Back to Journals » International Journal of Nanomedicine » Volume 15
Inactivation of Non-Enveloped Viruses and Bacteria by an Electrically Charged Disinfectant Containing Meso-Structure Nanoparticles via Modification of the Genome
Authors Sakudo A, Yamashiro R, Haritani M, Furusaki K, Onishi R , Onodera T
Received 4 September 2019
Accepted for publication 24 December 2019
Published 28 February 2020 Volume 2020:15 Pages 1387—1395
DOI https://doi.org/10.2147/IJN.S229880
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Akikazu Sakudo,1,2 Risa Yamashiro,2 Makoto Haritani,3 Koichi Furusaki,4 Rumiko Onishi,5 Takashi Onodera3
1Faculty of Veterinary Medicine, Okayama University of Science, Imabari, Ehime 794-8555, Japan; 2Laboratory of Biometabolic Chemistry, School of Health Sciences, University of the Ryukyus, Nishihara, Okinawa 903-0215, Japan; 3Research Center for Food Safety, The University of Tokyo, Bunkyo-Ku, Tokyo 113-8657, Japan; 4Mineral Activation Technical Research Center, Omuta, Fukuoka 836-0041, Japan; 5Santa Mineral Co., Ltd., Minato-Ku, Tokyo 105-0013, Japan
Correspondence: Akikazu Sakudo
Faculty of Veterinary Medicine, Okayama University of Science, Imabari, Ehime 794-8555, Japan
Tel/Fax +81-898-52-9198
Email [email protected]
Introduction: A previous study demonstrated the virucidal effect of an electrically charged disinfectant (CAC-717), which contains meso-structure nanoparticles, on enveloped viruses (influenza viruses). However, the effect of CAC-717 on other microorganisms and the mechanisms by which CAC-717 inactivates the microorganisms remain unclear. In this study, CAC-717 was further evaluated in terms of its biocidal and virucidal activity as well as its effect on bacterial and viral nucleic acids.
Methods: The inactivation effects of CAC-717 against various microorganisms [non-enveloped virus, feline calicivirus (FCV); bacteria, Salmonella enterica and Escherichia coli] were investigated by comparing the viral titer of the medium tissue culture infectious dose (TCID50) and the D value (estimated treatment time required to reduce the number of microorganisms by 90%). Furthermore, the effects of CAC-717 on viral and bacterial genomic RNA/DNA were examined using a polymerase chain reaction (PCR).
Results: Treatment of an equal volume of CAC-717 with cell lysate infected with a non-enveloped virus, feline calicivirus (FCV), reduced the TCID50. Viral titer dropped below the detection limit after 2 min of treatment. The D value of FCV was 0.256 min (average of multiple endpoint D values) and endpoint D value was 0.341 min. The D value for E. coli and S. enterica was 0.290 min and 0.080 min (average of multiple endpoint D values), respectively and the endpoint D value was 0.545 min and 0.054 min, respectively. In addition, PCR showed the inhibition of nucleic acid amplification of the RNA and DNA genome of FCV and bacteria, respectively.
Conclusion: Our findings suggest that CAC-717 inactivates viruses and bacteria by modifying the viral and bacterial nucleic acids.
Keywords: class I disinfectant, E. coli, feline calicivirus, food safety, meso-structure, Salmonella
Introduction
The development of the food industry and expansion of distribution channels in recent years has led to many more people consuming foods made in large production facilities. Consequently, the incidence of serious food poisoning involving large numbers of people traced to a single source is becoming more common. For example, ingestion of food contaminated with Escherichia coli O157:H7 or other serotypes can lead to life-threatening complications including hemorrhagic colitis (HC) and hemolytic uremic syndrome (HUS).1–4 Salmonella enterica causes gastroenteritis and typhoid fever,5 mostly due to ingestion of contaminated poultry and eggs. As well as a source of food, chicken eggs have been widely used for the production of vaccines in recent years. A technology that can disinfect Salmonella without heating is therefore needed. Furthermore, some non-enveloped viruses are commonly associated with food poisoning. For example, norovirus, which is resistant to alcohol disinfectants, is a foodborne agent for infectious gastroenteritis, where oysters and other shellfish are an important vehicle for transmission.6
Chlorine bleach (sodium hypochlorite) is generally recommended for the inactivation of a broad range of microorganisms including norovirus.7,8 However, application of this chemical generates toxic gases that can also act as an irritant and cause metal corrosion. Chemical disinfectants other than chlorine bleach may also be used, although they each have limitations in terms of safety and/or efficiency. For this reason, a safe and effective disinfectant against a broad range of microorganisms is required.
