Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Incidence and Factors Associated with Hyperglycemia in Patients with First Hospitalization for Major Depression Disorder: A Large Cross-Sectional Sample
Authors Qi S, Xu Y, Zeng K, Li Y, Ma J
Received 19 May 2023
Accepted for publication 10 August 2023
Published 21 August 2023 Volume 2023:19 Pages 1809—1818
DOI https://doi.org/10.2147/NDT.S421984
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Shuangyu Qi,1,2,* Yang Xu,1,2,* Kuan Zeng,1,2 Yi Li,1,2 Jun Ma1– 3
1Affiliated Wuhan Mental Health Center, Tongji Medical College of Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 2Wuhan Hospital for Psychotherapy, Wuhan, People’s Republic of China; 3Department of Psychiatry, Renmin Hospital, Wuhan University, Wuhan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yi Li; Jun Ma, Affiliated Wuhan Mental Health Center, Tongji Medical College of Huazhong University of Science and Technology, No. 89, Gongnongbing Road, Wuhan, Hubei Province, People’s Republic of China, Tel +86 027 82281733, Fax +86-027-82281733, Email [email protected]; [email protected]
Purpose: Major depressive disorder (MDD) is a mood disorder characterized by persistent spontaneous depression and has a high rate of disability and mortality. There is a complex relationship between MDD and disorders of glucose metabolism, and our study aimed to investigate the prevalence and risk factors for hyperglycemia in patients with MDD who were hospitalized for the first times.
Patients and Methods: A total of 981 first-time inpatients with MDD were recruited, socio-demographic information, anthropometric data, and biochemical parameters were collected for each participant. The 17-item Hamilton Assessment Scale for Depression (HAMD-17), the 14-item Hamilton Anxiety Scale (HAMA-14), the Positive Syndrome Scale (PSS), and Clinical General Impressions Inventory-Severity of Illness (CGI-SI) scores were used to assess patients’ clinical symptoms.
Results: The prevalence of hyperglycemia was 9.28% among patients with MDD who were hospitalized for the first time. Compared to the non-hyperglycemic subgroup, patients in the hyperglycemic subgroup were found to have more extensive and significant demographic and clinical characteristics, higher levels of metabolism-related parameters, and more severe psychological and psycho-pathological symptoms. Age, thyroid stimulating hormone (TSH), triglycerides (TG) were risk factors for hyperglycemia in MDD patients, while course of disease was a protective factor.
Conclusion: The study findings suggest that the prevalence of hyperglycemia is not high in patients with MDD who are hospitalized for the first time. The risk variables for predicting hyperglycemia include age, TSH and TG. The above three factors and course of disease have good combined diagnostic ability for hyperglycemia.
Keywords: major depression disorder, hyperglycemia, first hospitalization, incidence
Introduction
Major depressive disorder (MDD) is a serious psychiatric disorder with clinical manifestations characterized by mood and cognitive changes and loss of interest or pleasure lasting at least 2 weeks, with a range of depressive symptoms.1 The prevalence of major depressive disorder is high globally, with roughly 2% of the global population affected by MDD.2 Lifetime prevalence of MDD ranges from 2% to 21% worldwide, with some European countries having the highest prevalence and some Asian countries having the lowest prevalence.3 A meta-analysis has found that the 12-month and lifetime prevalence of MDD in China was 1.6% and 1.8%, respectively.4 Although the prevalence of MDD in China is relatively low compared to other countries, the incidence of MDD is increasing over time. And in the development of MDD, due to its high mortality and disabling characteristics,5–8 it not only causes great personal suffering to patients,9 but also a huge economic burden to families and society.10,11
Although depression and diabetes mellitus are usually considered as disorders of two separate systems, there are complex interrelated mechanisms between them. It has been suggested that depression and diabetes share common biological origins, such as a common over-activation of innate immunity leading to cytokine-mediated inflammatory responses12 and a common dysregulation of the hypothalamic-pituitary-adrenal axis,13 and that these pathways lead to an increased risk of depression, diabetes, and increased mortality throughout the lifespan.12,14 Genomic association studies have also reported that depression and diabetes share common genetic pathways or shared genetic load.15,16 At the same time, there is an overlap in the gut microbiota of depressed patients and patients with impaired glucose tolerance.17 The association between the two has been similarly affirmed in several epidemiologic and clinical studies. In a large survey involving 2783 patients with type 2 diabetes mellitus (T2D) in 18 countries, it was found that 10.6% of In a large survey involving 2783 patients with type 2 diabetes mellitus (T2D) in 18 countries, it was found that 10.6% of patients with T2D also had MDD.18 Another large meta-analysis found that the overall prevalence of T2DM in the MDD-diagnosed population was 8.7%, which is significantly higher than the prevalence in the general population.19 Even more disturbing is the fact that abnormal blood glucose levels increase the risk of suicide in patients with MDD.20–22 Therefore, it is reasonable to believe that a deeper understanding of the association between depression and diabetes remains an important future clinical study to improve the prognosis of MDD.
