Back to Journals » Infection and Drug Resistance » Volume 16
Incidence and Risk Factors of Cranial Nerve Palsy in Patients with Tuberculous Meningitis: A Retrospective Evaluation
Authors Wen A , Cao WF, Liu SM, Zhou YL, Xiang ZB, Hu F, Wu LF, Cai W, Leng EL
Received 3 November 2022
Accepted for publication 3 January 2023
Published 14 February 2023 Volume 2023:16 Pages 829—841
DOI https://doi.org/10.2147/IDR.S396022
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
An Wen,1,2 Wen-Feng Cao,1,2 Shi-Min Liu,1,2 Yong-Liang Zhou,1,2 Zheng-Bing Xiang,1,2 Fan Hu,1,2 Ling-Feng Wu,1,2 Wen Cai,1,2 Er-Ling Leng3
1Department of Neurology, Jiangxi Provincial People’s Hospital (The First Affiliated Hospital of Nanchang Medical College), Nanchang, People’s Republic of China; 2Institution of Neurology, Jiangxi Provincial People’s Hospital (The First Affiliated Hospital of Nanchang Medical College), Nanchang, People’s Republic of China; 3Department of Pediatrics, Jiangxi Provincial People’s Hospital (The First Affiliated Hospital of Nanchang Medical College), Nanchang, People’s Republic of China
Correspondence: Er-Ling Leng, Department of Pediatrics, Jiangxi Provincial People’s Hospital (The First Affiliated Hospital of Nanchang Medical College), No.92 Aiguo Road, Nanchang, 330006, People’s Republic of China, Tel +86791-8772-1401, Email [email protected]
Objective: Tuberculous meningitis (TBM) is a common form of central nervous system (CNS) tuberculosis (TB). Cranial nerve palsy is a serious complication of TBM. Literature regarding this subject is still limited in China. This study evaluated the incidence of cranial nerve palsy in patients with TBM in South China, its association with the clinical forms of TB, and other patient characteristics.
Methods: A retrospective chart review of patients with a diagnosis of TBM between January 2004 and December 2019 was conducted, and the demographic characteristics, clinical characteristics, and laboratory results of 114 patients were collected and followed up for 3 months. A multivariate logistic regression analysis model was used to explore the risk factors of cranial nerve palsy in patients with TBM.
Results: A total of 114 patients were enrolled in this study. Cranial nerve palsy was observed in approximately 38 (33.3%) of TBM patients. Among them, 13 (28.3%) had optic nerve palsy, 24 (52.2%) had oculomotor nerve palsy, 5 (10.9%) had abducens nerve palsy, 2 (4.3%) had auditory nerve palsy, 1 (2.2%) had glossopharyngeal nerve palsy, and 1 (2.2%) had vagus nerve palsy. Using logistic regression analysis, focal neurological deficit, extracranial TB and cerebrospinal fluid (CSF) total white cell count (WCC) were shown to be risk factors for cranial nerve palsy.
Conclusion: The prevalence rate of cranial nerve palsy was 33.3% in patients with TBM. Focal neurological deficits, extracranial TB and CSF total WCC are important predictors of cranial nerve palsy in patients with TBM.
Keywords: tuberculous meningitis, tuberculosis, cranial nerve palsy, risk factors, prediction
Introduction
In 2019, an estimated 10 million people developed tuberculosis (TB) and 1.4 million died, according to the World Health Organization (WHO) Global Tuberculosis Report 2020.1 Today, TB remains the most common cause of death from a single source of infection worldwide. China is a country with a high TB burden, with approximately 833,000 new cases in 2019, accounting for 8.4% of new TB cases.1,2 Tuberculous meningitis (TBM) is a serious and critical presentation of extrapulmonary TB caused by Mycobacterium tuberculosis (MTB) infection, with high rates of mortality and disability,3,4 especially among children and people living with HIV in developing countries.5,6
Cranial nerve palsy is a common complication of TBM. Recent studies have found that people with TBM are more likely to develop cranial nerve palsy than those with other central nervous system (CNS) infections and healthy controls.7–9 Unfortunately, a study has shown that cranial nerve palsy is associated with high mortality and disability in TBM.10 However, no study has assessed the incidence of cranial nerve palsy in patients with TBM and its associated factors in China. This study described the prevalence of cranial nerve palsy and its associated risk factors in patients with TBM in southern China.