Recently, we generated a meso-structure comprising a fine particle structure of about 50 ~ 500 nm, primarily composed of carbon and calcium from mineral components derived from plants and soil.9 An electrically charged disinfectant (CAC-717) was then made by placing this meso-structure in water.9 CAC-717 has been demonstrated to inactivate enveloped viruses such as influenza viruses.9 Although free alkaline ions are present in CAC-717, upon contact with human and animal tissue the pH is almost physiological (i.e. neutral pH).9 Consequently, CAC-717 does not cause irritation in animal eyes.9 Therefore, by comparison with current chemical disinfectants, such as chlorine bleach, CAC-717 could be a safer alternative. Due to its potential effectiveness, CAC-717 could be useful in reducing the risk of food poisoning in the food and dairy industries. However, it remains unclear whether CAC-717 is effective against a broad range of microorganisms. Moreover, the mechanism(s) of action of CAC-717 need to be established.
In this study, the inactivation effects of CAC-717 against various microorganisms [non-enveloped virus, feline calicivirus (FCV); bacteria, S. enterica, and E. coli] were investigated. Furthermore, we examined the effects of CAC-717 on viral and bacterial genomic RNA/DNA to gain insight into the likely mechanism of inactivation.
Materials and Methods
Study Design
To enhance food safety, key hygiene control at each step in the processing of food is recommended wherever possible. In particular, the development of safe and efficient disinfectants that can be used to minimize microbiological risk is an essential requirement. These considerations prompted us to design and produce a novel disinfectant CAC-717, which was previously reported to have inactivating activity against influenza virus without irritation and corrosion. Here, we have extended this work by addressing a number of additional issues as follows. Firstly, we aimed to assess the effectiveness of CAC-717 by analyzing the inactivation of FCV and bacteria such as E. coli and S. enterica. We also investigated changes to the viral and bacterial nucleic acids induced by CAC-717 treatment to elucidate its potential mechanism of viral and bacterial inactivation.
Synthesis of the Electrically Charged Disinfectant (CAC-717)
CAC-717 (FDA/USA Regulation No. 880.6890 Class I disinfectant, Japan Patent No. 5778328)10,11 was synthesized as described previously.9 Briefly, mineral water containing calcium hydrogen carbonate was subjected to a continuous electric field at a voltage of 2×104 V for 48 h using a Teflon insulation-coated electrostatic field electrode (N-800N, Mineral Activation Technical Research Center, Omuta, Fukuoka, Japan; Japan Patent No. 5864010).12 The obtained solution was CAC-717 as a 1x solution. The 1x CAC-717 was used in all the assays described in this study. The CAC-717 contains 6.9 mM calcium hydrogen carbonate particles with a mesoscopic structure and a pH of approximately 12.5.9,13
Viruses and Bacteria
The FCV F9 strain [VR-782; ATCC (American Type Culture Collection (ATCC), Manassas, VA, USA)], Escherichia coli HST04 (Takara Bio Inc., Otsu, Japan), and S. enterica serovar Abony NCTC 6017 (Microbiologics Inc., St Cloud, MN, USA) were used as test microorganisms.
Preparation of Bacterial Suspensions
E. coli on Luria-Bertani (LB) agar medium (Nacalai Tesque Inc., Kyoto, Japan) incubated at 37ºC for 24 h or S. enterica in buffered peptone water (Merk & Co., Kenilworth, NJ, USA) incubated at 35ºC for 24 h were collected and suspended in 1 mL of distilled water to obtain a bacterial suspension.
Preparation of FCV-Infected Cell Lysate
Crandell-Rees feline kidney-cell (CRFK) cells (CCL-94; ATCC) were maintained in minimum essential medium (MEM) supplemented with 10% fetal bovine serum (FCS) (JRH Biosciences Inc., Saint Louis, MO, USA). Penicillin-streptomycin (PS) was added to MEM as 100 units/mL of penicillin, 100 μg/mL of streptomycin (Nakalai Tesque Inc.). CRFK cells (3 × 105 cells) were grown in 100 mm dishes and adsorbed with FCV at a MOI (multiplicity of infection) of 0.1 for 1 h. The medium was exchanged with 2% FCS and PS-supplemented MEM medium and cultured at 37°C under 5% CO2 for 3 days. The cultured FCV–infected cells were suspended in phosphate buffered saline (PBS) (Life Technologies, Carlsbad, CA, USA), centrifugally washed three times, and then freeze-thawed at −80°C. Thereafter, the collected cells were disrupted by passage through a 28 G injection needle and suspended in PBS to prepare an FCV–infected cell lysate.
