Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 12
Incidence and Predictors of Anemia Among Children on Antiretroviral Therapy at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia, 2007–2017: A Retrospective Follow-Up Study
Authors Techane MA , Anlay DZ, Tesfaye E , Agegnehu CD
Received 18 September 2020
Accepted for publication 10 November 2020
Published 15 December 2020 Volume 2020:12 Pages 951—962
DOI https://doi.org/10.2147/HIV.S282675
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Masresha Asmare Techane,1 Degefaye Zelalem Anlay,2 Eleni Tesfaye,2 Chilot Desta Agegnehu3
1Department of Pediatrics and Child Health Nursing, School of Nursing, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 2Community Health Nursing Unit, School of Nursing, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 3School of Nursing, College of Medicine and Health Sciences and Comprehensive Specialized Hospital, University of Gondar, Gondar, Ethiopia
Correspondence: Chilot Desta Agegnehu
School of Nursing, College of Medicine and Health Sciences and Comprehensive Specialized Hospital, University of Gondar, Gondar, Ethiopia
Tel +251 918627403
Email [email protected]
Background: Anemia is the most common hematological abnormality among children on antiretroviral therapy. In Ethiopia, as far as our search, there are no studies done on the incidence and predictors of anemia among children on antiretroviral therapy. This study aimed to assess the incidence and predictors of anemia among children on antiretroviral therapy, attending antiretroviral therapy care at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia, from 2007 to 2017.
Methods: A retrospective follow-up study was conducted among 391 children on antiretroviral therapy. Mean survival time for children to be anemia free was estimated. A Log rank test was used to compare survival curves among different independent variables. The Cox regression model was used. The adjusted hazard ratio (AHR) with a 95% confidence interval (CI) was calculated. P-value ≤ 0.05 was considered as statically significant.
Results: The overall incidence rate of anemia was 10.5 (95% confidence interval (8.6, 12.8)) per 100 child-year. Being severe-immunosuppressed (AHR=2.9, 95% CI: 1.23– 6.77), undernutrition (AHR =2.7, 95% CI: 1.5– 5), taking zidovudine-based ART regimen (AHR =4, 95% CI: 1.23– 12.9), and tuberculosis (AHR =2.1, 95% CI: 1.4– 3.3) were independent predictors of anemia among children.
Conclusion: In this study, the incidence rate of anemia among children on antiretroviral therapy was found to be high. Tuberculosis, zidovudine-based drugs, severe immunosuppression, and undernutrition have remained statically significant predictors of anemia among children on antiretroviral therapy. Children with HIV were the most vulnerable group for anemia, especially in developing countries. Therefore, improving their nutritional status and considering other predictors of anemia were very important for children to reduce the incidence of anemia among children with HIV.
Keywords: anemia, anti-retroviral therapy, children, Ethiopia, incidence
Background
Human immunodeficiency virus (HIV) infection and Acquired Immune Deficiency Syndrome (AIDS) have a serious hematologic disorder in both patients with primary infection and the severe stage of the disease.1 Anemia is one of the most common hematological abnormalities of HIV infection which has a substantial long-term negative outcome on the patient’s quality of life, functional status, and clinical consequence.2 As to World Health Organization (WHO) definitions, anemia can be defined as a hemoglobin concentration less than 11 g/dl for children <5 years old, <11.5 g/dl for children 5–11.9 years old, and <12 g/dl for children 12–14.9 years old with altitude adjustment.3
According to the WHO Global database report on anemia, in 2008 anemia is estimated to affect around two billion population; 47.4% of these were children.4 Anemia is one of the common hematological disorder in children on Antiretroviral Therapy (ART).5 It may be due to pre-existing HIV related bone destruction or as a side effect of antiretroviral drugs.6 A study conducted in South Africa revealed that post ART initiation mortality in children was around 8.4 per 100 child-years; of these, anemia was the principal predictor.7
In Asia, the incidence of severe anemia among children after ART initiation is around 5.4 per 100 person-years.8 In Africa, the prevalence of anemia after initiation of ART in the pediatrics age group is up to 54.2%.9 Similarly, the incidence of anemia at the initiation of ART in West Africa (2.47–4.25per 100 person-year).10 According to the Ethiopian Demographic Health Survey 2016 report; the prevalence of anemia in Ethiopia among under-five children was 56% whereas ART-related anemia in this age group ranged from 18.9% to 30%.9,11
So far, measuring of hemoglobin before initiating ART,12 non-zidovudine (AZT) based regimen ART for patients with baseline severe anemia,13 early initiation of ART,14 cotrimoxazole prophylaxis,15 maintaining nutritional status,16 early diagnosis and management of Opportunistic Infections (OI),1,17 monitoring CD4 count and viral load18 has been taken as interventions to prevent the incident of anemia among children who are on ART. Despite, applying these intervention modalities to encounter the incident of anemia among children who are initiating ART; undernutrition,19 low CD4 count,20 AZT-based ART drug regimen,16 advanced disease stage,21 and OI,22 remained as risk factors of anemia among children on ART.