Methods and Materials
Patient Recruitment
A retrospective study was conducted from January 2004 to December 2019 at the Neurology ward of Jiangxi Provincial People’s Hospital and Infectious Diseases Hospital of Jiangxi Province, serving approximately 45.2 million people in South China. Consecutive patients (aged ≥ 16 years) with a suspected diagnosis of TBM were enrolled. Patients with incomplete case data, unclear diagnoses, other intracranial infections, and HIV seropositivity were excluded. All patients received four standard anti-tuberculosis treatment regimen for the first 2 ~ 3 months, including isoniazid (300 ~ 600 mg per day), rifampicin {450 mg per day (weight < 50kg) or 600 mg per day (weight ≥ 50kg)}, pyrazinamide (25 mg/kg per day), and ethambutol (15 mg/kg per day), and were followed up for at least 3 months.
Definitions
Diagnostic Criteria for TBM
TBM was divided into “definite”, “probable”, and “possible”, based on the case definitions proposed by Marais et al who described a diagnostic scoring system including the clinical criteria, cerebrospinal fluid (CSF) standards, radiologic criteria, and other evidence of TB outside the CNS (see Table 1).11 Definite TBM was diagnosed by smear microscopy for acid-fast bacteria (AFB) in CSF or culture or positive polymerase chain reaction for the MTB test. Probable TBM was diagnosed if a total score of 12 or more points (when cerebral imaging was not available, the total score decreased to 10 or more points), while it was compatible between patients who received a score of 6–11, and who were possible TBM (when cerebral imaging was unavailable, the score of 6–9 points).
 |
Table 1 The Marais Criteria for the Diagnosis of TBM on Admission |
TBM Grading Standards
The severity of TBM at admission was assessed according to British Medical Research Council (BMRC) criteria based on the patients’ Glasgow Coma Score (GCS) and focal neurological deficits.12 Stage I was defined as a GCS of 15 points without focal neurological deficits; stage II was defined as a GCS of 15 points with neurological deficits or a GCS of 11–14 points; stage III was defined as a GCS of ≤10 points. The diagnostic criteria for cranial nerve palsy refered to the eighth edition of Neurology published by the Chinese People’s Medical Publishing House.9,13 Patients with abnormal fundus exam without obvious visual impairment were excluded.
Procedures
All patients enrolled were subjected to a detailed history-taking and physcial examination at baseline. Clinical case data of all patients were collected. The following information (clinical signs and symptoms) was recorded: body temperature, night sweats, headache, nausea and vomiting, seizures, weight loss history, altered consciousness, duration of illness, psychiatric symptoms, focal neurological deficits (combination of slurred speech, crooked corners of the mouth, numbness, and weakness), extracranial TB, GCS score, TBM score, anorexia, and TBM severity grades. Routine laboratory tests, including blood white cell count (WCC), blood neutrophils percentage, blood lymphocytes percentage, C-reaction protein (CRP), erythrocyte sedimentation rate (ESR), serum sodium, serum chloride, anti-TB antibody (TB-Ab), hemoglobin (HB), and T-cell enzyme-linked immuno-spot assay (T-spot) were performed. All patients underwent lumbar puncture to obtain CSF samples, and the following tests were performed: total cell count and chloride, glucose, and protein levels. CSF samples were centrifuged, and the sediments were stained (mycobacterial smears) and cultured (common bacterial and fungal culture for all patients, Lowenstein–Jensen media for 6). To be enrolled, chest X-rays and cranial CT/MRI scans were obtained in all patients at admission.
