzan W, Al Fadhli M, Abdo N, Alali W, Dhar R. Surgical site infection following cesarean section in a general hospital in Kuwait: trends and risk factors. Epidemiol Infect. 2019;147:1.
11. Getaneh T, Negesse A, Dessie G. Prevalence of surgical site infection and its associated factors after cesarean section in Ethiopia: systematic review and meta-analysis. BMC Pregnancy Childbirth. 2020;20(1):311. doi:10.1186/s12884-020-03005-8
12. Rasa K, Kilpatrick C. Implementation of World Health Organization guidelines in the prevention of surgical site infection in low-and middle-income countries: what we know and do not know. Surg Infect. 2020;21(7):592–598. doi:10.1089/sur.2020.163
13. Ketema DB, Wagnew F, Assemie MA, et al. Incidence and predictors of surgical site infection following cesarean section in North-west Ethiopia: a prospective cohort study. BMC Infect Dis. 2020;20(1):1–11. doi:10.1186/s12879-020-05640-0
14. Molla M, Temesgen K, Seyoum T, Melkamu M. Surgical site infection and associated factors among women underwent cesarean delivery in Debretabor General Hospital, Northwest Ethiopia: hospital based cross sectional study. BMC Pregnancy Childbirth. 2019;19(1):317. doi:10.1186/s12884-019-2442-0
15. Rickard J, Beilman G, Forrester J, et al. Surgical infections in low-and middle-income countries: a global assessment of the burden and management needs. Surg Infect. 2020;21(6):478–494. doi:10.1089/sur.2019.142
16. Alemye T, Oljira L, Fekadu G, Mengesha MM. Post cesarean section surgical site infection and associated factors among women who delivered in public hospitals in Harar city, Eastern Ethiopia: a hospital-based analytic cross-sectional study. PLoS One. 2021;16(6):e0253194. doi:10.1371/journal.pone.0253194
17. Zaineb S, Akbar A, Ikram M, Mahboob S, Mahmood A, Khan AW. Incidence and risk factors for maternal surgical site infection after cesarean section. Professional Med J. 2021;28(10):1495–1500. doi:10.29309/TPMJ/2021.28.10.6187
18. Gelaw MW, Abdela A. Prevalence of surgical site infection and associated factors among mothers after cesarean delivery in zewditu memorial hospital. Ethiop J Reprod Health. 2018;10(4):1.
19. Wendmagegn TA, Abera GB, Tsehaye WT, Gebresslasie KB, Tella BG. Magnitude and determinants of surgical site infecion among women underwent cesarean section in Ayder comprehensive specialized hospital Mekelle City, Tigray region, Northern Ethiopia, 2016. BMC Pregnancy Childbirth. 2018;18(1):489. doi:10.1186/s12884-018-2075-8
20. Wodajo S, Belayneh M, Gebremedhin S. Magnitude and factors associated with post-cesarean surgical site infection at Hawassa university teaching and referral hospital, southern Ethiopia: a cross-sectional study. Ethiop J Health Sci. 2017;27(3):283–290. doi:10.4314/ejhs.v27i3.10
21. Lijaemiro H, Berhe Lemlem S, Tesfaye Deressa J. Incidence of surgical site infection and factors associated among cesarean deliveries in selected government hospitals in Addis Ababa, Ethiopia, 2019. Obstet Gynecol Int. 2020;2020:9714640. doi:10.1155/2020/9714640
22. New World Encyclopedia. Addis Ababa; 2020. Available from: https://www.newworldencyclopedia.org/entry/Addis_Ababa.
23. Health-Ethiopia Mo. Ethiopia national health accounts report Addis Ababa; 2022.
24. Agboeze J, Onoh RC, Umeora OU, et al. Microbiological pattern of postcesarean wound infection at Federal Teaching Hospital, Abakaliki. Afr J Med Health Sci. 2013;12(2):99.
25. Njoku CO, Njoku AN. Microbiological pattern of surgical site infection following Caesarean section at the University of Calabar Teaching Hospital. Open Access Maced J Med Sci. 2019;7(9):1430. doi:10.3889/oamjms.2019.286
26. World Health Organization. WHO recommendations on antenatal care for a positive pregnancy experience. World Health Organization; 2016. Available from: https://www.who.int/publications/i/item/9789241549912.
