Back to Journals » Infection and Drug Resistance » Volume 16
In vitro Antimicrobial Activity and Dose Optimization of Eravacycline and Other Tetracycline Derivatives Against Levofloxacin-Non-Susceptible and/or Trimethoprim-Sulfamethoxazole-Resistant Stenotrophomonas maltophilia
Authors Wu J, Zhang G, Zhao Q, Wang L, Yang J, Cui J
Received 26 June 2023
Accepted for publication 31 August 2023
Published 8 September 2023 Volume 2023:16 Pages 6005—6015
DOI https://doi.org/10.2147/IDR.S425061
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Jie Wu,1,2 Guangcun Zhang,3 Qiang Zhao,3 Lifeng Wang,3 Jiyong Yang,3 Junchang Cui1
1Department of Respiratory Diseases, The Eighth Medical Center, Chinese People’s Liberation Army General Hospital, Beijing, People’s Republic of China; 2Medical School of Chinese People’s Liberation Army, Beijing, People’s Republic of China; 3Laboratory Medicine Department, The First Medical Center, Chinese People’s Liberation Army General Hospital, Beijing, People’s Republic of China
Correspondence: Junchang Cui, The Eighth Medical Center, Department of Respiratory Diseases, Chinese People’s Liberation Army General Hospital, No. 17 Heishanhu Road, Haidian District, Beijing, 100091, People’s Republic of China, Tel +86 010 6677 5010, Email [email protected]
Purpose: To better guide clinical use, we determined the in vitro antimicrobial activity of the new drug eravacycline and other tetracycline derivatives against levofloxacin (LVFX)-non-susceptible and/or trimethoprim-sulfamethoxazole (TMP-SMZ)-resistant Stenotrophomonas maltophilia and evaluated their dosing regimens.
Methods: Seventy-seven unique strains of S. maltophilia were isolated from sputa samples and airway aspirate samples that were either LVFX-non-susceptible and/or TMP-SMZ-resistant. Monte Carlo simulations were performed for different dosing regimens according to the population pharmacokinetic parameters of antibiotics in patients with respiratory tract infections at the minimum inhibitory concentration (MIC).
Results: Eravacycline had excellent in vitro antibacterial activity against LVFX-non-susceptible and/or TMP-SMZ-resistant S. maltophilia. Monte Carlo simulations showed that for LVFX-non-susceptible strains, the cumulative fraction of response (CFR) of minocycline at the conventional recommended dose of 100 mg q12 h was 90.90%; for TMP-SMZ-resistant strains, the CFR of minocycline at a high dose of 200 mg q12 h was only 91.64%. For strains resistant to both LVFX and TMP-SMZ, the CFR of minocycline at a high dose of 200 mg q12 h was 89.81%. In contrast, the CFR of tigecycline was less than 40%, even at a dose of 100 mg q12 h.
Conclusion: For pneumonia, minocycline is better for S. maltophilia that is non-susceptible to LVFX; for TMP-SMZ-resistant strains and strains that are not susceptible to either LVFX or TMP-SMZ, the efficiency of eravacycline requires further evaluation. Eravacycline may be a better choice for extremely resistant S. maltophilia strains that are non-susceptible to LVFX, TMP-SMZ, and minocycline.