Statistical Analysis
Statistical calculations were performed using SPSS version 17.0. The 40 clinical and laboratory parameters of patients with and without cranial nerve palsy are compared in Table 2. Data are expressed as mean ± standard deviation (SD) in cases of normal distribution, and median (interquartile range) in cases of non-normal distribution. The t-test or Mann–Whitney U-test was used to compare the two groups of continuous variables and categorical variables were expressed as numbers and percentages, and analyzed using the χ2-test or Fisher's exact test. A p-value of < 0.05 was considered statistically significant. The odds ratios and 95% confidence intervals were calculated. Logistic regression analysis with a stepwise forward variable selection procedure was performed to investigate the independent effects of the determined significant variables between the groups.
 |
Table 2 Univariate Analysis, Comparison of the Clinical and Laboratory Characteristics in Tuberculous Meningitis with and without Cranial Nerve Palsy |
Results
Demographic Data and Clinical Features of the Enrolled Participants
During the study period, 134 patients with TBM were entered into the study; however, 20 (15%) patients were excluded as their diagnoses uncertain or their data were incomplete. Among the 114 patients we studied, 69 (60.5%) were men and 45 (39.5%) were women. Only 12 (10.5%) patients had a definitive diagnosis (all were based on CSF smear, no patient was MTB culture-positive), 79 (69.3%) had a probable diagnosis, and 23 (20.2%) had a possible diagnosis based on the Marais score criteria.11 All patients received anti-TB treatment after admission, and none had taken anti-TB drugs before admission. Fifty-nine (74.6%) patients had extracranial TB in hospitalization (see Table 3, Figure 1).
 |
Table 3 Sociodemographic and Clinical Characteristics in Patients with TBM from 2004 to 2019 (N=114) |
 |
Figure 1 Flow chart of the study. |
Among TBM patients, blood tests revealed anemia (Hb: men < 120 g/L, female < 110 g/L) in 33 (28.9%) patients. The WCC ranged from 10–500 mm3/mL in 27 (23.6%) patients, and neutrophilic granulocyte percentage was ≥ 70% in 82 (71.9%). No patients had an HIV infection. CSF analysis was performed in all patients: the CSF smear for AFB was positive in 12 (10.5%) patients, but none of the CSF culture tests were positive. On CSF examination, the median opening pressure of CSF was 17 cmH2O (9–40), and the intracranial pressure increased (≥ 25 cmH2O) in 71 (62.3%) patients (see Table 4).
 |
Table 4 Laboratory and Imaging Features in Patients of Tuberculous Meningitis with and without Cranial Nerve Palsy |
Electroencephalogram testing was performed in all adults and was abnormal in 53 (75.5%) patients. All patients also underwent chest radiographs and cranial CT or MRI scans after admission, wherein signs suggestive of active pulmonary TB were observed in 52 (46%) cases, tuberculoma in 12 (10.6%), hydrocephalus in 36 (31.9%), cerebral infarction in 57 (50.4%), and meningeal reinforcement in 74 (66.1%) (see Figure 2).
Prevalence and Features of Cranial Nerve Palsy
Cranial nerve palsy occurred in 33.3% of the 114 patients with TBM in the present study. Among them, there were 13 (28.3%) cases of optic nerve palsy (the 2nd nerve), 24 (52.2%) cases of oculomotor nerve palsy (the 3rd nerve), 5 (10.9%) cases of abducens nerve palsy (the 6th nerve), 2 (4.3%) cases of auditory nerve palsy (the 8th nerve), 1 (2.2%) case of glossopharyngeal nerve palsy (the 9th nerve), and 1 (2.2%) case of vagus nerve palsy (the 10th nerve). In the cranial nerve palsy group, six (13.0%) patients had two groups of cranial nerve palsy (optic nerve palsy and oculomotor nerve palsy in two cases, optic nerve palsy and abducens nerve palsy in one case, oculomotor nerve palsy and abducens nerve palsy in two cases, and glossopharyngeal nerve palsy and vagus nerve palsy in one case). One (2.2%) patient had three groups of cranial nerve palsy (optic nerve palsy, oculomotor nerve palsy, and auditory nerve palsy). There were no significant differences in sex or age between the cranial nerve palsy and non-cranial nerve palsy groups (P > 0.05) (see Table 5).