27. Center of disease control. Guideline for prevention of surgical site infection. Infection Control; 2015. Available from: https://www.cdc.gov/infectioncontrol/guidelines/ssi/index.html.
28. Pervention CODCA. Healthy weight, nutrition, and physical activity USA; 2022. Available from: https://www.cdc.gov/other/accessibility.html.
29. Association WM. Declaration of Helsinki. Ethical principles for medical research involving human subjects. Jahrbuch für Wissenschaft und Ethik. 2009;14(1):233–238. doi:10.1515/9783110208856.233
30. Azeze GG, Bizuneh AD. Surgical site infection and its associated factors following cesarean section in Ethiopia: a cross-sectional study. BMC Res Notes. 2019;12(1):288. doi:10.1186/s13104-019-4325-x
31. Chu K, Maine R, Trelles M. Cesarean section surgical site infections in sub-Saharan Africa: a multi-country study from medecins sans frontieres. World J Surg. 2015;39:350–355. doi:10.1007/s00268-014-2840-4
32. Di Gennaro F, Marotta C, Pisani L, et al. Maternal caesarean section infection (MACSI) in Sierra Leone: a case-control study. Epidemiol Infect. 2020;148:e40. doi:10.1017/S0950268820000370
33. Gidey G, Hailu B, Nigus K, Hailu T, Gher W, Gerensea H. Timing of first focused antenatal care booking and associated factors among pregnant mothers who attend antenatal care in Central Zone, Tigray, Ethiopia. BMC Res Notes. 2017;10(1):608. doi:10.1186/s13104-017-2938-5
34. Shrestha S, Shrestha R, Shrestha B, Dongol A. Incidence and risk factors of surgical site infection following cesarean section at Dhulikhel Hospital. Kathmandu Univ Med J. 2014;12(2):113–116. doi:10.3126/kumj.v12i2.13656
35. Onyegbule OA, Akujobi CN, Ezebialu IU, et al. Determinants of post-caesarean wound infection in Nnewi, Nigeria. Br J Med Med Res. 2015;5(6):767. doi:10.9734/BJMMR/2015/10297
36. Mamo T, Abebe TW, Chichiabellu TY, Anjulo AA. Risk factors for surgical site infections in obstetrics: a retrospective study in an Ethiopian referral hospital. Patient Saf Surg. 2017;11:24. doi:10.1186/s13037-017-0138-9
37. Mujagic E, Marti WR, Coslovsky M, et al. Associations of hospital length of stay with surgical site infections. World J Surg. 2018;42(12):3888–3896. doi:10.1007/s00268-018-4733-4
38. Jasim HH, Sulaiman SAS, Khan AH, Dawood OT, Abdulameer AH, Usha R. Incidence and risk factors of surgical site infection among patients undergoing cesarean section. Therapeutics. 2017;9:1179559X17725273.
39. Fisha K, Azage M, Mulat G, Tamirat KS. The prevalence and root causes of surgical site infections in public versus private hospitals in Ethiopia: a retrospective observational cohort study. Patient Saf Surg. 2019;13:26. doi:10.1186/s13037-019-0206-4
40. Diejomaoh MFE, Al-Jassar W, Bello Z, Karunakaran K, Mohammed A. The relevance of the second cesarean delivery in the reduction of institutional cesarean delivery rates. medical principles and practice. Int J Kuwait Univ. 2018;27(6):555–561.
41. Harzif AK, Wicaksono MD, Kallista A, Emeraldi M, Pratama G. Overview of risk factor and bacterial pattern in patient with surgical site infection after caesarean section in Ciptomangunkusumo Hospital from 2016 to 2018. Infect Prev Pract. 2020;2(4):100090. doi:10.1016/j.infpip.2020.100090
42. Mutahi JW. Post Caesarean Section Surgical Site Infection, Microbial Patterns and Sensitivity to Antibiotics Among Women Who Delivered at Kenyatta National Hospital Between 2014 and 2020. University of Nairobi; 2022.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.