Keywords: eravacycline, tetracyclines, levofloxacin, trimethoprim, sulfamethoxazole drug combination, Stenotrophomonas maltophilia, Monte Carlo method
Introduction
With the beginning of the postantibiotic era, Stenotrophomonas maltophilia, an opportunistic pathogen, has begun to develop a significant increase in resistance to commonly used first-line drugs by intrinsic and acquired resistance mechanisms, making the optimal clinical treatment option difficult to determine. Increasingly, resistant isolates to drugs with historically good susceptibility, such as levofloxacin (LVFX), trimethoprim-sulfamethoxazole (TMP-SMZ), and minocycline, are being reported.1–4 A resistance study in China comparing 300 S. maltophilia strains collected over two periods, 2005–2009 and 2010–2014, showed a significant increase in rates of resistance, with TMP-SMZ-resistance increasing from 29.7% to 47.1%, ceftazidime-resistance increasing from 28.9% to 52.3%, and a decrease in the percentage of minocycline-susceptible strains from 13.5% to 10.9%.5 In addition to increasing resistance rates, multiple adverse drug reactions and a lack of definitive pharmacokinetic/pharmacodynamic (PK/PD) data have limited the use of these antibiotics.6,7 Tetracycline derivatives, including minocycline, tigecycline, and eravacycline, are currently drugs to which strains have high sensitivity in vitro and show promise as an important option for treating non-susceptible S. maltophilia.8–11 National/global surveillance programs have been conducted to compare the in vitro minimum inhibitory concentration (MIC) of tetracycline derivatives against S. maltophilia. These surveillance programs suggest that our new drug, eravacycline, has potential for treating extremely drug-resistant strains. In vitro, the efficacy of eravacycline is superior to or similar to that of minocycline and tigecycline, especially for strains that are non-susceptible to LVFX, resistant to TMP-SMZ or non-susceptible to both.9,11,12 With respect to clinical dosing regimens for tetracycline derivatives, Zidaru et al suggests minocycline (the agent with the greatest fAUC24h /MIC90) be preferentially used in critically ill patients with bacteremia and/or sepsis. The f in fAUC24h /MIC90 represents the free fraction of the drug, and the AUC24h is the area under the plasma concentration-time curve from 0 to 24 h (mg ·h /L). Eravacycline can be used (alone or in combination with other drugs) for infections caused by resistant S. maltophilia when conventional therapy has failed.13
S. maltophilia infections are mainly associated with respiratory systems, such as pneumonia and acute exacerbations of chronic obstructive pulmonary disease (COPD).14–17 However, S. maltophilia is not covered by empirical antibiotic regimens for respiratory tract infections.16 In a joint guideline from the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) published in 2016, clinicians should adopt dosing regimens and administered doses based on the PK/PD of antimicrobial drugs rather than relying solely on drug instructions.18 Although the findings of Zidaru et al provide a good guide for clinical drug selection for S. maltophilia bloodstream infections, their sample included only 11 blood isolates, they did not specify whether the strains were drug resistant, and no specific dosing regimen was indicated.13 In this study, we compared the in vitro MIC values of LVFX-non-susceptible and/or TMP-SMZ-resistant S. maltophilia to that of various tetracycline derivatives, performed PK/PD evaluation of the dosing regimen of tetracycline derivatives for the treatment of respiratory tract infection using Monte Carlo simulation (MCS), and calculated the probability of the reaching target to identify the optimal regimen for the treatment of non-susceptible S. maltophilia infection.
Materials and Methods
Bacterial Strains
In this work, a total of 77 strains of S. maltophilia non-susceptible to LVFX and/or resistant to TMP-SMZ, isolated from sputa samples and airway aspirate samples between June 2020 and August 2022, were collected from the Clinical Microbiology Laboratory of the First Medical Center of the Chinese People’s Liberation Army General Hospital (Table 1). Susceptibility to clinically relevant antibiotics was determined using the VITEK 2 Compact automated microbiological analysis system (bioMérieux, Marcy-l’Étoile, France). After we screened the drug-resistant strains that met the requirements from the clinical strain database, we used the gold standard of drug sensitivity determination, the micro broth dilution method, to determine their MIC values, and further screened the strains that met the requirements according to the Clinical and Laboratory Standards Institute (CLSI) document M100-Ed32 (2022).19 Seventy-seven strains isolated in the clinic were non-susceptible to LVFX, resistant to TMP-SMZ, or non-susceptible to both of these substances. Non-susceptible strains included intermediate and drug-resistant strains according to CLSI. However, CLSI did not define intermediates susceptibility for TMP-SMZ, and only resistant strains were considered in this study. Strains that were not susceptible to LVFX were those with MICs ≥ 4 mg/L, including intermediate and resistant strains, for a total of 71 strains; strains that were resistant to TMP-SMZ were those with MICs ≥ 4/76 mg/L, for a total of 34 strains; and strains that were not susceptible to either were those that were not susceptible to LVFX with MICs ≥ 4 mg/L and resistant to TMP-SMZ with MICs ≥ 4/76 mg/L, a total of 28 strains. In addition, 7 strains were not susceptible to minocycline (MICs ≥ 8 mg/L). Because of the small number of strains that were not susceptible to a single drug, they were not grouped and statistically analyzed. Species-appropriate quality control strains were used to ensure that isolates met the standards recommended by the CLSI document M07-Ed11 (2018).20 Escherichia coli ATCC 25922 was used as the experimental quality control strain for minocycline and TMP-SMZ, and Pseudomonas aeruginosa ATCC 27853 was used as the control for the other drugs.