Though the occurrence of anemia increases among children who are on ART, studies on the incident and its predictors of anemia among children initiating ART were not conducted as did on the prevalence of anemia after initiations of ART. Similarly, in our country Ethiopia, data on the incidence of anemia and its predictors among children who are on ART is limited. Therefore, this study was aimed to assess the incidence rate of anemia and its predictors among children initiating ART at the University of Gondar Comprehensive and Specialized Hospital, Ethiopia. Besides, determining the incidence rate of anemia and its predictors among the pediatrics age group who are initiating ART in the various settings is critical for health surveillance to be more confident for decision making.
Method
Study Design and Setting
A hospital-based retrospective follow-up study was conducted from 2007 to 2017 by using a simple random sampling technique. This study was conducted at the University of Gondar Comprehensive Specialized Hospital pediatrics ART clinic. Gondar town is located in North Gondar zone North West Ethiopia far from 737 km from Addis Ababa, the capital city of Ethiopia. University of Gondar Comprehensive Specialized Hospital is a Teaching Hospital, which serves around five million people. The hospital started ART service in 2005. Currently, the pediatrics ART clinic has two staff; one BSc and one MSc. More than 9010 adults and 789 pediatrics patients were enrolled in HIV care from 2005 to 2017. Currently, around 636 pediatrics patients actively following their ART in this hospital. In the study setting different activities for a child with HIV were conducted: in the first visit eligibility assessment for ART (0 weeks), second visit one week after baseline visit to decide ART initiation, third visit two weeks after ART initiation, fourth visit four weeks after initiation, fifth visit (eight weeks after initiation), six visits (12 weeks after initiation), seventh (16 weeks after initiation) and eighth visit (24 weeks after initiation) to determine ART (toxicities, adherence, intolerance, and IRIS). After 24 weeks of ART initiation, patients are scheduled to return every twelve weeks.
Study Population and Sample
All children age less than 15 years who were enrolled in pediatrics HIV care at the University of Gondar compressive specialized hospital were included in this study. Children who had anemia at baseline and with incomplete hemoglobin recording at baseline were excluded.
The sample size was determined by using single population proportion formula with considerations of the following statistical assumption; confidence level 95%, proportion =18.9% (on a study conducted at the University of Gondar Comprehensive Specialized Hospital on the prevalence and associated factors among children’s who are on pre-ART and post-ART) and 4% margin of error (W).
Where;
ni= initial sample size
Z=1.96 the corresponding Z-score for the 95% CI
α=confidence interval (95%)
p = proportions of new anemia case among children who are on ART
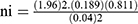 =368, by considering the incomplete patient record, 10% (37) of the initial sample size was added and the final sample size was 405.
=368, by considering the incomplete patient record, 10% (37) of the initial sample size was added and the final sample size was 405.
Variable of the Study
The dependent variable was the time of occurrence of anemia while the independent variables include child Socio-demographic characteristics (age, sex, residence, disclosure status, birth order, family size, parental status, and level of adherence), socio-demographic characteristics of caregiver (Age, HIV status, occupation, educational status, relation with the child, marital status and level of income), clinical and treatment characteristics (WHO stage, CD4count&percentage, TB, other Opportunistic infection, nutritional status, developmental status, ART (time of initiation, duration, and regimen), cotrimoxazole prophylaxis Therapy (CPT), Isoniazid Prophylaxis Therapy (IPT) and medication side effect).
