Back to Journals » Infection and Drug Resistance » Volume 15
In vitro Antifungal Activity of a Novel Antimicrobial Peptide AMP-17 Against Planktonic Cells and Biofilms of Cryptococcus neoformans
Authors Yang L, Tian Z, Zhou L, Zhu L, Sun C, Huang M, Peng J, Guo G
Received 13 October 2021
Accepted for publication 8 January 2022
Published 25 January 2022 Volume 2022:15 Pages 233—248
DOI https://doi.org/10.2147/IDR.S344246
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Longbing Yang,1 Zhuqing Tian,1 Luoxiong Zhou,1,2 Lijuan Zhu,1 Chaoqin Sun,1 Mingjiao Huang,1 Jian Peng,1,3 Guo Guo1,3,4
1School of Basic Medical Sciences, The Key and Characteristic Laboratory of Modern Pathogen Biology, Guizhou Medical University, Guiyang, 550025, People’s Republic of China; 2School of Public Health, Guizhou Medical University, Guiyang, 550025, People’s Republic of China; 3Key Laboratory of Environmental Pollution Monitoring and Disease Control, Ministry of Education, Guizhou Medical University, Guiyang, 550025, People’s Republic of China; 4Translational Medicine Research Center, Guizhou Medical University, Guiyang, 550025, People’s Republic of China
Correspondence: Guo Guo, Building Wuben, School of Basic Medical Sciences, Guizhou Medical University, College Town, Gui’an New District, Guiyang, 550025, People’s Republic of China, Tel/fax +86 851 882 59268, Email [email protected]
Background: Cryptococcus neoformans is a common human fungal pathogen in immunocompromised people, as well as a prevalent cause of meningitis in HIV-infected individuals. With the emergence of clinical fungal resistance and the shortage of antifungal drugs, it is urgent to discover novel antifungal agents. AMP-17, a novel antimicrobial peptide from Musca domestica, has antifungal activity against C. neoformans. However, its antifungal and anti-biofilm activities remain unclear. Thus, this study aimed to evaluate the antifungal activity of AMP-17 against planktonic cells and biofilms of C. neoformans.
Methods: The minimum inhibitory concentration (MIC), the biofilm inhibitory and eradicating concentration (BIC and BEC) were determined by the broth microdilution assay or the 2, 3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) reduction assay, respectively. The inhibitory and killing activities of AMP-17 against C. neoformans were investigated through the time-inhibition/killing kinetic curves. The potential antifungal mechanism of AMP-17 was detected by flow cytometry, scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM). The efficiency of AMP-17 against biofilm formation or preformed biofilm was evaluated by crystal violet staining and XTT reduction assays. The morphology of pre-biofilms was tested by optical microscopy (OM) and CLSM.
Results: AMP-17 exhibited in vitro antifungal activity against C. neoformans planktonic cells and biofilms, with MICs of 4∼ 16 μg/ml, BIC80 and BEC80 of 16∼ 32 μg/ml, 64∼ 128 μg/ml, respectively. In addition, the 2× and 4× MIC of AMP-17 exhibited similar inhibition levels compared to the 2× and 4× MIC of the clinical drugs FLC and AMB in C. neoformans growth. Moreover, the time-kill results showed that AMP-17 (8× MIC) did not significantly eliminate colony forming units (CFU) after 6 h of treatment; however, there was 2.9-log reduction in CFU of C. neoformans. Furthermore, increasing of the permeability of the fungal cell membrane was observed with the treatment of AMP-17, since the vast change as fungal leakage and cell membrane disruption. However, the DNA binding assay of AMP-17 indicated that the peptide did not target DNA. Besides, AMP-17 was superior in inhibiting and eradicating biofilms of C. neoformans compared with FLC.
Conclusion: AMP-17 exhibited potential in vitro antifungal activity against the planktonic cells and biofilms of C. neoformans, and it may disrupt fungal cell membranes through multi-target interactions, which provides a promising therapeutic strategy and experimental basis for Cryptococcus-associated infections.
Keywords: antimicrobial peptides, AMP-17, Cryptococcus neoformans, antifungal mechanism, anti-biofilm
Introduction
Cryptococcus neoformans, an encapsulated pathogenic yeast-like fungus, causes cryptococcosis in immunocompetent and immunocompromised patients.1,2 In recent years, with the spread and epidemic of acquired immunodeficiency syndrome (AIDS), intensive chemotherapy of cancer patients and widespread use of immunosuppressive therapy and broad-spectrum antimicrobial agents, opportunistic pathogenic infections caused by C. neoformans have been increasing year after year.3,4 Furthermore, cryptococcal meningitis is the most common cause of meningitis in individuals with HIV in regions of the world with high rates of HIV infection.5,6 It remains the second most prevalent cause of death in patients with AIDS.6,7 The global burden of cryptococcosis is estimated to be 223,000 cases per year, with nearly 181,000 people dying each year.6,7 Hence, cryptococcosis, like other invasive fungal infections, is a challenging problem that necessitates focused research and the development of novel antifungal agents.