Evaluation of CAC-717 Efficiency Against Viruses and Bacteria
A 10 µL aliquot of microorganism suspension (FCV, E. coli, or S. enterica) was mixed with 10 µL of 1× CAC-717. The mixture was incubated at 25°C for 0, 0.5, 1, 2, 5 or 10 min. Samples were quickly diluted with 1 mL of distilled water for S. enterica and E. coli or with 400 µL of PBS for FCV. Then, FCV, S. enterica and E. coli were subjected to various assays described below.
Counting of Viable Bacterial Number
The number of colony forming units (CFU) was calculated after culturing for 24 h at 37°C using LB agar medium for E. coli14 or culturing for 48 h at 35°C using sheet medium (Sanita-kun; JNC Corporation, Tokyo, Japan) for S. enterica in accordance with the supplier’s instructions.15
Scanning Electron Microcopy (SEM)
CAC-717 was treated with 2% glutaraldehyde/0.1 M phosphate buffer for at least 14 h at 4°C followed by treatment with 2% osmium tetroxide solution for 2 h, and then dehydration with 30–100% ethanol at room temperature. The samples were subsequently subjected to drying with tert-butyl alcohol before depositing a coat of osmium plasma. SEM was performed using a JSM-7500F electron microscope (JEOL Ltd., Tokyo, Japan) at 5 kV and ×100,000 magnification.
Calculation of the Viral Titer of FCV
Samples were diluted 10-fold with PBS and added to CRFK cells (7.5 × 103 cells/well) seeded on a 96-well microtiter plate. Cells were then cultivated at 37°C under 5% CO2 for 3 days. A cytopathic effect (CPE) was observed and TCID50 (median tissue culture infectious dose) was calculated from the CPE based on the Behrens-Kärber method.16
DNA Extraction of Bacteria and Polymerase Chain Reaction (PCR)
Bacterial DNA of E. coli and S. enterica was extracted by heat treatment at 95 ° C for 15 min. Bacterial 16S rDNA PCR kit fast (800) (Takara Bio Inc.) was used for amplification of E. coli 16S rDNA according to the manufacturer’s guidelines,17 while One shot PCR (Takara Bio Inc.) was used to amplify the invasion protein gene (invA) of S. enterica as described previously.18 After DNA gel electrophoresis, band intensities were calculated using image data analysis software (ImageJ version 1.52a; National Institute of Health, Bethesda, MD, USA). Band intensities were determined as an average field intensity value after adjustment of images to a standard threshold value. The band intensity at 0 min in E. coli or S. enterica was set to 100% and comparison made with the corresponding bands at 0.5, 1, 2, 5, and 10 min.
Viral RNA Extraction of FCV and Real-Time PCR
Viral RNA of FCV was extracted using a QIAamp Viral RNA mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. RNA was eluted into 60 μL of nuclease-free water and transcribed with PrimeScriptII 1st strand cDNA Synthesis kit (Takara Bio Inc.) using random primers to generate the cDNA. The following temperature regime was employed: 65°C for 5 min; 4°C for 5 min; 42°C for 60 min. The resultant cDNA was analyzed by real-time PCR using SYBR Premix Ex Taq II (Tli RNase H plus) (Takara Bio Inc.) according to the manufacturer’s instructions. The real-time PCR components included SYBR Premix Ex Taq II as well as forward and reverse gene primers targeted the open reading frame 1 (ORF1) region of FCV (83 bp) encoding the nonstructural protein of FCV (GenbankM86379). The primers had the following sequences: Forward primer (FCV-F1): 5ʹ-TAATTCGGTGTTTGATTTGGCCTGGGCT-3ʹ; Reverse primer (FCV-R1): 5ʹ-CATATGCGGCTCTGATGGCTTGAAACTG-3ʹ. Real-time PCR was performed using a Thermal Cycler Dice Real Time System (Takara Bio Inc.) as described previously.19 The cycling program included initial denaturation at 95°C for 30 sec followed by 40 cycles of 95°C for 30 sec and 60°C for 30 sec. Each reaction was carried out more than three times and the results were analyzed using Thermal Cycler Dice Realtime System Single software (Takara Bio Inc.). The relative DNA levels of each sample were compared with serially diluted viral cDNA and estimated using the standard curve of diluted viral cDNA versus absorbance. PCR specificity was verified by dissociation curve analysis of the amplified DNA fragments of step 1 (95°C/15 sec), step 2 (60°C/30 sec), and step 3 (95°C/15 sec).