Although there is evidence of dysglycemia in patients with MDD, there is some heterogeneity in the prevalence of hyperglycemia in patients with MDD in the relevant studies due to differences in region, ethnicity, sample size, and differentiation criteria for hyperglycemia. Meanwhile, there is a lack of large-sample studies on hyperglycemia in first-time hospitalized MDD patients in existing studies. Therefore, in this study, we investigated the prevalence of hyperglycemia in a large sample of first-time hospitalized MDD patients in China and further analyzed the influencing factors, which will help to further study the mechanism of combined hyperglycemia in MDD patients and the corresponding prevention and treatment methods.
Materials and Methods
Subjects
A total of 1012 patients diagnosed with MDD were admitted to the Wuhan Mental Health Center from July 2017 to August 2022, 31 patients were excluded as shown in Figure 1.
 |
Figure 1 Flow chart. |
Patients must meet the following criteria at the time of inclusion in the study:
- Fulfill the diagnostic criteria for MDD according to the 10th revision of the International Classification of Diseases (ICD-10).
- Patients on the day of admission had no history of hospitalization prior to completing the interview.
- Patients were between the ages of 18–60 years of Chinese Han ethnicity.
- 17-item Hamilton Depression Scale (HAMD-17) score ≥ 24.
Patients were not included in the study if they meet any of the following conditions:
- During pregnancy or lactation.
- Having a history of psychoactive substance dependence.
- Suffering from a serious physical illness or personality disorders.
- Those with a clear history of diabetes before the onset of MDD and treated with glucose-lowering medications.
- Unable to cooperate with the completion of a psycho-psychological evaluation because of a severe behavioral disorder or other reasons.
The study followed the principles of the Declaration of Helsinki, was approved by the Ethics Committee of the Wuhan Mental Health Center under the approval number KY20170201.01, and all participants signed an informed consent form by the patients themselves or their families.
Research Design
This study used a cross-sectional design to determine the prevalence of hyperglycemia in patients with MDD who were hospitalized for the first time, assessed patients’ fasting glucose levels, and compared demographic and general clinical data for clinical subgroups with and without hyperglycemia. Finally, we determined important characteristics to distinguish patients with and without hyperglycemia by plotting ROC curves.
On the day of admission, patients who met the inclusion criteria were interviewed and evaluated by a psychiatrist in a separate interview room on the hospital ward to collect general clinical data, which included the patient’s age, gender, age of onset, course of disease, marital status, educational background, treatment history, the history of suicidal behavior, blood pressure level (specifically: systolic blood pressure, SBP; diastolic blood pressure, DBP) and body mass index (BMI). At the same time, we respectively assessed patients’ depressive symptoms, anxiety symptoms, psychotic symptoms, and disease severity using HAMD-17, the 14-item Hamilton Anxiety Scale (HAMA-14), the Positive Symptom Subscale (PSS) for items P1-P7 of the Positive and Negative Symptom Scale (PANSS), and the Clinical General Impressions Inventory-Severity of Illness (CGI-SI). On the second day after admission, we collected the following serological data in the fasting state of the patients from the electronic medical record system: lipid profile (specifically: total cholesterol, TC; triglycerides, TG; low-density lipoprotein cholesterol, LDL-C; high-density lipoprotein cholesterol, HDL-C), fasting blood glucose (FBG) level, and thyroid function (specifically: thyrotropic hormone, TSH; free triiodothyronine, FT3; free thyroxine, FT4).