 |
Table 1 Type and Features of Isolated Strains |
Antimicrobial Susceptibility Testing
Eravacycline was purchased from MedChemExpress (USA), tigecycline was purchased from Shanghai Yuanye Technology & Biology Co., Ltd. (China), minocycline and doxycycline were purchased from the National Institutes for Food and Drug Control (China), and Mueller-Hinton Broth (MHB) lot 212322 medium was purchased from Becton, Dickinson and Company (USA). Stock solutions of each agent were prepared fresh in individual aliquots at the beginning of each week and stored frozen at −80°C. Cation-adjusted MHB was used within 12 hours after preparation. The MICs of minocycline, tigecycline, doxycycline, and eravacycline against S. maltophilia were determined by the microbroth dilution method, and the test was performed in strict accordance with the criteria of CLSI document M07-Ed11 (2018).20 Minocycline, tigecycline and doxycycline were diluted in a gradient of 10 concentrations with a concentration range of 0.5 mg/L-256 mg/L. Eravacycline was diluted in a gradient of 11 concentrations, and the concentration range was 0.03 mg/L-32 mg/L. The colonies were picked after reviving the strains. The colonies were inoculated in fresh sterilized broth, and enriched at 37°C for 6–8 hours. After the turbidity of the cultured bacteria solution was 0.5 McF, the bacterial suspension was inoculated in the drug susceptibility testing plate. The drug susceptibility testing plates were incubated for 20–24 hours in a constant temperature incubator at 37°C in an air environment.
Pharmacokinetic/Pharmacodynamic (PK/PD) Model
All pharmacokinetic parameters, associated standard deviations (SD), and protein binding indices were referenced from published studies (Table 2). The pharmacokinetic parameters for minocycline were from 12 patients with respiratory bacterial infections and those for tigecycline were from 89 patients with hospital-acquired pneumonia.21–26 Minocycline 100 mg q12 h and tigecycline 50 mg q12 h are the recommended dosing regimens based on treatment instructions (Table 3). For tetracycline derivatives, the chosen PK/PD ratio 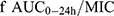 , which is considered the most likely predictor of efficacy,23,25 can be calculated by the following equation:
, which is considered the most likely predictor of efficacy,23,25 can be calculated by the following equation:
 |
Table 2 Summary of PK Parameters and PK/PD Target Level |
where dose24h is the dose administered over 24 h (mg), CL represents the total body clearance (L/h) and MIC is the minimum inhibitory concentration (mg/L).
Monte Carlo Simulation
In this study, MCSs were performed using Oracle Crystal Ball software (version: Oracle Crystal Ball 11.1.24) based on the PK/PD parameters and dosing regimens for minocycline and tigecycline. Simulations were performed assuming a random distribution for MICs, a log-normal distribution for pharmacokinetic parameters, and a uniform distribution for f, with 10,000 run calculations and confidence intervals set at 95%. The MCS results were generally expressed as the probability target attainment (PTA) for a given MIC and the cumulative fraction of response (CFR) for reaching a given target for the MIC population was calculated using the following equation:
where PTAi represents the probability of target attainment for each MIC value and Fi is the proportion of strains with this MIC in the population (i). A dosing regimen with a CFR greater than 90% is generally considered optimal for an antimicrobial drug against that bacterium.27
Results
Antimicrobial Susceptibility Testing
Table 4 displays the MIC50, MIC90, and MIC range of each agent against all 77 isolates. According to the drug sensitivity breakpoints from CLSI document M100-Ed32 (2022),19 69 of the 77 strains (89.6%) were susceptible to minocycline, and there were no defined breakpoints for the remaining the drugs. In each bacterial subgroup, eravacycline showed the highest in vitro efficacy with MIC50/90 values of 2/4 mg/L. The results of tigecycline were similar to that of eravacycline, but it had MIC50/90 values of 2/8 mg/L for the TMP-SMZ-resistant strains. Although minocycline had MIC50 values of 1–2 mg/L, its MIC90 was one to two gradients higher than that of eravacycline due to the presence of a minocycline-resistant strain. For LVFX-non-susceptible, TMP-SMZ-resistant, minocycline-non-susceptible strains, the MIC50/90 values were 4/16 mg/L.