DNA Sequencing
The amplified products obtained by PCR or real-time PCR were verified by direct DNA sequencing after gel extraction using a QIAquick Gel Extraction Kit (Qiagen) or after subcloning into Takara T-Vector pMD20 (Takara Bio Inc.), respectively. Sequencing was performed on an ABI373OXL Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
Indirect Immunofluorescence Assay
Detection of FCV in FCV–infected CRFK cells was performed by indirect immunofluorescence assays. After incubation with samples for 1 day, cells were fixed with 4% paraformaldehyde for 20 min and cold methanol for 10 min. Cells were blocked with 3% bovine serum albumin (BSA) for 30 min and incubated with anti-FCV antibody (ab33990 [FCV1-43]; Abcam, Cambridge, UK) at 1: 100 dilution in PBS for 1 h at 37°C. Cells were subsequently labeled with fluorescein isothiocyanate (FITC)-labeled donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) at 1: 200 dilution by PBS for 30 min at 37°C. The stained cell monolayer was visualized at a magnification of x20 under fluorescence microscopy (Biozero BZ-8100; Keyence, Osaka, Japan) with excitation of 480/30 nm and emission of 510 nm. Exposure times were held constant at 4 sec throughout the entire experiment. Quantification was performed using ImageJ version 1.52a software (National Institute of Health). Fluorescent images were adjusted to a standard threshold value. Green intensities in the image were given an average field intensity value. The intensity at 0 min was taken as 100% and then compared with that at 1, 2, and 5 min.
Calculation of the Average of Multiple Endpoint D Values
The endpoint was considered the first point at which all replicates showed no growth (<10 CFU/mL or <10 TCID50/mL). The D value was calculated according to the treatment time required to lower the number of microorganisms to 1/10. The following formula, which is modified from a previous report,20 was used to calculate the D value.
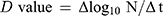
where Δlog10N: logarithm of the change in the number of microorganisms, Δt: change in time, N: number of microorganisms.
Endpoint D values were calculated using the same formula for the D value between the endpoint and 0 min. The average of multiple endpoint D values was calculated using the same formula for endpoint D value between each time point and 0 min, and then an average was taken.20
Statistical Analysis
All data were collected and assembled in an Excel spreadsheet for statistical analysis. The statistical analysis of significant difference was performed by non-repeated measured analysis of variance (ANOVA) followed by Bonferroni correction. The analysis was carried out using the GraphPad Prism 7 software (GraphPad Prism Software Inc., La Jolla, CA, USA). The obtained results are the mean ± standard error of mean (SEM) of experiments conducted at least in triplicate.
Results
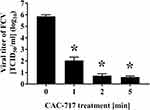
Firstly, SEM analysis of CAC-717 was performed, which showed it to contain aggregated nanoparticles (approximately 50 nm in size) (Figure 1). The change of FCV infectivity after treatment with an equal volume of CAC-717 was also investigated. A 10 μL aliquot of virus-infected cell lysate was mixed with 10 μL of CAC-717 for 0, 1, 2, or 5 min (Figure 2). After treatment, the suspension was recovered with 400 μL of PBS and the TCID50 determined. The results showed that the initial viral titer of FCV (0 min) was 7.26×105 ± 2.70×105 TCID50/mL. CAC-717 treatment caused a significant decrease in viral titer: 1.09×102 ± 1.04×102 TCID50/mL at 1 min; below the detection limit (less than 10 TCID50/mL) at 2 and 5 min. From the data, the D value, which is the time required to achieve 90% reduction of viral titer, was calculated. The average of multiple endpoint D values was used to estimate the D value as described in Materials and Methods, which was 0.256 min, while the endpoint D value was 0.341 min.
Furthermore, an immunofluorescent assay using anti-FCV antibody against FCV capsid protein was performed after incubation of CRFK cells with CAC-717-treated FCV (Figure 3). Proliferation of FCV in CRFK cells was observed after incubation with untreated cell lysate (0 min) for 24 h. However, reduced FCV proliferation was observed in CRFK cells after incubation with cell lysate exposed to CAC-717 treatment in a treatment time-dependent manner. The average field intensity value in the green fluorescent image at 0 min was set to 100%, while those at 1, 2, and 5 min were 0.17%, 0.08%, and 0.01%, respectively. These findings suggested that CAC-717 treatment decreased infectivity of FCV.