We used fasting glucose level to distinguish two clinical subgroups, the classification criteria were based on the diagnostic criteria for hyperglycemia in the Standards of medical care for type 2 diabetes in China 2019, in which meeting FBG ≥ 6.1 mmol/L was considered as hyperglycemia, and <6.1 mmol/L was considered as non-hyperglycemia.23
The relevant psychometric indicators were assessed by two uniformly trained attending psychiatrists at the medical center where the sample was located.
Data Analysis
Data collected for normally distributed continuous measures were given in the form of means and standard deviations, while categorical variables were expressed as counts.Considering the susceptibility of age to extreme values, age was chosen to be expressed as the median (range). In order to compare the continuous variables in different groups, we utilized independent samples from T-test. In addition, we performed the chi-squared tests for categorical variables. In order to study the variables affecting patients’ hyperglycemia, a binary logistic regression model was constructed with hyperglycemia as the dependent variable and variables with differences in univariate analysis as independent variables. Finally, the area under the receiver operating characteristics (AUCROC) was used to determine the discriminatory capacity of significant parameters to distinguish between patients with and without hyperglycemia. The threshold of significance was set at less than 0.05 and all P values were two-tailed. Figures were plotted using GraphPad Prism (version 8.4.3; GraphPad Software, Inc., La Jolla, CA, USA) and statistically analyzed using SPSS 25 (SPSS, Inc., Chicago, IL).
Results
The Differences Between Clinical Subgroups with and without Hyperglycemia
Among 981 MDD patients included in this study, 91 met the diagnosis of hyperglycemia, representing 9.28% (91/981) of the total sample. The fasting blood glucose level in the hyperglycemic group was 6.60±0.46 mmol/L and in the non-hyperglycemic group was 5.13±0.46 mmol/L. There were significant differences in demographic and general clinical data between the subgroups with and without hyperglycemia. As shown in Table 1, compared to the non-hyperglycemic subgroup, the hyperglycemic subgroup generally had higher values for indicators such as age, age at onset, prevalence of suicidal behavior, scores on four scales (PSS, HAMD, HAMA, and CGI-SI), TSH levels, FT3 levels, WC, TG levels, TC levels, LDL-C levels, SBP and DBP, but the indicators of disease course and educational attainment were even lower.
 |
Table 1 The Demographic and General Clinical Data in Different Clinical Subgroups |
Determinants of Hyperglycemia Among MDD Patients: Based on a Binary Logistic Model
We constructed a binary logistic regression model (Backward: Wald) with the variables that differed in the univariate analysis as independent variables and hyperglycemia as the dependent variable. The results showed that age (B = 0.03, p = 0.007, OR = 1.03), TSH (B = 0.27, p < 0.001, OR = 1.31), TG (B= 0.23, p = 0.037, OR = 1.26) were risk factors for hyperglycemia. On the contrary, the course of disease (B = −0.08, p = 0.009, OR = 0.92) was the protective factor of hyperglycemia. These results were summarized in Table 2.
 |
Table 2 Binary Logistic Regression Analyses of Determinants of Hyperglycemia in MDD Patients |
ROC Curve Analysis to Distinguish Hyperglycemia from Non- Hyperglycemia Patients
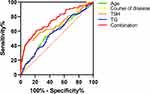
We performed a ROC analysis of the correlates identified in the regression analysis that had a significant effect on hyperglycemia in the study population, as shown in Table 3. And the ROC curves of these four variables were plotted, including age, disease course, TSH, and TG, as shown in Figure 2. The ROC curves showed the following four-factor results: the AUC for age was 0.58 (p = 0.008, 95% CI = 0.52–0.65), the AUC for course of disease was 0.60 (p = 0.001, 95% CI = 0.54–0.66), the AUC for TSH was 0.68 (p < 0.001, 95% CI = 0.62–0.75), the AUC for TG was 0.58 (p = 0.011, 95% CI = 0.52–0.64). The AUC for combination was 0.74 (p < 0.001, 95% CI = 0.69–0.80).