 |
Table 4 Activities of Selected Agents Against Clinical S. maltophilia Isolates Non-Susceptible to Levofloxacin and/or Trimethoprim-Sulfamethoxazole |
Probability Target Attainment
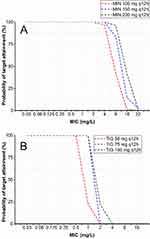
Due to the lower in vitro efficacy of doxycycline compared to the other three drugs and the lack of pharmacokinetic parameters associated with eravacycline for the treatment of respiratory tract infections, MCSs were performed in this study for only minocycline and tigecycline (Figure 1A and B). When simulating the recommended dose of minocycline (100 mg q12 h), the PTA with MIC ≤ 4 mg/L was 96.58%; at an MIC value up to 8 mg/L, the PTA decreased rapidly to 41.82%; and at an MIC of 16 mg/L, the PTA was only 1.31%. The recommended dose of tigecycline (50 mg q12 h) had a PTA greater than 99% at an MIC ≤ 0.5 mg/L; when the MIC increased to 1 mg/L, the PTA was 71.83%; and at an MIC of 2 mg/L, the PTA was 11.6%. We increased the simulated dose of administration and found that the PTA in each MIC group also increased. When the simulated minocycline dose was increased to the maximum recommended dose of 200 mg q12 h, the PTA for the MIC of 8 mg/L was almost 2.3 times higher than the recommended dose, reaching 96.27%. For tigecycline simulation at a dose of 75 mg q12 h, the PTA for the MIC of 1 mg/L was elevated nearly 4.3 times higher than the recommended dose, and it reached 100%.
Cumulative Fraction of Response
According to the calculation formula, combined with the frequency of MIC distribution (Figure 2A–F) and the corresponding PTA (Figure 1A and B), we calculated the CFRs for different dosing regimens (Table 3). First, for LVFX-non-susceptible strains, the CFR of minocycline at 100 mg q12 h reached 90.90%, while the CFR of tigecycline at 100 mg q12 h was only 27.29%. Second, for TMP-SMZ-resistant strains, it was necessary to increase the minocycline dose to 200 mg q12 h to achieve a CFR of 91.64%, but the CFR of tigecycline at a dose of 100 mg q12 h was only 39.17%. Third, for LVFX-non-susceptible and TMP-SMZ-resistant strains, the CFR of minocycline at 200 mg q12 h reached 89.81%, while the CFR of tigecycline at 100 mg q12 h was only 32.51%.
Discussion
Respiratory infections caused by S. maltophilia strains that are non-susceptible to LVFX and/or resistant to TMP-SMZ pose a serious threat to immunocompromised patients. Tetracycline derivatives are increasingly used in clinical therapy, but there are fewer data related to drug-resistant bacteria. This study examined the MIC values of tetracycline derivatives against our LVFX-non-susceptible and/or TMP-SMZ-resistant S. maltophilia and evaluated their treatment regimens for respiratory infections based on MCSs with PK/PD parameters.
For tetracyclines, the pH, calcium ion and magnesium ion content of the MHB affects the final MIC value. For tigecycline, whether the MHB is freshly prepared or not will also affect the drug sensitivity results, and nonfreshly prepared MHB will make the MIC value higher.19 Therefore, we required that the MIC values of quality control bacteria were within the quality control range to ensure the validity of the experiments. In this study, among the four tetracycline derivatives, eravacycline showed the highest in vitro activity, followed by tigecycline, minocycline, and doxycycline, especially in TMP-SMZ-resistant strains of S. maltophilia. The MIC of eravacycline reported in this study is slightly lower than the MICs of the 41 LVFX-non-susceptible and/or TMP-SMZ-resistant S. maltophilia strains that were assessed by Biagi et al (MIC50, 2 mg/L, MIC90, 8 mg/L), but the MIC90 results for minocycline reported here are higher than what was reported in their study (MIC50, 2 mg/L, MIC90, 4 mg/L).9 In the absence of specific CLSI or EUCAST breakpoints for eravacycline, the level of resistance was not interpreted to avoid misestimation of the true resistance rate. However, the low MIC values (≤4 mg/L) suggest that eravacycline is highly effective against the strains in our collection, which indicates that eravacycline may be an acceptable therapeutic choice against LVFX-non-susceptible and/or TMP-SMZ-resistant S. maltophilia infections; eravacycline should also be considered for routine susceptibility screening in clinical microbiology laboratories. The IDSA recommends minocycline as a first line agent,28 and the rate of susceptibility to minocycline in this study agrees with the results of Biagi et al and Flamm et al;9,10 however, minocycline-non-susceptible strains are rapidly emerging. We identified seven clinical strains that were not susceptible to LVFX, TMP-SMZ, or minocycline, and eravacycline showed good in vitro activity (MIC ≤ 4 mg/L) against 5 of these strains. Only one strain with a high eravacycline MIC (MIC 16 mg/L) was collected in our strain set, and such specific resistant strains are rarely collected based on the literature, so the exact mechanism of resistance should be further investigated.