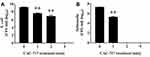
In addition, the effect of CAC-717 on viable cell number of bacteria, such as E. coli and S. enterica, was examined (Figure 4). Viable cell number after CAC-717 treatment was determined as described in Materials and Methods. The viable cell number of E. coli at 0 min was 1.52×109 ± 0.35×109 CFU/mL, but there was a significant decrease to 4.00×108 ± 1.58×108 CFU/mL at 1 min, 7.50×106 ± 2.50×106 CFU/mL at 2 min, and undetectable at 5 min (Figure 4A). Similarly, a bactericidal effect of CAC-717 on S. enterica was evaluated. Viable cell number of S. enterica was 2.14×107 ± 0.12×107 CFU/mL at 0 min, but 2.00×105 ± 0.58×105 CFU/mL at 1 min, and undetectable at 2 and 5 min (Figure 4B). Overall, the average of the multiple endpoint D values for E. coli and S. enterica were 0.290 min and 0.080 min, respectively. In addition, the endpoint D values for E. coli and S. enterica were 0.545 min and 0.054 min, respectively.
Moreover, the effect of CAC-717 treatment on DNA of E. coli and S. enterica was examined. PCR was used to amplify E. coli 16S rDNA and S. enterica invA to assess DNA damage caused by CAC-717 treatment for 0, 0.5, 1, 2, 5, and 10 min (Figure 5). The amplified band of E. coli 16S rDNA (800 bp) and S. enterica invA DNA (378 bp) was slightly less intense at 0.5 min and 1 min compared to 0 min and markedly less intense at 2, 5, and 10 min. Analysis using ImageJ software supported the results, which showed that band intensities were 100% at 0 min, 95.74% at 0.5 min, 90.04% at 1 min, 65.77% at 2 min, 31.38% at 5 min, and 4.13% at 10 min in E. coli. The corresponding values for S. enterica were 100% at 0 min, 99.90% at 0.5 min, 44.46% at 1 min, 38.75% at 2 min, 0% at 5 min, and 0% at 10 min. In addition, the obtained band of E. coli 16S rDNA was gel extracted and direct sequence analysis performed. As a result, the obtained DNA sequence of E. coli 16S rDNA was 99% identical (N=4) to that of E. coli strain HST 04, complete genome (ie identical to Genbank Accession number CP 013952), indicating that the amplified PCR product was derived from E. coli DNA. The 378-bp PCR product from S. enterica using the same PCR kit was previously confirmed to correspond to invA of Salmonella enterica subsp. enterica (identical to Genbank accession number EU348369) by DNA sequencing.18
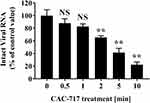
To further investigate the effect of CAC-717 treatment on virus, biochemical changes to the viral genome of FCV were analyzed (Figure 6). Real-time PCR using sequence-specific primers for FCV showed the detection of intact FCV RNA by PCR amplification in the untreated FCV sample (0 min). Dissociation curve analysis of the reaction products confirmed the specificity of the reaction. Reduced levels of intact FCV RNA were found in CAC-717 treated FCV for 0.5 and 1 min by comparison with the untreated FCV (100.00 ± 9.45% at 0 min; 88.09 ± 6.92% at 0.5 min; 82.89 ± 4.11% at 1 min), but the difference was not significant. However, a significant decrease was observed in intact FCV RNA after CAC-717 treatment for 2 min (65.56 ± 2.55%), 5 min (41.41 ± 4.00%), and 10 min (22.35 ± 2.42%).