 |
Table 3 ROC Analysis of Factors Influencing Hyperglycemia |
Discussion
This was a large sample cross-sectional study to investigate the prevalence and the associated factors of hyperglycemia in the first hospitalized MDD patients. The main findings of our study were as follows: 1. The prevalence of hyperglycemia in first hospitalized MDD patients was 9.28%; 2. The metabolic data, psychological and psycho-pathological symptom scores, as well as age and onset age of the hyperglycemic subgroup are generally higher than those of the non-hyperglycemic subgroup; 3. Age, TSH level, and TG level were risk factors for the diagnosis of hyperglycemia in patients with MDD who were first hospitalized, while the course of the disease was a protective factor; 4. First hospitalization MDD patients with co-morbid hyperglycemia could be predicted by the combination of age, course of disease, TSH level, TG level, and the combination discrimination ability was 74%.
In our study, the prevalence of hyperglycemia in patients with MDD was 9.28%, whereas in some previous studies, the prevalence of hyperglycemia in patients with MDD was higher. In other studies, the prevalence of hyperglycemia in patients with MDD ranged from 13.62% to 37%.24–26 The lower prevalence of hyperglycemia in our study compared to other studies may be partly due to the fact that we included patients with MDD who were hospitalized for the first time, whereas other study subjects tended to be primary unmedicated patients. 64.83% of the patients in our study were treated on an outpatient basis and previous visits and monitoring of appropriate indicators helped in glycemic control. Another reason for the significantly higher prevalence than our study is the broader definition of elevated blood glucose.
We found that patients in the hyperglycemic subgroup were older than those in the nonhyperglycemic subgroup in terms of age and age of onset, and previous studies have shown that morbidity and mortality in diabetes are age-related.27 Similar to previous studies,22,28–30 we found a higher rate of patients with a history of suicidal behavior in the hyperglycemic subgroup. Uncontrolled blood glucose may lead to suicidal thoughts and suicidal thoughts may promote insulin resistance.31–33 In terms of psychopathological and psychological symptoms, the hyperglycemic group was also more severe, which is also consistent with previous findings.24,34,35 In animal and clinical trials, it is found that hyperglycemia may affect patients’ mood and behavior by affecting the hippocampus and amygdala,36,37 the hypothalamus and its nucleus accumbens,38 the HPA axis,39 and MDD and hyperglycemic co-morbidity genes.40 We also observed that waist circumference, other metabolic index levels and blood pressure were also higher in the hyperglycemic group than in the non-hyperglycemic subgroup, which is similar to the results of some previous studies,21,34 suggesting that the overall metabolic level of patients with comorbid hyperglycemic MDD was disturbed, and interactions between different metabolic disorders have been found.25,41–49 We also found that the hyperglycemic subgroup had a shorter duration of disease and a lower level of education. However, these did not differ significantly in some other studies,24,34 which may be related to differences in the study population and for the classification of education level.
In our study, we found that age, disease course, TSH and TG were influential factors for co-morbid hyperglycemia in MDD patients. The effect of increasing age on elevated blood glucose levels may involve adipose tissue dysfunction, cellular senescence, inflammation, endogenous cannabinoids, intestinal permeability, and mitochondrial dysfunction.27 Elevated TSH levels and TG levels are consistent with the results of other studies.34,41 Thyroid hormone function is closely related to glycemia, and elevated TSH levels in particular may cause metabolic abnormalities in MDD patients, including elevated blood glucose, obesity, elevated LDL-C, and metabolic syndrome.25,41,42 TSH may cause insulin resistance and glucose dysregulation, where mechanisms include hepatic endoplasmic reticulum stress,42,50 endothelial dysfunction, and inhibition of lipid triglyceride lipase leading to increased TG stores,51,52 among others. Similar to the results of existing studies, we found that TG levels were also associated with hyperglycemia in patients.21,34 There is a close relationship between hyperglycemia and hyperlipidemia.43,44 On the one hand, hyperglycemia may lead to elevated lipids through conversion to lipids.24 On the other hand, TG levels have been shown to be a marker of insulin resistance and may predict the risk of impaired fasting glucose concentrations.53 Of course, age, TG, and TSH also interact with each other, and we speculate that this may be somewhat related to the patient’s elevated blood sugar.54 In the study findings, course of disease was a protective factor for comorbid hyperglycemia in patients with MDD who were hospitalized for the first time. One study finds abnormal glucose tolerance in the early stages of depression.55 However, other studies have not found significant differences in the course of disease between first-time or first-treatment MDD patients with elevated glucose and MDD patients with normal glucose.24,34 This variability in outcomes may be related to differences between the enrolled samples. In our study, the patients were all first-time hospitalized MDD patients, 64.83% had received medication prior to admission, and the shorter disease course may be related to acute inflammatory or metabolic reactions associated with the administration of antidepressants.56
One study finds that the combination of TSH levels and TSH, TG, thyroglobulin antibody (TgAb), and thyroid peroxidase antibody (TPOAb) can distinguish overweight or obese MDD patients with elevated fasting glucose.34 Although the study findings were not entirely consistent with this study, this may be due to differences in the population we included and the selected serological data content. Our study further reported that age, course of disease, TSH levels, and TG levels had a good combination diagnostic capability for hyperglycemia in first-time hospitalized MDD patients.