Anemia
According to WHO definitions, anemia can be defined as a hemoglobin concentration less than 11 g/dl for children <5 years old, <11.5 g/dl for children 5–11.9 years old, and <12 g/dl for children 12–14.9 years old with altitude adjustment.3
Event
The occurrence of anemia during the follow-up time was diagnosed through hemoglobin concentration in children living with HIV at any time after registering into ART care. Censured: when the study participants lost, transfer out, died of other causes, or completed the study period before developing anemia.
Under-Nutrition
According to WHO growth curve weight/age<-2 Z- score, height/age<-2, Z-score, weight/height < −2 Z-score. Severe Immuno-compromised is defined as when age <11 months, 12-35 months, 36-59 months and greater than equal to 5 years their CD4 value is <25%,<20%,<15% and <200cells/mm3, repectively with age.
Data Collection Procedure and Quality Control
Data were extracted from patients’ medical records by using a data extraction tool prepared in the English language after checking the availability of variables on the HIV patient registration book. Three nurses who have experience of working in the ART clinic and took ART training were selected for data extraction. The patient’s chart was retrieved using the patient’s registration number from the database. Finally, charts that have complete baseline data were selected and variables were documented. The data extraction tool was checked for the existence of variables in the registration format on the patient’s HIV care chart via a pretest of 5% of the sample.
Data Processing and Analysis
After checking data for completeness and consistency, it was coded and entered into EPI info version 7 and exported into SPSS version 20 for analysis. Summary statistics were calculated to describe the study population about different variables. Besides, the incidence rate was calculated. Nutritional status was evaluated by using anthropometric measurements. Kaplan-Meir estimator to estimate, the anemia-free survival time was used. Survival curves between categories of different independent variables were conducted using the Log rank test. Also, Cox proportional model assumptions were checked through the Schonfeld residual test (global test, P =0.32). Bi-variable and multivariable cox proportional hazard model was applied to detect predictors. Factors with P-values ≤0.2 in the Bi-variable analysis were entered into the multivariable analysis and Adjusted Hazard Ratio (AHR) with a 95% confidence interval (CI) was calculated. Finally, factors with a P-value ≤0.05 was considered as statically significant.
Result
Socio-Demographic Characteristics of Children Who are on ART
A total of 405 charts of children on ART were reviewed. Of these, 14 (3.6%) were excluded because of data incompleteness (incomplete Hemoglobin level, ART regimen, and weight). Therefore, 391 charts of children on ART were analyzed. The mean age with standard deviation (SD) age of the study participants was 5.58 (3.214SD) year. Almost, half (49.6%) of the children were under five years of age and more than half (61.6%) of them were males. Three-fourth (75.2%) of the study participants were living in urban and majorities (91.5%) of children were lives with their parents. More than half (52.7%) of children’s caregivers were between the age group of (30–40) and 211 (54%) of them were housewives. The majorities (88%) of the children’s caregivers were HIV positive (Table 1).
 |
Table 1 Socio-Demographic Characteristics of Children Who are on ART at University of Gondar Comprehensive Specialized Hospital, from 2007–2017 |
Clinical Characteristics of Children Who are on ART
Of the total 391children, 239 (61%) had advanced disease (WHO stage 3 and 4) of HIV. One-hundred-twenty-two (31.2%) of the study participants had baseline severe immune suppression. More than half (54%) of children were undernutrition. The majority (98.5) of the study participants were taken cotrimoxazole preventive therapy whereas only 87 (22.3%) of them were taking isoniazid prophylaxis therapy (Table 2).