Discussion
A previous study showed that influenza virus could be inactivated by CAC-717.9 The present results show that CAC-717 efficiently inactivates both E. coli and S. enterica as well as FCV. The findings from this study together with the previous study9 indicate that CAC-717 might effectively inactivate a broad range of microorganisms. Moreover, during the submission process for the present manuscript, CAC-717 was shown to inactivate mouse norovirus and human norovirus.13
FCV infectivity after CAC-717 treatment for 1 min decreased by more than 3 log10, satisfying the criteria listed by the US Environmental Protection Agency (US-EPA) for a virucidal agent (at least log10 reduction of FCV viral titer).21 Therefore, CAC-717 treatment is considered to be an effective virucidal method. The average of multiple endpoint D values for FCV after CAC-717 treatment was 0.256 min. The inactivation efficiency of FCV by CAC-717 was good even in comparison to sodium hypochlorite (675 ppm), where the D value after treatment at room temperature was 1.563 min22 and with heat treatment at 56°C was 6.7 min.23 This observation further confirms that CAC-717 is an efficient method for the inactivation of FCV, which is a non-enveloped virus. These findings are consistent with a recently published study using a non-enveloped virus, mouse norovirus and human norovirus, which were efficiently inactivated by CAC-717.13 In addition, previous studies showed that the infectious titer of influenza virus, which is an enveloped virus, decreased after treatment with CAC-717.9 Taken together, these results suggest that the inactivating effect of CAC-717 on viruses is unaffected by the presence or absence of an envelope.
In the case of Salmonella, the average of multiple endpoint D values with sodium hypochlorite (4 ppm) when used as a fungicide for chicken eggs mixed with Salmonella enterica was 0.195 min.24 However, CAC-717, with an average of multiple endpoint D values of 0.080 min, exerts a more powerful bactericidal effect than sodium hypochlorite.
Moreover, CAC-717 treatment causes damage to the FCV genomic RNA as well as to bacterial genomic DNA of E. coli and S. enterica. These findings infer that damage to the viral and bacterial genome, such as the mutation of nucleic acids, is involved in the reduction of infectivity. Furthermore, during submission of the present manuscript, the effect of CAC-717 on human norovirus RNA has been reported,13 which supports the overall conclusion of this study.
CAC-717 contains minerals forming a mesoscopic structure. The structure of the mineral elicits a bactericidal effect by electrolysis via self-discharge and hydrogen occlusion.9 Indeed, this characteristic may be related to the emission and absorption of terahertz waves when minerals extracted from plants and soil components possess a mesoscopic structure. Far-infrared radiation from mesoscopic structures appear to function as small “nanobatteries”.9 The terahertz radiation is thought to be absorbed by biomolecules such as proteins and carbohydrates as well as nucleic acids. The far-infrared radiation seems to, at least in part, contribute to the inactivation mechanisms of CAC-717. However, the precise damage to the nucleic acids as well as other biomolecules remains to be determined.
The mechanisms of action of CAC-717 are apparently different from any antibiotics, suggesting that CAC-717 may be effective in inactivating antibiotic resistant strains of harmful bacteria.25–28 However, further studies using various antibiotic-resistant bacteria would be required to confirm the effect of CAC-717 on these strains.
Bacterial and fungal toxins, such as Shiga toxins and aflatoxins, are known to cause food poisoning. If CAC-717 is effective at inactivating these toxins it could be used for cleaning and toxin removal as well as for disinfection. Consequently, CAC-717 could be a novel advanced disinfectant that can simultaneously inactivate pathogens and remove their associated toxins. However, further studies on CAC-717 are required to fully understand the inactivation mechanisms and evaluate the usefulness of CAC-717.
Previous observations using influenza virus indicated that the addition of 10% BSA to CAC-717 had no effect on viral inactivation.9 The effect of CAC-717 treatment on FCV in the presence of serum was further examined (Fig. S1). FCS (JRH Biosciences Inc., Saint Louis, MO, USA) at a concentration of 0%, 5%, 10%, 20%, 50% was mixed with FCV–infected cell suspension and subjected to CAC-717 treatment. After the treatment, infectivity was unchanged among 0-50% FCS in PBS (Fig. S1A) and CAC-717 (Fig. S1B). Thus, the FCV–inactivating effect of CAC-717 was maintained even in the presence of substantial amounts of serum proteins.
By using CAC-717, safe disinfection without irritation and corrosion may be possible. As CAC-717 is derived from plants and soils, safety of this disinfectant is thought to be high. Indeed, our previous study involved a toxicity test of CAC-717 using Organisation for Economic Co-operation and Development (OECD) guidelines for testing chemicals No.405; test for acute eye irritation29 and tests based on International Organization for Standardization (ISO).30,31 None of these tests using CAC-717 indicated any harmful effects. These findings suggest CAC-717 could make a valuable contribution as a safe and efficient disinfectant.