According to the results of our study, it is necessary to pay attention to the patient’s age and disease duration in clinical practice, and at the same time, it is possible to monitor and control the patient’s TSH level and TG level, which can help to detect the elevation of the patient’s blood glucose level in a timely manner, and control the blood glucose as soon as possible, so as to avoid any further impact on the patient’s prognosis.
There are some limitations in our study. Firstly, this study was a cross-sectional study, so it could only analyze the prevalence of hyperglycemia and related factors in first hospitalized MDD patients, but unable to draw a causal relationship. Subsequent studies should be conducted longitudinally to further investigate the relationship between associated factors and glucose metabolism in first-time hospitalized MDD patients. Secondly, in this study, we did not collect data on glycated hemoglobin (HbA1c) and oral glucose tolerance test (OGTT), which are more sensitive than fasting glucose,57 so we were unable to fully cover hyperglycemia in patients with MDD who were hospitalized for the first time. Furthermore, since our study population may have had a past history of outpatient treatment, and we did not control for the effects of age, TG, and TSH on each other, these factors may have confounded the results of the study. We will further control for these interferences in subsequent studies. Finally, we did not collect data on diet, exercise, and sedentary lifestyles, which have a large metabolic impact, and this is a part of the study that needs to be included in the future.
Conclusion
The study findings suggest that the prevalence of hyperglycemia is not high in patients with MDD who are hospitalized for the first time. Age, TSH levels, TG levels and course of disease were factors associated with hyperglycemia in first-time hospitalized MDD patients.
Acknowledgments
The authors thank all the patients who participated in this study and the health care professionals who performed the diagnostic and clinical evaluations.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Malhi GS, Mann JJ. Depression. Lancet. 2018;392(10161):2299–2312. doi:10.1016/S0140-6736(18)31948-2
2. James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi:10.1016/S0140-6736(18)32279-7
3. Gutierrez-Rojas L, Porras-Segovia A, Dunne H, Andrade-Gonzalez N, Cervilla JA. Prevalence and correlates of major depressive disorder: a systematic review. Braz J Psychiatry. 2020;42(6):657–672. doi:10.1590/1516-4446-2020-0650
4. Zhao YJ, Jin Y, Rao WW, et al. Prevalence of Major Depressive Disorder Among Adults in China: a Systematic Review and Meta-Analysis. Front Psychiatry. 2021;12:659470. doi:10.3389/fpsyt.2021.659470
5. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11):e1001547. doi:10.1371/journal.pmed.1001547
6. Lundberg J, Cars T, Loov SA, et al. Clinical and societal burden of incident major depressive disorder: a population-wide cohort study in Stockholm. Acta Psychiatr Scand. 2022;146(1):51–63. doi:10.1111/acps.13414
7. Patten SB, Williams JV, Bulloch AG. Major depressive episodes and mortality in the Canadian household population. J Affect Disord. 2019;242:165–171. doi:10.1016/j.jad.2018.08.030
8. Salk RH, Hyde JS, Abramson LY. Gender differences in depression in representative national samples: meta-analyses of diagnoses and symptoms. Psychol Bull. 2017;143(8):783–822. doi:10.1037/bul0000102
9. Battle DE. Diagnostic and Statistical Manual of Mental Disorders (DSM). Codas. 2013;25(2):191–192. doi:10.1590/s2317-17822013000200017