 |
Table 2 Clinical and Treatment Characteristics of Children Who are on ART at the University of Gondar Comprehensive Specialized Hospital, from 2007–2017 |
Incidence Rate and Free-Survival Time of Anemia
Three-hundred-ninety-one (391) children were followed for different periods which give us 935-child-years of observation. Respondents were followed for a minimum of 1 month and a maximum of 116 months with a median follow-up period of 1.75 and IQR (0.58–3) years. In this study, a total of 98 (25.1%) of anemia have occurred while 293 (74.9%) were censored. Of the censored cases, 124 (31.7%) did not develop anemia until the end of the study, 136 (34.8%) were transferred out with developing anemia, 20 (5.1%) were lost to follow-up, and 13 (3.3%) of them were dead before developing anemia. The overall incidence rate of anemia in this study was 10.5 (95% CI: 8.6, 12.8) per 100 child-year of observation. Of the total observed anemia cases during the follow-up period, nearly three-fourth (72.4%) of them were ≤5years of age. Sixty-four (65.3%) of anemia cases were advanced disease (WHO stage 3 and 4) of HIV. The incidence rate of anemia within the first 12 months of ART was 76/100 child years. The estimated probabilities of survival (anemia free) at the end of 6 months, 12months, 36months, 48months, and 114months were 81%,79%,70%,68%, and 67%, respectively (Table 3).
Predictors of Anemia Incidence
Equality of survival for the different categories of independent variables was checked through the Log-rank (mantel-cox) test, and ART regimen, TB, baseline nutritional status, and CD4 count were significantly associated with time to anemia occurrence. The mean survival time (not developing anemia) of the entire follow-up was 83.4 (CI: 77.6–89) months among children with ART (Figure 1).
 |
Figure 1 Kaplan–Meier curve of anemia free survival proportion among children with ART at the University of Gondar Comprehensive Specialized Hospital, from 2007–2017. |
Regarding survival capability, there was the presence of a significant difference between patients who had tuberculosis and no tuberculosis (p-value<0.001) (Figure 2). Besides, there were also significant variance between zidovudine-based ART and ABC-based regimen (p-value<0.001) (Figure 3).
 |
Figure 2 Kaplan–Meier estimate of anemia among children with ART at the University of Gondar Comprehensive Specialized Hospital, from 2007–2017. |
 |
Figure 3 Kaplan–Meier survival estimates of ART regimen among children with ART at the University of Gondar Comprehensive Specialized Hospital, from 2007–2017. |
Cox Regression Analysis
Cox proportional model assumptions were checked through the Schonfeld residual test (global test, P =0.32). In the bi-variable cox regression analysis age, residence, WHO staging, ART regimen, immunosuppression, nutritional status, and TB were associated with anemia incidence among children on ART. However, in the multivariable cox regression analysis TB, ART regimen, immunosuppression, and nutritional status have remained statistically significant predictors of anemia among children.
Children with severe immunosuppression were 2.9 (AHR= 2.9, 95% CI; 1.23, 6.77) times at risk of anemia as compared with children with normal immune status. Similarly, children with undernutrition were 2.8 times at higher risk of developing anemia as compared with children with normal nutritional status (AHR=2.7 (95% CI; 1.5, 5)) throughout the follow-up period. The hazard of developing anemia among children who had taken zidovudine-based ART regimen were 4 (AHR=4, 95% CI; 1.097, 11.44) times as compared to who had taken Abacavir-based ART regimen. Study participants with tuberculosis were 2.1 (AHR=2.1, 95% CI; 1.4, 3.3) times at higher risk of anemia incidence as compared with those who had no tuberculosis throughout the follow-up period (Table 4).
 |
Table 4 Cox Regression Analysis of Predictors of Anemia Among Children Who are on ART at University of Gondar Comprehensive Specialized Hospital, from 2007–2017 |
Discussion
This study reported that the overall incidence rate of anemia among children with ART in this study was 10.5 (95% CI; 8.6, 12.8) per 100 child-year follow-up which was higher than retrospective cohort study conducted in Asia with 5.4 per 100-child year,8 a retrospective cohort study conducted in West Africa which was 2.72 per100-child year for those on AZT based and 4.25 per100-child year follow-up for those on non-AZT-based ART combination.10 This difference could be due to, those previous studies were done specifically on severe anemia, but this study stresses the overall anemia incidence. The other possible justification for the Asian study might be due to the difference in age of study participants; in the previous study the age of study participants was ≤18years, but in our study, the age of participants was <15 years.