 |
Table 5 Various Patterns of Cranial Nerve Palsy in Patients with Tuberculous Meningitis |
At enrollment, among the 38 patients with cranial nerve palsy, the main symptoms were headache in 30 (78.9%) patients, nausea and vomiting in 15 (39.5%), and weight loss in 14 (36.8%). The most common clinical signs were: stiff-neck in 28 (73.7%) patients, focal neurological deficit in 25 (65.8%), and altered consciousness in 21 (55.3%). During hospital admission, according to the modified BMRC criteria for TBM severity, 8 (21.1%) cases were TBM grade I, 12 (31.5%) were TBM grade II, and 18 (47.4%) were TBM grade III.
Evidence of Extracranial TB Infection
Twenty-three (60.5%) patients had extracranial TB in the cranial nerve palsy group, while in the non-cranial nerve palsy group, 36 (47.4%) patients had extracranial TB. In the cranial nerve palsy group, 19 (82.6%) cases had pulmonary tuberculosis. There were 14 (60.9%) cases of extrapulmonary tuberculosis: 5 (35.7%) cases had tuberculous pleurisy, 1 (7.1%) had tuberculous peritonitis, 1 (7.1%) had tuberculous pericarditis, 2 (14.3%) had bone tuberculosis, 1 (7.1%) had lymphatic tuberculosis, 3 (21.4%) had renal tuberculosis, and 1 (7.1%) had liver tuberculosis. Additionally, two (14.3%) patients had more than two sites of extrapulmonary TB (tuberculous pleurisy, tuberculous peritonitis, and liver tuberculosis in one case; tuberculous peritonitis, tuberculous pericarditis, and renal tuberculosis in another case) (see Figures 3–5).
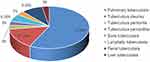 |
Figure 3 Evidence of TB elsewhere. |
 |
Figure 4 Type of TB. (A) CT showed pia miliary tuberculosis in both lungs. (B) CT showed marked pleural thickening at the right side (arrows). (C) CT showed bilateral pleural thickening and adhesion. |
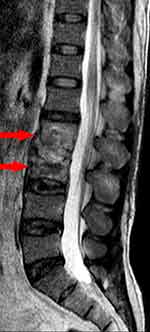 |
Figure 5 MRI showed tuberculosis of L2 and L3 vertebrae (arrows). |
Assessment of Factors Associated Cranial Nerve Palsy
Per univariate analysis, night sweats, headache, stiff-neck, altered consciousness, duration of illness, definite/probable TBM, focal neurological deficits, extracranial TB, anorexia, grade I/III, serum sodium, erythrocyte sedimentation rate, CRP, and CSF total WCC might be associated with cranial nerve palsy (P < 0.2) (see Table 4). A multivariate analysis model using binary logistic regression was then constructed and revealed that three factors (focal neurological deficits, extracranial TB, and CSF total WCC) were found to be associated with cranial nerve palsy (see Table 6).
 |
Table 6 Multivariate Logistic Regression Analysis |
Evolution of Cranial Nerve Palsy with Anti-TB Treatment
All study patients were treated with a standard four-drug anti-TB therapy (ATT), including isoniazid, rifampicin, pyrazinamide and ethambutol. Twenty-five (21.9%) patients received adjunctive corticosteroids (0.4 mg/kg/day of dexamethasone), and fluoroquinolones were used in combination in 45 patients (levofloxacin in 30 patients and moxifloxacin in 15). Moreover, patients were routinely treated with a combination of dehydration to reduce cranial pressure, and B vitamins to nourish the nerves whenever required.