Eravacycline is a new fluorocycline drug with a broad antimicrobial spectrum that overcomes the common mechanisms of tetracycline resistance; however, studies on the efficacy of this drug against non-susceptible S. maltophilia infections are rarely reported.12,29,30 The results of this study demonstrate that eravacycline effectively inhibits the growth of non-susceptible S. maltophilia in vitro, and clinical trial results have shown good clinical efficacy of eravacycline in the treatment of severe intra-abdominal infections, including infections caused by extended-spectrum β-lactamase (ESBL)-producing bacteria.31–33 This suggests that in the face of increasing drug resistance, eravacycline holds promise as an emerging drug for the treatment of non-susceptible S. maltophilia respiratory tract infections. Clinical PK/PD targets are generally used as targets for optimal efficacy. If clinical PK/PD targets are not available, animal PK/PD targets are preferred and are calculated by measuring blood concentrations in animal models of infections with different MIC values treated by different administration methods. However, only PK/PD targets obtained for eravacycline in a mouse model of thigh infection have been examined,31 and no animal model of respiratory tract infection is available. Meanwhile, studies have only provided pharmacokinetic parameters for eravacycline in healthy humans,34,35 without clinical data for patients with respiratory tract infections. The feasibility of performing efficacy simulations for the administration regimen of eravacycline is low and the confidence level is poor. Studies clarified drug clearance and protein binding in healthy volunteers receiving an infusion of 1 mg/kg q12h eravacycline (Table 2). Additionally, for the non-susceptible bacteria, the MIC of eravacycline measured in this study was close to that of tigecycline. According to the results calculated by the PK/PD formula, the infusion of 1 mg/kg q12h of eravacycline in healthy volunteers closely resembled that of patients with respiratory tract infections using the recommended tigecycline regimen. Combined with the PK/PD target values for the comparable drug tigecycline, it is reasonable to assume that the clinical efficacy achieved by these two regimens is similar and that adequate pharmacodynamic exposure cannot be achieved. The correlation coefficients of AUC24h/MIC and Cmax/MIC with antibacterial response are greater than 0.8, suggesting that both parameters are highly correlated with antibacterial response;36 therefore, when the AUC24h/MIC is difficult to determine, the Cmax value combined with in vitro drug sensitivity results can be used to assess in vivo drug efficacy. The Cmax for the recommended dose of eravacycline was 2.125 ± 0.36 mg/L in healthy volunteers and 9.793 ± 16.4 mg/L for a single injection of 3 mg/kg;35 the Cmax for the recommended dose of minocycline was 7.22 ± 3.53 mg/L in patients with respiratory tract infections.24 Based on the MCS results of minocycline, the Cmax values of the drugs, and with reference to the lower MIC values for eravacycline than minocycline, we infer that a single injection of 3 mg/kg eravacycline is likely to achieve similar effects as multiple injections of 100 mg minocycline q12 h when treating patients with respiratory infections of S. maltophilia that are non-susceptible to LVFX. In addition, drug concentrations are determined based on blood samples, and minocycline and eravacycline have been shown to have higher concentrations in the lungs,37–39 making both 100 mg q12 h minocycline or a single injection of 3 mg/kg eravacycline highly effective in the treatment of respiratory infections caused by these strains.