10. Ärnlöv J. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi:10.1016/S0140-6736(15)60692-4
11. Zhdanava M, Pilon D, Ghelerter I, et al. The Prevalence and National Burden of Treatment-Resistant Depression and Major Depressive Disorder in the United States. J Clin Psychiatry. 2021;82(2). doi:10.4088/JCP.20m13699
12. Moulton CD, Pickup JC, Ismail K. The link between depression and diabetes: the search for shared mechanisms. Lancet Diabetes Endocrinol. 2015;3(6):461–471. doi:10.1016/S2213-8587(15)00134-5
13. Penninx BW, Milaneschi Y, Lamers F, Vogelzangs N. Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med. 2013;11:129. doi:10.1186/1741-7015-11-129
14. Duarte-Silva E, de Melo MG, Maes M, Filho A, Macedo D, Peixoto CA. Shared metabolic and neuroimmune mechanisms underlying Type 2 Diabetes Mellitus and Major Depressive Disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2021;111:110351. doi:10.1016/j.pnpbp.2021.110351
15. Amare AT, Schubert KO, Klingler-Hoffmann M, Cohen-Woods S, Baune BT. The genetic overlap between mood disorders and cardiometabolic diseases: a systematic review of genome wide and candidate gene studies. Transl Psychiatry. 2017;7(1):e1007. doi:10.1038/tp.2016.261
16. Su MH, Shih YH, Lin YF, et al. Familial aggregation and shared genetic loading for major psychiatric disorders and type 2 diabetes. Diabetologia. 2022;65(5):800–810. doi:10.1007/s00125-022-05665-x
17. Slyepchenko A, Maes M, Jacka FN, et al. Gut Microbiota, Bacterial Translocation, and Interactions with Diet: pathophysiological Links between Major Depressive Disorder and Non-Communicable Medical Comorbidities. Psychother Psychosom. 2017;86(1):31–46. doi:10.1159/000448957
18. Lloyd CE, Nouwen A, Sartorius N, et al. Prevalence and correlates of depressive disorders in people with Type 2 diabetes: results from the International Prevalence and Treatment of Diabetes and Depression (INTERPRET-DD) study, a collaborative study carried out in 14 countries. Diabet Med. 2018;35(6):760–769. doi:10.1111/dme.13611
19. Vancampfort D, Mitchell AJ, De Hert M, et al. TYPE 2 DIABETES IN PATIENTS WITH MAJOR DEPRESSIVE DISORDER: a META-ANALYSIS OF PREVALENCE ESTIMATES AND PREDICTORS. Depress Anxiety. 2015;32(10):763–773. doi:10.1002/da.22387
20. Dong R, Haque A, Wu HE, Placide J, Yu L, Zhang X. Sex differences in the association between suicide attempts and glucose disturbances in first-episode and drug naive patients with major depressive disorder. J Affect Disord. 2021;292:559–564. doi:10.1016/j.jad.2021.05.110
21. Liu W, Wu Z, Sun M, et al. Association between fasting blood glucose and thyroid stimulating hormones and suicidal tendency and disease severity in patients with major depressive disorder. Bosn J Basic Med Sci. 2022;22(4):635–642. doi:10.17305/bjbms.2021.6754
22. Fugger G, Dold M, Bartova L, et al. Major Depression and Comorbid Diabetes - Findings from the European Group for the Study of Resistant Depression. Prog Neuropsychopharmacol Biol Psychiatry. 2019;94:109638. doi:10.1016/j.pnpbp.2019.109638
23. Jia W, Weng J, Zhu D, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev. 2019;35(6):e3158. doi:10.1002/dmrr.3158
24. Wang Q, Li Y, Ren H, et al. Metabolic characteristics, prevalence of anxiety and its influencing factors in first-episode and drug-naive major depressive disorder patients with impaired fasting glucose. J Affect Disord. 2023;324:341–348. doi:10.1016/j.jad.2022.12.096