In this study, the proportion of anemia among children on ART was 25.1% (95% CI; 20.5, 29.5) which was comparable to various studies conducted at Addis Ababa Ethiopia 22.2%,20 Jimma Ethiopia 21.6%,2 and Bahirdar northwest Ethiopia 28.9%.23 This might be due to similarity in socio-demographic characteristics of study participants, the use of similar guidelines of HIV management in the country, and similarities of age category in the study participants.
However, the proportion of anemia in this study among children on ART was lower than a retrospective data analysis study conducted in India which was 66%,19 and a cross-sectional study conducted at Harar, Ethiopia which was 39.2%.24 This may be due to differences in study setting; in the Indian study the study setting was both tertiary health care center and community clinics, however, in our study the study setting was the comprehensive and specialized hospital. And, the higher proportion of anemia in Harar might be due to the difference in the follow-up period, in Harar, Ethiopia, study it was 4 years follow-up period. Whereas, in our study, it was 11 years of the follow-up study.
This study stated that the hazard of developing anemia in children on ART with severe immunosuppression had nearly three times as compared with those who had normal immune status. This finding is consistent with a study conducted at Bahirdar Felegehiwot hospital, Ethiopia.23 This might be because severe immunosuppression possibly leads to viral duplication, which can result in anemia via increased cytokine-mediated myelosuppression and higher loads of opportunistic infections.22,25
An important predictor of anemia was ART regimens in which children who were treated AZT-based were four times at higher hazard of developing anemia compared to children who were treated in ABC-based ART regimen. This result was supported by a study conducted in Asia.8 This could be explained by the fact that Zidovudine is related to suppressions of bone marrow that leads to a reduction in the production of red blood cells and other types of blood cells in the bone marrow.26
Another significant predictor of anemia among children with ART was under-nutrition which had 2.7 times at higher risk to develop anemia than normal nutritional status. The finding was in line with a study conducted in West Africa.10 The possible reason for this might be due to decreased essential macronutrients and micronutrients leads to iron and folate deficiency which causes anemia, and immunosuppression which increases susceptibility to infectious disease in turn may result in anemia.27,28
This analysis also showed that children who had tuberculosis had nearly two times at higher risk for anemia as compared with those who had no tuberculosis among children after initiations of ART. This result agrees with a retrospective study conducted in India.19 This could be explained by the fact that inflammatory mediators in tuberculosis infection cause suppression of erythropoiesis that leads to decreased production of red blood cells by bone marrow.22
The limitation of this study could be since it was based on secondary data analysis, due to this other possible causes of anemia like iron deficiency anemia, malaria, intestinal parasite, and use of bone marrow suppressive drugs were not included due to incomplete medical records. Also, those study participants whose chart was lost were not included in the study which may affect the finding of the study.
Conclusion
Anemia incidence was high among children who are on ART at the University of Gondar Comprehensive Specialized Hospital, North West Ethiopia. TB, AZT-based ART regimen, severe immunosuppression, and undernutrition remained statistically significant predictors of anemia among children with ART. Children with HIV were the most vulnerable group to anemia and it also remains a public health problem among HIV patients. Therefore, to reduce the burden of anemia among children with HIV different strategies, approaches, and plans should consider and better to emphasize the predictors of anemia among children identified from this study.
Abbreviations
ABC, abacavire; AHR, adjusted hazard ratio; AIDS, acquired immune deficiency syndrome; ART, ant-retroviral therapy; AZT, zidovudine; CHR, crude hazard ratio; CI, confidence interval; CPT, cotrimoxazole prophylaxis therapy; HGB, hemoglobin; HIV, human immune deficiency virus; IDR, incidence density rate; IPT, isoniazid prophylaxis therapy; IQR, interquartile range; OI, opportunistic infection; SD, standard deviation; TB, tuberculosis; WHO, World Health Organization.
Data Sharing Statement
Regarding data availability, you can get the data from the corresponding author based on request when you want the data.