Conclusion
The present study suggests that CAC-717 can efficiently inactivate bacteria and non-enveloped viruses. In addition to an inactivation effect on enveloped viruses as shown in a previous study, CAC-717 might potentially be useful for the inactivation of a broad range of harmful bacteria and viruses. Furthermore, the current study shows CAC-717 damages nucleic acids of microorganisms. Optimizing the conditions of reaction and production of CAC-717 will inevitably enhance the efficiency of this disinfectant.
Acknowledgments
This work was supported in part by Grand-in-aids from Osimo Foundation and Kieikai Research Foundation. The authors would like to acknowledge Ms. Toko Tamanaha, Mr. Morihide Shiroma, Ms. Satoko Shinzato (Laboratory of Biometabolic Chemistry, School of Health Sciences, University of the Ryukyus) for assistance in collecting data.
Disclosure
K.F. and R.O. are employed by the Mineral Activation Technical Research Center and Santa Mineral Co., Ltd., respectively. The authors declare that they have no other conflicts of interest with the content of this article.
References
1. Boerlin P. Evolution of virulence factors in Shiga-toxin-producing Escherichia coli. Cell Mol Life Sci. 1999;56(9–10):735–741. doi:10.1007/s000180050020
2. Scallan E, Hoekstra RM, Angulo FJ, et al. Foodborne illness acquired in the United States–major pathogens. Emerg Infect Dis. 2011;17(1):7–15. doi:10.3201/eid1701.P11101
3. Gyles CL. Shiga toxin-producing Escherichia coli: an overview. J Anim Sci. 2007;85(13 Suppl):E45–E62. doi:10.2527/jas.2006-508
4. Hunt JM. Shiga toxin-producing Escherichia coli (STEC). Clin Lab Med. 2010;30(1):21–45. doi:10.1016/j.cll.2009.11.001
5. European Food Safety Authority (EFSA). The community summary report on trends and sources of zoonosis, zoonotic agents and food-borne outbreaks in the European Union in 2008. EFSA J. 2010;8:1496.
6. Patel MM, Hall AJ, Vinje J, Parashar UD. Noroviruses: a comprehensive review. J Clin Virol. 2009;44(1):1–8. doi:10.1016/j.jcv.2008.10.009
7. List G. United States Environmental Protection Agency (US-EPA) Registered Hospital Disinfectants Effective Against Norovirus (Norwalk-Like Virus). Washington (DC): US-EPA; 2016. Available from: https://www.epa.gov/sites/production/files/2016-06/documents/list_g_norovirus.pdf/.
8. Sanekata T, Fukuda T, Miura T, et al. Evaluation of the antiviral activity of chlorine dioxide and sodium hypochlorite against feline calicivirus, human influenza virus, measles virus, canine distemper virus, human herpesvirus, human adenovirus, canine adenovirus and canine parvovirus. Biocontrol Sci. 2010;15(2):45–49. doi:10.4265/bio.15.45
9. Nakashima R, Kawamoto M, Miyazaki S, et al. Evaluation of calcium hydrogen carbonate mesoscopic crystals as a disinfectant for influenza A viruses. J Vet Med Sci. 2017;79(5):939–942. doi:10.1292/jvms.16-0603
10. US Food and Drug Administration. Title21-Food and Drugs, Chapter I-Food and Drug Administration, Department of Health and Human Services, Subchapter H-Medical Devices, Part880-General Hospital and Personal Use Devices, Subpart G-General Hospital and Personal Use Miscellaneous Devices, Sec. 880.6890 General Purpose Disinfectants. New Hampshire Avenue, Silver Spring, MD, USA: U.S. Food and Drug Administration; 2019. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?FR=880.6890.
11. Furusaki K, inventor; Riken Techno System, Santa Mineral Inc., patentee. The pest control method of functional water and unicellular organisms. Japanese patent JP 5778328. 2015 Jul 17.
12. Furusaki K, inventor; Riken Techno System, Santa Mineral Inc., patentee. The method for producing mineral water. Japanese patent JP 5864010. 2016 Jan 8.
13. Shimakura H, Gen-Nagata F, Haritani M, et al. Inactivation of human norovirus and its surrogate by the disinfectant consisting of calcium hydrogen carbonate mesoscopic crystals. FEMS Microbiol Lett. 2019;366:fnz235. doi:10.1093/femsle/fnz235
14. Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual.
15. Instruction Manual of Sanita-kun [homepage on the Internet]. Tokyo, Japan: JNC Corporation; 2019. Available from: https://www.jnc-corp.co.jp/sanita/english_pdf/InstructionManual.pdf.