All patients were followed up for 3 months after receiving anti-TB treatment to analyze the prognosis of cranial nerve palsy. If cranial nerve palsy persists or even worsens, or if death occurs, the patient is generally considered to have a poor prognosis in this regard. Both groups have paradoxical reactions during treatment. For non-cranial nerve palsy group: there were no deaths during follow up, 3 (3.9%) patients (1 patient with 2nd nerve, 1 patient with 3th nerve, and 1 patient with 6th nerve) had poor outcome. For cranial nerve palsy group: three patients died during the study period: one patient died due to a complication of foramen magnum hernia, one of infectious shock, and one of multiple organ failure; the final mortality rate was 7.9%. During the follow up of 35 patients who survived in the cranial nerve palsy group, 30 (85.7%) patients with cranial nerve palsy recovered completely, 3 (8.6%) experienced no change in vision, and 2 (5.7%) had deterioration in their hearing and swallowing status. For this group, 8 (21%; including 3 deaths) had poor outcome. In conclusion, the prognosis of non-cranial nerve palsy group was better than that of cranial nerve palsy group (χ2= 6.65, P= 0.01) (see Figures 1 and 6).
 |
Figure 6 Effect of cranial nerve palsy of functional outcome after 3 months of follow up. |
Discussion
The main findings of this study were as follows: 1) cranial nerve palsy was a common complication, with an incidence of 33.3% in TBM patients; 2) auditory nerve, glossopharyngeal nerve, and vagus nerve palsies were rare, and most cases affected the oculomotor nerve, optic nerve, and abducens nerve; 3) after 3 months of follow-up, the majority of patients with cranial nerve palsy recovered completely with no obvious sequelae; and 4) cranial nerve palsy was associated with focal neurological deficits, extracranial TB, and CSF total WCC.
TBM is a devastating infection that occurs secondary to TB in areas other than the brain, is associated with the highest rate of mortality and disability,14–16 and is often complicated by cranial nerve palsy; however, its incidence has been reported inconsistently.9,10,17 In a study from India, Sharma et al enrolled 158 patients with TBM and found that cranial nerve palsy was present in 38% of patients with TBM. The abducens nerve was found to be the most common cranial neuropathy (32.3%), with two or more cranial nerve palsies in 10.12% of patients.10 A study by Li et al reported that the involvement of TBM with cranial nerve palsy was observed in only 14.8% of patients during follow-up. The oculomotor nerve palsy (56.9%) was the most common among these.9 In this study, cranial nerve palsy was detected in 33.3% of enrolled patients: the oculomotor nerve (the 3rd nerve) was the most frequently affected cranial nerve (52.2%), followed by optic nerve (the 2nd nerve) (28.3%), the abducens nerve (the 6th nerve) (10.9%), the auditory nerve (the 8th nerve) (4.3%), glossopharyngeal nerve (the 9th nerve) (2.2%), and the vagus nerve (the 10th nerve) (2.2%). 18.4% of cases had two or more groups of cranial nerve palsies. Finally, cranial nerve palsy was recovered at discharge in 30 (85.7%) patients.
The mechanisms attributed to the processes of cranial nerve palsy in TBM include the following. Firstly, MTB causes exudative and proliferative lesions in the CNS. The exudate deposits in the subarachnoid space at the bottom of the brain and the lateral cistern, which spreads around the brain stem and compresses the cranial nerve palsy. Second, CSF circulation suffers interference, resulting in ventricular orifices and aqueduct stenosis, and leading to obstructive hydrocephalus. Conversely, the excessive secretion of CSF brings about communicating hydrocephalus, causing the cranial nerve to be pulled and producing paralytic. Third, sometimes occlusive vasculitis leads to cerebral stem infarction and stroke, causing cranial nerve damage. Fourth, direct placeholder effect of cranial nerve in subarachnoid space by tuberculoma or abscess could be a mechanism. Fifth, intracranial hypertension and cerebral hernia can also damage the cranial nerves in advanced patients.