This study has some limitations. First, the host sources of the 77 non-susceptible strains are mainly concentrated in northern China and may not be representative of the MIC distribution in other regions. Second, the sample size taken for the simulation is small due to the small number of TMP-SMZ-resistant strains collected clinically, especially LVFX-non-susceptible strains that are also resistant to TMP-SMZ. The larger the sampling volume of MCS is, the more accurate the results. Third, although some studies have shown that minocycline is well tolerated at single intravenous doses of up to 600 mg in healthy adult subjects, with a maximum tolerated multiple dose of 300 mg q12 h intravenously,40 treatment of pneumonia with high doses of minocycline is also very infrequent. Therefore, high doses are not recommended for the treatment of respiratory infections. There have also been studies in healthy volunteers in which the incidence of adverse reactions was lower with single injections of eravacycline than with multiple injections, but the incidence of nausea and vomiting was higher with a single intravenous injection of 2 mg/kg and 3 mg/kg eravacycline than with the recommended dose (1 mg/kg).35 In addition, there are no clinical trials of eravacycline for the treatment of respiratory tract infections, and a detailed risk-benefit assessment needs to be completed in patients with clinical pneumonia before clinical dosing can be guided. Fourth, the pharmacokinetic studies of minocycline in patients with respiratory tract infections are rare, thus, this study only used parameters with earlier publication times and smaller sample sizes.
In summary, in this study, eravacycline was the most effective in vitro agent for pneumonia caused by either LVFX-non-susceptible or TMP-SMZ-resistant S. maltophilia. MCS analysis combining PK/PD parameters showed that the recommended dose of minocycline for LVFX-non-susceptible S. maltophilia pneumonia could achieve a high CFR. Good simulated clinical efficacy could also be achieved by doubling the dose of minocycline for TMP-SMZ-resistant strains, and for strains non-susceptible to LVFX and resistant to TMP-SMZ. Although there is no definitive evidence that eravacycline can be used in LVFX-non-susceptible and/or TMP-SMZ-resistant pneumonia caused by S. maltophilia, this study suggests that eravacycline can combat the increase in drug resistance, with satisfactory clinical efficacy seen at high doses. To treat S. maltophilia first-line with eravacycline, more preliminary work needs to be done by domestic and foreign scholars. We look forward to many clinical studies on this important new antibiotic in the next few years.
Ethics Statement
The study was approved by the Ethics Committee of Chinese People’s Liberation Army General Hospital. The study confirmed that informed consent was obtained from the study participants (for clinical sample collection) and the guidelines outlined in the Declaration of Helsinki were followed. The approval number of the study is S2023-064-01.
Acknowledgments
The author would like to thank her tutor, Prof. Junchang Cui, who provided important guidance for the experimental design and thesis writing of this research. At the same time, the author would like to thank the Clinical Microbiology Laboratory of the First Medical Center of the Chinese People’s Liberation Army General Hospital and Prof. Jiyong Yang for providing the strains, experimental sites and experimental design guidance.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Perez F, Adachi J, Bonomo RA. Antibiotic-resistant gram-negative bacterial infections in patients with cancer. Clin Infect Dis. 2014;59(Suppl 5):S335–339. doi:10.1093/cid/ciu612
2. Chang YT, Lin CY, Chen YH, Hsueh PR. Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front Microbiol. 2015;6:893. doi:10.3389/fmicb.2015.00893
3. Mojica MF, Rutter JD, Taracila M, et al. Population structure, molecular epidemiology, and β-lactamase diversity among stenotrophomonas maltophilia isolates in the United States. mBio. 2019;10(4). doi:10.1128/mBio.00405-19
4. Mojica MF, Ouellette CP, Leber A, et al. Successful treatment of bloodstream infection due to metallo-β-lactamase-producing Stenotrophomonas maltophilia in a renal transplant patient. Antimicrob Agents Chemother. 2016;60(9):5130–5134. doi:10.1128/AAC.00264-16
5. Hu L-F, Xu X-H, Li H-R, et al. Surveillance of antimicrobial susceptibility patterns among Stenotrophomonas maltophilia isolated in China during the 10-year period of 2005–2014. J Chemother. 2018;30(1):25–30. doi:10.1080/1120009X.2017.1378834
6. Ko JH, Kang CI, Cornejo-Juárez P, et al. Fluoroquinolones versus trimethoprim-sulfamethoxazole for the treatment of Stenotrophomonas maltophilia infections: a systematic review and meta-analysis. Clin Microbiol Infect. 2019;25(5):546–554. doi:10.1016/j.cmi.2018.11.008
7. Nys C, Cherabuddi K, Venugopalan V, Klinker KP. Clinical and microbiologic outcomes in patients with monomicrobial stenotrophomonas maltophilia infections. Antimicrob Agents Chemother. 2019;63(11). doi:10.1128/AAC.00788-19
8. Sader HS, Castanheira M, Mendes RE, Flamm RK. Frequency and antimicrobial susceptibility of Gram-negative bacteria isolated from patients with pneumonia hospitalized in ICUs of US medical centres (2015–17). J Antimicrob Chemother. 2018;73(11):3053–3059. doi:10.1093/jac/dky279
9. Biagi M, Tan X, Wu T, et al. Activity of potential alternative treatment agents for stenotrophomonas maltophilia isolates nonsusceptible to levofloxacin and/or trimethoprim-sulfamethoxazole. J Clin Microbiol. 2020;58(2). doi:10.1128/JCM.01603-19
10. Flamm RK, Shortridge D, Castanheira M, Sader HS, Pfaller MA. In vitro activity of minocycline against U.S. isolates of Acinetobacter baumannii-Acinetobacter calcoaceticus species complex, stenotrophomonas maltophilia, and Burkholderia cepacia complex: results from the SENTRY antimicrobial surveillance program, 2014 to 2018. Antimicrob Agents Chemother. 2019;63(11):1.