Ethical Approval and Consent
Ethical clearance has been obtained from the school of nursing on behalf of the University of Gondar institutional review board. From the University of Gondar Comprehensive Specialized Hospital management, a permission letter was obtained. Information in the data abstraction tool was anonymized. The files of entered data in the software and the final result of the study were protected with a password. The overall confidentiality of the information was kept throughout the entire study process and the information was used only for the study purpose. The data were reviewed from medical charts and as this was not a primary study the Ethical Review Committee of the University of Gondar waived the requirement for consent. This study was conducted following the Declaration of Helsinki.
Acknowledgments
The authors would like to thank the University of Gondar for its financial support. Also, we want to acknowledge porters who were working at the University of Gondar Comprehensive Specialized Hospital for their willingness to retrieve charts from the chart room.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. We have agreed on the journal to which the article will be submitted, reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage, agree to take responsibility and be accountable for the contents of the article.
Funding
Financial support was obtained from the University of Gondar. The funding institution has no role in any activities of the preparation of the manuscript as well as the decision to publish.
Disclosure
The authors have declared that they have no competing interests.
References
1. Adane A, Desta K, Bezabih A, Gashaye A, Kassa D. HIV-associated anaemia before and after initiation of antiretroviral therapy at Art Centre of Minilik II Hospital, Addis Ababa, Ethiopia. Ethiop Med J. 2012;50(1):13–21.
2. Abebe M, Alemseged F. Hematologic abnormalities among children on HAART, in Jimma university specialized hospital, southwestern Ethiopia. Ethiop J Health Sci. 2009;19:2.
3. WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. 2011. Available from: http://wwwwhoint/vmnis/indicators/haemoglobinpdf.2015.
4. Benoist BME, Egll I, Cogswell M:. WorldwidePrevalence of Anaemia World Health Organization Global Data-Base on Anaemia. World Health Organization: Geneva, Switzerland; 2008.
5. Oshikoya K, Lawal S, Oreagba I, Awodele O, Olayemi S, Iroha E. Adverse events in HIV-infected children on antiretroviral therapy at a teaching hospital in Lagos, Nigeria: a retrospective study. Adv Pharmacoepidemiol Drug Saf. 2012;1(4):1000117.
6. Ruhinda EN, Bajunirwe F, Kiwanuka J. Anaemia in HIV-infected children: severity, types and effect on response to HAART. BMC Pediatr. 2012;12(1):170.
7. Wamalwa DC, Obimbo EM, Farquhar C, et al. Predictors of mortality in HIV-1 infected children on antiretroviral therapy in Kenya: a prospective cohort. BMC Pediatr. 2010;10(1):33. doi:10.1186/1471-2431-10-33
8. Bunupuradah T, Kariminia A, Chan K-C, et al. Incidence and predictors of severe anemia in Asian HIV-infected children using first-line antiretroviral therapy. Int J Infect Dis. 2013;17(10):e806–e810. doi:10.1016/j.ijid.2013.04.006
9. Geletaw T, Zelalem MZ, Demisse AG. Hematologic abnormalities and associated factors among HIV infected children pre- and post-antiretroviral treatment, North West Ethiopia. J Blood Med. 2017;8:99. doi:10.2147/JBM.S137067
10. Renner LA, Dicko F, Koueta F, et al. Anaemia and zidovudine-containing antiretroviral therapy in paediatric antiretroviral programmes in the IeDEA Paediatric West African Database to evaluate AIDS. J Int AIDS Soc. 2013;16(1):18024. doi:10.7448/IAS.16.1.18024
11. Geleta D, Demissie DB, Seyoum B, Egata G. Prevalence of anemia and associated factors among PHIVs attendants antiretroviral therapy clinics in Public Health Institutions in Dire Dawa Town, East Ethiopia. Prevalence. 2016;22.
12. Obirikorang C, Yeboah FA. Blood haemoglobin measurement as a predictive indicator for the progression of HIV/AIDS in resource-limited setting. J Biomed Sci. 2009;16(1):102. doi:10.1186/1423-0127-16-102
13. WHO. Antiretroviral Therapy of HIV Infection in Infants and Children: Towards Universal Access: Recommendations for a Public Health Approach-2010 Revision. World Health Organization; 2010.