Extracranial TB was found to be an independent predictor of cranial nerve palsy. In the course of the initial pulmonary infection, MTB may enter the circulatory system and then reach the oxygen-rich CNS, where it seeds and forms a localized lesion, known as a “Rich foci”.18
It may be located in the meninges, spinal cord, and brain parenchyma below the pia meninges or ependymal. Later, the lesion may rupture into the subarachnoid space or ventricle system leading to TBM.19,20 The probability of MTB reaching the brain depends on the degree of bacteremia and the host’s immune response to it.21,22 Cell-mediated immune response in the brain produces a thick, gelatinous, inflammatory exudate, often involving the basal cistern and lateral fissure pools,23 located in the basal cistern exudation can cause obstruction of CSF circulation leading to hydrocephalus and oppressive nerve,24 and located at the base of the brain blood vessels serious involvement can lead to obstructive vasculitis and cerebral infarction.17,25–27 In addition, if the patient with TBM lacks adequate immunity or the lesion is large enough, foci of tuberculosis located in the brain parenchyma may develop into tuberculoma or brain abscesses.27–29
CSF total WCC was significantly elevated in patients with cranial nerve palsy, suggesting an associated between the two. The high cell count of CSF total WCC could have caused the accumulation of inflammatory exudate and filling of the cistern with granulation tissue, which gradually developed into meningeal thickening and cerebral cistern casting enhancement, eventually leading to cranial nerve palsy.23,29
The results of this study highlight the importance of focal neurological deficits as predictors of cranial nerve palsy. Focal neurological deficits were found in 50% of our enrolled patients. A previous study has observed that the enhancement of exudative meningitis of the circle of Willis in TBM can cause vascular strangulation, cramping, narrowing, periarteritis, and even necrotizing arteritis, leading to thrombosis.30 Misra et al also found that focal weakness and cranial nerve palsy are predictors of persistent neurological sequelae.31 Another study reported that focal motor deficit at admission is also an important predictor of cranial nerve palsy.8
A previous study showed that cranial nerve palsy has a positive predictive role in the diagnosis of TBM and is the most important factor to differentiate TBM from acute bacterial meningitis.8 In terms of distribution, cranial nerve palsy was mainly in the anterior cranial nerve group, of which the oculomotor nerve was the most frequently involved cranial nerve, followed by the optic nerve and abducens nerve. Cranial nerve palsy in the posterior cranial nerve group was rare, which was consistent with several publications.8,9
In our study, the incidence of cranial nerve palsy in patients with grade III (47.4%) was higher than that in patients with grade II (31.5%) or grade I (21.1%). This is consistent with the conclusions of Sher et al.32 It has been speculated that patients with severe TBM may be more prone to cranial nerve palsy. After admission, all patients underwent anti-TB treatment. Finally, cranial nerve palsy was almost resolved (85.7%) at the 3 months follow-up in the group of 38 patients. This result is similar to that reported by Li et al.9 Surprisingly, in a study from India,7 in a cohort with a high incidence of cranial nerve palsy, 10.2% of 88 TBM patients were blind and 12.5% experienced visual loss, which may be related to the longer average duration of illness (51±52 days vs 20±23.5 days) compared to this study. This further suggests that early diagnosis and intervention of TBM are of greatly important in alleviating or even reducing the sequelae of cranial nerve palsy.
Limitations
There were still some limitations in the present study. Firstly, our study was a retrospective two-center design. Information about enrolled patients was limited to medical records, which inevitably led to selective bias. Secondly, we were unable to assess the long-term prognosis of patients. Another limitation of this study is that PCR testing was not performed, which is considered important for accurate diagnosis of TBM. Fourthly, no patients with HIV were included. With these limitations considered, the subsequent step was to conduct a multicenter prospective cohort study in the region.
Conclusion
In summary, cranial nerve palsy is a common clinical complications of TBM, and focal neurological deficits, extracranial TB, and CSF total WCC are common predictors of cranial nerve palsy. In most patients with TBM, TBM-combined cranial nerve palsy is reversible and can be completely treated with effective anti-TB treatment. Clinicians should look for the clues of TBM and cranial nerve palsy carefully, as rapid and accurate diagnosis and effective treatment are the important means to reduce the occurrence of palsy and the resulting damage.
Data Sharing Statement
The original data supporting the conclusions of this paper will be provided by the authors, with no unnecessary reservations for any qualified researcher.