25. Peng P, Wang Q, Lang XE, Liu T, Zhang XY. Association between thyroid dysfunction, metabolic disturbances, and clinical symptoms in first-episode, untreated Chinese patients with major depressive disorder: undirected and Bayesian network analyses. Front Endocrinol (Lausanne). 2023;14:1138233. doi:10.3389/fendo.2023.1138233
26. Han KM, Kim MS, Kim A, Paik JW, Lee J, Ham BJ. Chronic medical conditions and metabolic syndrome as risk factors for incidence of major depressive disorder: a longitudinal study based on 4.7 million adults in South Korea. J Affect Disord. 2019;257:486–494. doi:10.1016/j.jad.2019.07.003
27. Chia CW, Egan JM, Ferrucci L. Age-Related Changes in Glucose Metabolism, Hyperglycemia, and Cardiovascular Risk. Circ Res. 2018;123(7):886–904. doi:10.1161/CIRCRESAHA.118.312806
28. Koponen H, Kautiainen H, Leppanen E, Mantyselka P, Vanhala M. Association between suicidal behaviour and impaired glucose metabolism in depressive disorders. BMC Psychiatry. 2015;15:163. doi:10.1186/s12888-015-0567-x
29. Chen SW, Li X, Lang X, Li J, Zhang XY. Metabolic parameters and thyroid hormones in relation to suicide attempts in patients with first-episode and drug-naive major depressive disorder with comorbid glucose disturbances: a large cross-sectional study. Eur Arch Psychiatry Clin Neurosci. 2023;273(1):199–207. doi:10.1007/s00406-022-01490-w
30. Wang B, An X, Shi X, Zhang JA. MANAGEMENT OF ENDOCRINE DISEASE: suicide risk in patients with diabetes: a systematic review and meta-analysis. Eur J Endocrinol. 2017;177(4):R169–R181. doi:10.1530/EJE-16-0952
31. Russell KS, Stevens JR, Stern TA. Insulin overdose among patients with diabetes: a readily available means of suicide. Prim Care Companion J Clin Psychiatry. 2009;11(5):258–262. doi:10.4088/PCC.09r00802
32. Marano CM, Workman CI, Lyman CH, et al. The relationship between fasting serum glucose and cerebral glucose metabolism in late-life depression and normal aging. Psychiatry Res. 2014;222(1–2):84–90. doi:10.1016/j.pscychresns.2014.01.009
33. Li C, Lumey LH. Impact of disease screening on awareness and management of hypertension and diabetes between 2011 and 2015: results from the China health and retirement longitudinal study. BMC Public Health. 2019;19(1):421. doi:10.1186/s12889-019-6753-x
34. Gao W, Deng Z, Cai X, Zhang D, Xiao H, Zhang X. Clinical correlates and metabolic indicators of elevated fasting glucose in overweight/obese Chinese Han patients with first-episode and drug-naive major depressive disorder. Front Endocrinol (Lausanne). 2023;14:1102670. doi:10.3389/fendo.2023.1102670
35. Perry BI, Salimkumar D, Green D, et al. Associated illness severity in schizophrenia and diabetes mellitus: a systematic review. Psychiatry Res. 2017;256:102–110. doi:10.1016/j.psychres.2017.06.027
36. Yang W, Liu M, Tian Y, et al. The increased prevalence of depression and anxiety in T2DM patients associated with blood glucose fluctuation and sleep quality. BMC Endocr Disord. 2022;22(1):232. doi:10.1186/s12902-022-01147-8
37. Grillo CA, Piroli GG, Lawrence RC, et al. Hippocampal Insulin Resistance Impairs Spatial Learning and Synaptic Plasticity. Diabetes. 2015;64(11):3927–3936. doi:10.2337/db15-0596
38. Rawlinson S, Andrews ZB. Hypothalamic insulin signalling as a nexus regulating mood and metabolism. J Neuroendocrinol. 2021;33(4):e12939. doi:10.1111/jne.12939
39. Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8(4):383–395. doi:10.31887/DCNS.2006.8.4/ssmith
40. Amin M, Ott J, Gordon D, et al. Comorbidity of Novel CRHR2 Gene Variants in Type 2 Diabetes and Depression. Int J Mol Sci. 2022;23(17):56.