16. Kärber G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Archiv f Experiment Pathol u Pharmakol. 1931;162:480–483. doi:10.1007/BF01863914
17. Bacterial 16S rDNA PCR Kit fast (800) Product Manual [homepage on the Internet]. Shiga, Japan: Takara Bio Inc.; 2019. Available from: https://www.takarabio.com/assets/documents/User%20Manual/RR182A_e.v1606Da.pdf.
18. Sakudo A, Chou H, Nagatsu M. Antibody-integrated and functionalized graphite-encapsulated magnetic beads, produced using ammonia gas plasma technology, for capturing Salmonella. Bioorg Med Chem Lett. 2015;25(5):1012–1016. doi:10.1016/j.bmcl.2015.01.031
19. Yamashiro R, Misawa T, Sakudo A. Key role of singlet oxygen and peroxynitrite in viral RNA damage during virucidal effect of plasma torch on feline calicivirus. Sci Rep. 2018;8(1):17947. doi:10.1038/s41598-018-36779-1
20. Sutton SV, Franco RJ, Porter DA, et al. D-value determinations are an inappropriate measure of disinfecting activity of common contact lens disinfecting solutions. Appl Environ Microbiol. 1991;57(7):2021–2026. doi:10.1128/AEM.57.7.2021-2026.1991
21. Initial virucidal effectiveness test using feline calicivirus as surrogate for norovirus [homepage on the Internet]. Washington, DC: Antimicrobials division US-EPA; 2015. Available from: https://www.epa.gov/sites/production/files/2015-09/documents/fcv1_initial_surf_pcol.pdf/.
22. Chiu S, Skura B, Petric M, McIntyre L, Gamage B, Isaac-Renton J. Efficacy of common disinfectant/cleaning agents in inactivating murine norovirus and feline calicivirus as surrogate viruses for human norovirus. Am J Infect Control. 2015;43(11):1208–1212. doi:10.1016/j.ajic.2015.06.021
23. Cannon JL, Papafragkou E, Park GW, Osborne J, Jaykus LA, Vinje J. Surrogates for the study of norovirus stability and inactivation in the environment: A comparison of murine norovirus and feline calicivirus. J Food Prot. 2006;69(11):2761–2765. doi:10.4315/0362-028X-69.11.2761
24. Hines JD, McKelvey PJ, Bodnaruk PW. Inappropriate use of D-values for determining biocidal activity of various antimicrobials. J Food Sci. 2011;76(1):M8–M11. doi:10.1111/j.1750-3841.2010.01923.x
25. Barlaam A, Parisi A, Spinelli E, Caruso M, Taranto PD, Normanno G. Global emergence of colistin-resistant Escherichia coli in food chains and associated food safety implications: A review. J Food Prot. 2019;82(8):1440–1448. doi:10.4315/0362-028X.JFP-19-116
26. Davin-Regli A, Lavigne JP, Pages JM. Enterobacter spp.: update on taxonomy, clinical aspects, and emerging antimicrobial resistance. Clin Microbiol Rev. 2019;32(4). doi:10.1128/CMR.00002-19
27. Hasannejad-Bibalan M, Mojtahedi A, Biglari H, Halaji M, Sedigh Ebrahim-Saraie H. Antibacterial activity of tedizolid, a novel oxazolidinone against methicillin-resistant Staphylococcus aureus: A systematic review and meta-analysis. Microb Drug Resist. 2019;25:1330–1337. doi:10.1089/mdr.2018.0457
28. Peyclit L, Baron SA, Rolain JM. Drug repurposing to fight colistin and carbapenem-resistant bacteria. Front Cell Infect Microbiol. 2019;9:193. doi:10.3389/fcimb.2019.00193
29. Organisation for Economic Co-operation and Development (OECD) guideline for the testing of chemicals: Acute eye irritation/corrosion, No.405 [homepage on the Internet]. Paris: OECD; 2017. Available from: https://www.oecd.org/env/test-no-405-acute-eye-irritation-corrosion-9789264185333-en.htm/.
30. Biological evaluation of medical devices – Part 10: Tests for irritation and skin sensitization International Organization for Standardization (ISO) 10993-10:2010 [homepage on the Internet]. Geneva: ISO; 2010. Available from: https://www.iso.org/standard/40884.html/.
31. Biological evaluation of medical devices - Part 2: Animal welfare requirements, ISO10993-2:2006 [homepage on the Internet]. Geneva: ISO; 2006. Available from: https://www.iso.org/standard/36405.html/.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.