Ethical Statement
This study was approved by the Ethics Committee of Jiangxi Provincial People’s Hospital (NO.2022038). The study complied with the Declaration of Helsinki. Owing to the retrospective nature of the study, we only reviewed the medical records and all identifiable personal information was removed for privacy protection. So, patient consent for inclusion was waived.
Acknowledgments
The authors thank the staffs from the Neurology ward of Jiangxi Provincial People’s Hospital for technical assistance as well as Editage (www.editage.cn) for English language editing.
Funding
This work was supported by grant from Jiangxi Provincial Health Commission Foundation (NO. 202310122).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chakaya J, Khan M, Ntoumi F, et al. Global tuberculosis report 2020 - reflections on the global TB burden, treatment and prevention efforts. Int J Infect Dis. 2021;113(Suppl 1):S7–S12. doi:10.1016/j.ijid.2021.02.107
2. Pang Y, An J, Shu W, et al. Epidemiology of extrapulmonary tuberculosis among inpatients, China, 2008–2017. Emerg Infect Dis. 2019;25(3):457–464. doi:10.3201/eid2503.180572
3. Brancusi F, Farrar J, Heemskerk D. Tuberculous meningitis in adults: a review of a decade of developments focusing on prognostic factors for outcome. Future Microbiol. 2012;7(9):1101–1116. doi:10.2217/fmb.12.86
4. Seddon JA, Tugume L, Solomons R, Prasad K, Bahr NC. The current global situation for tuberculous meningitis: epidemiology, diagnostics, treatment and outcomes. Wellcome Open Res. 2019;4:167. doi:10.12688/wellcomeopenres.15535.1
5. Ho J, Marais BJ, Gilbert GL, Ralph AP Diagnosing tuberculous meningitis - have we made any progress? Trop Med Int Health. 2013;18(6):783–793. doi:10.1111/tmi.12099.
6. Robertson FC, Lepard JR, Mekary RA, et al. Epidemiology of central nervous system infectious diseases: a meta-analysis and systematic review with implications for neurosurgeons worldwide. J Neurosurg. 2018;1:1–20. doi:10.3171/2017.10.jns17359
7. Sinha MK, Garg RK, Anuradha H, et al. Vision impairment in tuberculous meningitis: predictors and prognosis. J Neurol Sci. 2010;15(1–2):27–32. doi:10.1016/j.jns.2009.12.012
8. Moghtaderi A, Alavi-Naini R, Rashki S. Cranial nerve palsy as a factor to differentiate tuberculous meningitis from acute bacterial meningitis. Acta Med Iran. 2013;51(2):113–118. PMID 23585318.
9. Li X, Ma L, Zhang L, Wu X, Chen H, Gao M. Clinical characteristics of tuberculous meningitis combined with cranial nerve palsy. Clin Neurol Neurosurg. 2019;9(184):105443. doi:10.1016/j.clineuro.2019.105443
10. Sharma P, Garg RK, Verma R, Singh MK, Shukla R. Incidence, predictors and prognostic value of cranial nerve involvement in patients with tuberculous meningitis: a retrospective evaluation. Eur J Intern Med. 2011;22(3):289–295. doi:10.1016/j.ejim.2011.01.007
11. Marais S, Thwaites G, Schoeman JF, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis. 2010;10(11):803–812. doi:10.1016/S1473-3099(10)70138-9