11. Morrissey I, Olesky M, Hawser S, et al. In vitro activity of eravacycline against gram-negative bacilli isolated in clinical laboratories worldwide from 2013 to 2017. Antimicrob Agents Chemother. 2020;64(3):1.
12. Zhanel GG, Cheung D, Adam H, et al. Review of eravacycline, a novel fluorocycline antibacterial agent. Drugs. 2016;76(5):567–588. doi:10.1007/s40265-016-0545-8
13. Zidaru A, Phe K, Lasco TM, Tam VH. An integrated approach to evaluate different tetracycline derivatives for formulary decisions. Am J Health Syst Pharm. 2022;79(6):467–471. doi:10.1093/ajhp/zxab451
14. Baidya A, Kodan P, Fazal F, et al. Stenotrophomonas maltophilia: more than just a colonizer! Indian J Crit Care Med. 2019;23(9):434–436. doi:10.5005/jp-journals-10071-23241
15. Pathmanathan A, Waterer GW. Significance of positive Stenotrophomonas maltophilia culture in acute respiratory tract infection. Eur Respir J. 2005;25(5):911–914. doi:10.1183/09031936.05.00096704
16. Kwa AL, Low JG, Lim TP, Leow PC, Kurup A, Tam VH. Independent predictors for mortality in patients with positive Stenotrophomonas maltophilia cultures. Ann Acad Med Singapore. 2008;37(10):826–830. doi:10.47102/annals-acadmedsg.V37N10p826
17. Oladunjoye OO, Oladunjoye AO, Oladiran O, Donato AA. Stenotrophomonas maltophilia infection in a patient with acute exacerbation of Chronic Obstructive Pulmonary Disease (COPD): a colonizer or true infection? Am J Case Rep. 2020;21:e924577. doi:10.12659/AJCR.924577
18. Erb CT, Patel B, Orr JE, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia. Ann Am Thorac Soc. 2016;13(12):2258–2260. doi:10.1513/AnnalsATS.201608-641CME
19. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. CLSI document M100-Ed32. Wayne, PA: Clinical and Laboratory Standards Institute; 2022.
20. Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. CLSI document M07-Ed11. Wayne, PA: Clinical and Laboratory Standards Institute; 2018.
21. Wyeth Pharmaceuticals Inc. Tygacil® (Tigecycline) for Injection. Philadelphia (PA): Wyeth Pharmaceuticals Inc; 2013.
22. Zhou Y, Xu P, Li H, et al. Population pharmacokinetics and exposure-response analysis of tigecycline in patients with hospital-acquired pneumonia. Br J Clin Pharmacol. 2021;87(7):2838–2846. doi:10.1111/bcp.14692
23. Bhavnani SM, Rubino CM, Hammel JP, et al. Pharmacological and patient-specific response determinants in patients with hospital-acquired pneumonia treated with tigecycline. Antimicrob Agents Chemother. 2012;56(2):1065–1072. doi:10.1128/AAC.01615-10
24. Yamamoto T, Takano K, Matsuyama N, et al. Pharmacokinetic characteristics of minocycline in debilitated elderly patients. Am J Ther. 1999;6(3):157–160. doi:10.1097/00045391-199905000-00006
25. Maaland MG, Papich MG, Turnidge J, Guardabassi L. Pharmacodynamics of doxycycline and tetracycline against Staphylococcus pseudintermedius: proposal of canine-specific breakpoints for doxycycline. J Clin Microbiol. 2013;51(11):3547–3554. doi:10.1128/JCM.01498-13
26. Andes D, Craig W. Pharmacokinetics and Pharmacodynamics of Tetracyclines. In: Nightingale CH, editor. Antimicrobial Pharmacodynamics in Theory and Clinical Practice.