41. Kim MD, Yang HJ, Kang NR, Park JH, Jung YE. Association between subclinical hypothyroidism and metabolic syndrome among individuals with depression. J Affect Disord. 2020;264:494–497. doi:10.1016/j.jad.2019.11.080
42. Xu C, Zhou L, Wu K, et al. Abnormal Glucose Metabolism and Insulin Resistance Are Induced via the IRE1alpha/XBP-1 Pathway in Subclinical Hypothyroidism. Front Endocrinol (Lausanne). 2019;10:303. doi:10.3389/fendo.2019.00303
43. Akin S, Boluk C. Prevalence of comorbidities in patients with type-2 diabetes mellitus. Prim Care Diabetes. 2020;14(5):431–434. doi:10.1016/j.pcd.2019.12.006
44. Mata-Cases M, Franch-Nadal J, Real J, Cedenilla M, Mauricio D. Prevalence and coprevalence of chronic comorbid conditions in patients with type 2 diabetes in Catalonia: a population-based cross-sectional study. BMJ Open. 2019;9(10):e31281. doi:10.1136/bmjopen-2019-031281
45. Lee JJ, Beretvas SN, Freeland-Graves JH. Abdominal adiposity distribution in diabetic/prediabetic and nondiabetic populations: a meta-analysis. J Obes. 2014;2014:697264. doi:10.1155/2014/697264
46. Despres JP, Lemieux I, Bergeron J, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28(6):1039–1049. doi:10.1161/ATVBAHA.107.159228
47. Alshehri T, Boone S, de Mutsert R, et al. The association between overall and abdominal adiposity and depressive mood: a cross-sectional analysis in 6459 participants. Psychoneuroendocrino. 2019;110:104429. doi:10.1016/j.psyneuen.2019.104429
48. Cheung BM, Li C. Diabetes and hypertension: is there a common metabolic pathway? Curr Atheroscler Rep. 2012;14(2):160–166. doi:10.1007/s11883-012-0227-2
49. Przezak A, Bielka W, Pawlik A. Hypertension and Type 2 Diabetes-The Novel Treatment Possibilities. Int J Mol Sci. 2022;23(12):6500. doi:10.3390/ijms23126500
50. Zhou L, Ding S, Li Y, et al. Endoplasmic Reticulum Stress May Play a Pivotal Role in Lipid Metabolic Disorders in a Novel Mouse Model of Subclinical Hypothyroidism. Sci Rep. 2016;6:31381. doi:10.1038/srep31381
51. Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA. Clinical depression and inflammatory risk markers for coronary heart disease. Am J Cardiol. 2002;90(12):1279–1283. doi:10.1016/S0002-9149(02)02863-1
52. Vogelzangs N, Kritchevsky SB, Beekman AT, et al. Depressive symptoms and change in abdominal obesity in older persons. Arch Gen Psychiatry. 2008;65(12):1386–1393. doi:10.1001/archpsyc.65.12.1386
53. Tirosh A, Shai I, Bitzur R, et al. Changes in triglyceride levels over time and risk of type 2 diabetes in young men. Diabetes Care. 2008;31(10):2032–2037. doi:10.2337/dc08-0825
54. Deng B, Yuan Y, Zhong M, Ren R, Deng W, Duan X. The Relationship Between Metabolic Parameters, Age, and Thyroid Status: a Cross-Sectional Study-Based National Survey of Iodine Nutrition, Thyroid Disease. Risk Manag Healthc Policy. 2021;14:1723–1730. doi:10.2147/RMHP.S306122
55. Garcia-Rizo C, Fernandez-Egea E, Miller BJ, et al. Abnormal glucose tolerance, white blood cell count, and telomere length in newly diagnosed, antidepressant-naive patients with depression. Brain Behav Immun. 2013;28:49–53. doi:10.1016/j.bbi.2012.11.009
56. Salvi V, Grua I, Cerveri G, Mencacci C, Barone-Adesi F. The risk of new-onset diabetes in antidepressant users - A systematic review and meta-analysis. PLoS One. 2017;12(7):e182088. doi:10.1371/journal.pone.0182088
57. American Diabetes Association. Standards of medical care in diabetes--2009. Diabetes Care. 2009;32 Suppl 1(Suppl 1):S13–S61. doi:10.2337/dc09-S013
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.