12. British Medical Research Council. Streptomycin treatment of tuberculous meningitis. BMJ. 1948;1:582–597.
13. Jia J. Neurology(8th Edition)[M]. People’s medical publishing house; 2013.
14. Tucker EW, Marais S, Seddon JA, et al. International survey reveals opportunities to improve tuberculous meningitis management and the need for standardized guidelines. Open Forum Infect Dis. 2020;7(11):ofaa445. doi:10.1093/ofid/ofaa445
15. Thwaites GE, van Toorn R, Schoeman J. Tuberculous meningitis: more questions, still too few answers. Lancet Neurol. 2013;12(10):999–1010. doi:10.1016/s1474-4422(13)70168-6
16. Abo YN, Curtis N, Butters C, Rozen TH, Marais BJ, Gwee A. Successful treatment of a severe vision-threatening paradoxical tuberculous reaction with infliximab: first pediatric use. Pediatr Infect Dis J. 2020;39(4):e42–e45. doi:10.1097/inf.0000000000002578
17. Selvaraj JU, Sujalini BB, Rohitson MS, George AA, Arvind VH, Mishra AK. Identification of predictors of cerebrovascular infarcts in patients with tuberculous meningitis. Int J Mycobacteriol. 2020;9(3):303–308. doi:10.4103/ijmy.ijmy_107_20
18. Róna G, Lörinc J. The pathogenesis of tuberculous meningitis. Ada Morphol. 1933;52:5–37. doi:10.1016/S0140-6736(48)92003-0
19. Donald PR, Schaaf HS, Schoeman JF. Tuberculous meningitis and miliary tuberculosis: the rich focus revisited. J Infect. 2005;50(3):193–195. doi:10.1016/j.jinf.2004.02.010
20. Thwaites G, Chau TT, Mai NT, Drobniewski F, McAdam K, Farrar J. Tuberculous meningitis. J Neurol Neurosurg Psychiatry. 2000;68(3):289–299. doi:10.1136/jnnp.68.3.289
21. Donovan J, Thwaites GE, Huynh J. Tuberculous meningitis: where to from here? Curr Opin Infect Dis. 2020;33(3):259–266. doi:10.1097/qco.0000000000000648
22. Donovan J, Figaji A, Imran D, Phu NH, Rohlwink U, Thwaites GE. The neurocritical care of tuberculous meningitis. Lancet Neurol. 2019;18(8):771–783. doi:10.1016/S1474-4422(19)30154-1
23. Zaharie SD, Franken DJ, van der Kuip M, et al. The immunological architecture of granulomatous inflammation in central nervous system tuberculosis. Tuberculosis. 2020;125:102016. doi:10.1016/j.tube.2020.102016
24. Rajshekhar V. Management of hydrocephalus in patients with tuberculous meningitis. Neurol India. 2009;57(4):368–374. doi:10.4103/0028-3886.55572
25. Mishra AK, Lal A, George AA. Letter by Mishra et al regarding article, “infection as a stroke trigger: associations between different organ system infection admissions and stroke subtypes”. Stroke. 2019;50(11):e328. doi:10.1161/strokeaha.119.026990
26. Wasay M, Khan M, Farooq S, et al. Frequency and impact of cerebral infarctions in patients with tuberculous meningitis. Stroke. 2018;49(10):2288–2293. doi:10.1161/strokeaha.118.021301
27. Wasay M, Farooq S, Khowaja ZA, et al. Cerebral infarction and tuberculoma in central nervous system tuberculosis: frequency and prognostic implications. J Neurol Neurosurg Psychiatry. 2014;85(11):1260–1264. doi:10.1136/jnnp-2013-307178
28. Be NA, Kim KS, Bishai WR, Jain SK. Pathogenesis of central nervous system tuberculosis. Curr Mol Med. 2009;9(2):94–99. doi:10.2174/156652409787581655
29. DeLance AR, Safaee M, Oh MC, et al. Tuberculoma of the central nervous system. J Clin Neurosci. 2013;20(10):1333–1341. doi:10.1016/j.jocn.2013.01.008
30. Chan KH, Cheung RT, Lee R, Mak W, Ho SL. Cerebral infarcts complicating tuberculous meningitis. Cerebrovasc Dis. 2005;19(6):391–395. doi:10.1159/000085568
31. Misra UK, Kalita J, Srivastava M, Mandal SK. Prognosis of tuberculous meningitis: a multivariate analysis. J Neurol Sci. 1996;137(1):57–61. doi:10.1016/0022-510X(95)00334-X
32. Sher K, Firdaus AA, Bullo N, Kumar S. Stages of tuberculous meningitis: a clinicoradiologic analysis. J Coll Physicians Surg Pak. 2013;23(6):405–408. PMID 23763800.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