14. Sauvageot D, Schaefer M, Olson D, Pujades-Rodriguez M, O’Brien DP. Antiretroviral therapy outcomes in resource-limited settings for HIV-infected children <5 years of age. Pediatrics. 2010;125(5):e1039–e1047.
15. Prendergast A, Walker AS, Mulenga V, Chintu C, Gibb DM. Improved growth and anemia in HIV-infected African children taking cotrimoxazole prophylaxis. Clin Infect Dis. 2011;52(7):953–956. doi:10.1093/cid/cir029
16. Rajesh R, Vidyasagar S, Varma DM, Mohiuddin S. Evaluation of incidence of zidovudine induced anemia in Indian human immunodeficiency virus positive patients in comparison with stavudine based highly active antiretroviral therapy. Int J Risk Saf Med. 2011;23(3):171–180. doi:10.3233/JRS-2011-0531
17. Emmanuel AA, Ayodotun O. Prevalence and risk factors of anaemia among human immunodeficiency virus (HIV)-infected antiretroviral therapy nave children in Makurdi, Nigeria: a retrospective study. J AIDS HIV Res. 2014;6(6):128–137. doi:10.5897/JAHR2014.0304
18. Daka D, Lelissa D, Amsalu A. Prevalence of anaemia before and after the initiation of antiretroviral therapy at ART centre of Hawassa University Referral Hospital, Hawassa, South Ethiopia. Sch J Med. 2013;3(1):1–6.
19. Shet A, Mehta S, Rajagopalan N, et al. Anemia and growth failure among HIV-infected children in India: a retrospective analysis. BMC Pediatr. 2009;9(1):37. doi:10.1186/1471-2431-9-37
20. Mihiretie H, Taye B, Tsegaye A. Magnitude of anemia and associated factors among pediatric HIV/AIDS patients attending Zewditu Memorial Hospital ART Clinic, Addis Ababa, Ethiopia. Anemia. 2015;2015:1–6. doi:10.1155/2015/479329
21. Calis JC, van Hensbroek MB, de Haan RJ, Moons P, Brabin BJ, Bates I. HIV-associated anemia in children: a systematic review from a global perspective. AIDS. 2008;22(10):1099–1112. doi:10.1097/QAD.0b013e3282fa759f
22. Yadav J, Nanda S, Sharma D. Opportunistic infections and complications in human immunodeficiency virus-1-infected children: correlation with immune status. Sultan Qaboos Univ Med J. 2014;14(4):e513.
23. Tsegay Y, Tadele A, Addis Z, Alemu A, Melku M. Magnitude of cytopenias among HIV‑infected children in Bahir Dar, northwest Ethiopia; a comparison of HAART-naïve and HAART‑experienced children. HIV/AIDS. 2017;55(unknown):31–42.
24. Teklemariam Z, Mitiku H, Mesfin F. Prevalence of anemia and nutritional status among HIV-positive children receiving antiretroviral therapy in Harar, eastern Ethiopia. HIV/AIDS. 2015;7:191.
25. Alexaki A, Wigdahl B. HIV-1 infection of bone marrow hematopoietic progenitor cells and their role in trafficking and viral dissemination. PLoS Pathog. 2008;4(12):e1000215.
26. Dash KR, Meher LK, Hui P, Behera S, Nayak S. High incidence of zidovudine induced anaemia in HIV infected patients in Southern Odisha. Indian J Hematol Blood Transfus. 2015;31(2):247–250. doi:10.1007/s12288-014-0426-9
27. Wang J, Wang H, Chang S, et al. The influence of malnutrition and micronutrient status on anemic risk in children under 3 years old in poor areas in China. PLoS One. 2015;10(10):e0140840. doi:10.1371/journal.pone.0140840
28. Tine RCK, Ndiaye M, Hansson HH, et al. The association between malaria parasitaemia, erythrocyte polymorphisms, malnutrition and anaemia in children less than 10 years in Senegal: a case control study. BMC Res Notes. 2012;5(1):565. doi:10.1186/1756-0500-5-565
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.