27. Mouton JW, Dudley MN, Cars O, Derendorf H, Drusano GL. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: an update. J Antimicrob Chemother. 2005;55(5):601–607. doi:10.1093/jac/dki079
28. Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America guidance on the treatment of AmpC β-lactamase-producing Enterobacterales, carbapenem-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia infections. Clin Infect Dis. 2022;74(12):2089–2114. doi:10.1093/cid/ciab1013
29. Lee YR, Burton CE. Eravacycline, a newly approved fluorocycline. Eur J Clin Microbiol Infect Dis. 2019;38(10):1787–1794. doi:10.1007/s10096-019-03590-3
30. McCarthy MW. Clinical pharmacokinetics and pharmacodynamics of eravacycline. Clin Pharmacokinet. 2019;58(9):1149–1153. doi:10.1007/s40262-019-00767-z
31. Thabit AK, Monogue ML, Newman JV, Nicolau DP. Assessment of in vivo efficacy of eravacycline against Enterobacteriaceae exhibiting various resistance mechanisms: a dose-ranging study and pharmacokinetic/pharmacodynamic analysis. Int J Antimicrob Agents. 2018;51(5):727–732. doi:10.1016/j.ijantimicag.2018.01.001
32. Solomkin J, Evans D, Slepavicius A, et al. Assessing the efficacy and safety of eravacycline vs ertapenem in complicated intra-abdominal infections in the Investigating Gram-Negative Infections Treated With Eravacycline (IGNITE 1) trial: a randomized clinical trial. JAMA Surg. 2017;152(3):224–232. doi:10.1001/jamasurg.2016.4237
33. Solomkin JS, Gardovskis J, Lawrence K, et al. IGNITE4: results of a Phase 3, Randomized, Multicenter, Prospective Trial of Eravacycline vs Meropenem in the Treatment of Complicated Intraabdominal Infections. Clin Infect Dis. 2019;69(6):921–929. doi:10.1093/cid/ciy1029
34. Connors KP, Housman ST, Pope JS, et al. Phase I, open-label, safety and pharmacokinetic study to assess bronchopulmonary disposition of intravenous eravacycline in healthy men and women. Antimicrob Agents Chemother. 2014;58(4):2113–2118. doi:10.1128/AAC.02036-13
35. Newman JV, Zhou J, Izmailyan S, Tsai L. Randomized, double-blind, placebo-controlled studies of the safety and pharmacokinetics of single and multiple ascending doses of eravacycline. Antimicrob Agents Chemother. 2018;62(11). doi:10.1128/AAC.01174-18
36. Weiss WJ, Pulse M, Renick P, et al. Efficacy of fluorocyclines TP-434 in the neutropenic thigh infection model is predicted by AUC/MIC.
37. Watanabe A, Anzai Y, Niitsuma K, Saito M, Yanase K, Nakamura M. Penetration of minocycline hydrochloride into lung tissue and sputum. Chemotherapy. 2001;47(1):1–9. doi:10.1159/000048494
38. Zhou J, Ledesma KR, Chang KT, Abodakpi H, Gao S, Tam VH. Pharmacokinetics and pharmacodynamics of minocycline against Acinetobacter baumannii in a neutropenic murine pneumonia model. Antimicrob Agents Chemother. 2017;61(5):1.
39. Petraitis V, Petraitiene R, Maung BBW, et al. Pharmacokinetics and comprehensive analysis of the tissue distribution of eravacycline in rabbits. Antimicrob Agents Chemother. 2018;62(9). doi:10.1128/AAC.00275-18
40. Cornely OA, Arenz D, Barraud O, et al. Phase I study to evaluate the safety and pharmacokinetics of single and multiple ascending doses of intravenous minocycline in healthy adult subjects.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.