It has been reported that current recommendations induction therapy should consist of a combination of amphotericin B and 5-flucytosine, followed by a consolidation period of relatively high-dose fluconazole and a longer maintenance period of lower-dose fluconazole.8,9 Although the best therapy for cryptococcosis is a combination of AMB and 5-FU, it is not generally utilized in resource-poor nations due to side effects and high costs.10 Therefore, fluconazole is still the most commonly used antifungal drug in clinical practice for the treatment and prevention of C. neoformans infection, and FLC has been the recommended classic treatment for recent years.5,11 However, the widespread use of FLC has caused the rapid emergence of resistant strains.12 More seriously, C. neoformans can easily form biofilms on solid surfaces like implants and medical devices, as well as sessile biofilms can serve as a storage facility for antimicrobial drugs resistant, resulting in long-term systemic infections.13,14 Biofilms are microbial communities attached to an external polymer matrix on a solid surface.15 C. neoformans biofilms consist of a complex network of yeast cells fused with a large amount of polysaccharide matrix.16 In particular, the glucuronoxylomannan (GXM) in the capsule of C. neoformans plays a central role in biofilm formation and pathogenesis.17 Biofilm formation allows microorganisms to survive in harsh environments, colonize and sow new niches, and confer protection against predation.18,19 The self-produced polysaccharide-rich extracellular polymeric matrix (EPM) of biofilm renders sessile cryptococcal cells resistant to antimicrobial therapy, leading to fungal resistance. Furthermore, biofilm formation has been linked to persistent infections, as biofilms increase resistance to host immune mechanisms and antimicrobial therapies.20 Therefore, it is urgent to investigate novel, effective, and safe anti-biofilm drugs, which might be a promising strategy to prevent or treat C. neoformans infections.
Antimicrobial peptides (AMPs), which are a key component of most organisms’ innate immune systems, have been testified to be effective antimicrobial infection agents.21 AMPs have been shown to have antiviral, antitumor, and immunomodulatory properties in addition to antimicrobial activity.22,23 Therefore, AMPs are being considered as a possible drug candidate in the near future. Antimicrobial peptide-17 (AMP-17), which was screened from the C. albicans-induced M. domestica transcriptome database, was identified as a potential antifungal agent in previous studies.24,25 Full-length ORF of the AMP-17 was 495 bp, encoding 164 amino acids, and had an isoelectric point (PI) of 6.09.25 In addition, it was discovered that AMP-17ʹs secondary structure is primarily composed of three types: α-helix, random coil, and extended strand.25 The α-helical structure mainly forms a potential-dependent channel on the cell membrane, which changes the permeability of the cell membrane and causes the cell contents to leak, and has antimicrobial activity against gram-positive, negative bacteria, and fungi. Previous studies showed that AMP-17 has α-helical structure relating with antimicrobial peptides. Furthermore, one research reported that AMP-17 exhibits heat resistance, freeze-thaw resistance, and is non-toxic to human red blood cells.26 Meanwhile, AMP-17 exhibits potential antifungal activity against a variety of clinical fungi and has been shown to destroy the integrity of the C. albicans cell wall, causing the cell membrane structure to be destroyed and the cell membrane permeability to increase.25–27 However, the antifungal and anti-biofilm activities of AMP-17 against C. neoformans in vitro remain unclear. Thus, more reliable and accurate data are needed to explain the antifungal and anti-biofilm effects of AMP-17 against C. neoformans.
Given the above, this study evaluated the in vitro antifungal activity of AMP-17 against C. neoformans planktonic cells and observed the damaging effect of AMP-17 on the planktonic cell membrane by flow cytometry, CLSM, and SEM images. Furthermore, we also investigated the effect of AMP −17 on the formation and maturation of C. neoformans biofilm and performed CLSM and OM image observations during the mature stage of C. neoformans biofilm to visualize possible biofilm destruction activities.
Materials and Methods
Antimicrobial Peptide
AMP-17 was overproduced from pET-28a in Escherichia coli BL21 (DE3) and purified with Ni-NTA beads, as previously reported (Novagen, Germany).25,27 In addition, AMP-17 obtained a Chinese patent license in 2019. (Patent number: ZL 2016 10428119. 8).
Microbial Strains
A total of 18 C. neoformans (2 standard strains: H99 and KN99, 9 clinical strains: IFM56716, IFM56769, IFM56771, IFM56772, IFM56787, IFM56793, IFM56794, IFM56800, IFM56803 and 7 environmental strains: IFM56722, IFM56726, IFM56728, IFM56731, IFM56734, IFM56735, IFM56743) were used in this study. These strains originated from the Institute of Fungi, Chiba University School of Medicine, Japan. And these strains were kindly donated by Professor Yingqian Kang, the Department of Microbiology, Guizhou Medical University, Guiyang, China.
Antifungal Susceptibility Testing
The MIC of AMP-17 against C. neoformans planktonic cells was determined by referring to the microscale broth dilution method recommended by the M27-A3 documents of the Clinical and Laboratory Standard Institute (CLSI).28 Briefly, planktonic cells cultured in Sabouraud dextrose Broth (SDB; Solarbio, Beijing, China) were collected at mid-logarithmic phase, washed with 10 mM phosphate-buffered saline (PBS, pH 7.4), and re-suspended at a density of 0.5~2.5 × 103 CFU/mL in RPMI 1640 medium (Gibco, BRL Invitrogen, US). 100 μL of fungal suspension and 100 μL final concentration of 0.5~64 μg/mL AMP-17, 0.5~64 μg/mL FLC (Sigma, US) or 0.125~16 μg/mL AMB (Sigma, US) were added to a 96-well plate for susceptibility tests and incubated at 35 °C for 72 h. The OD630 nm value of each well was measured by a microplate reader. The lowest drug concentration corresponding to the growth inhibition ≥80% well was the MIC value of AMP-17 and FLC, whereas the MIC value of AMB corresponded to no visible fungal growth, compared to the negative control. The assays were conducted in triplicate, with each experiment replicated twice.
Time-Inhibition/Killing Kinetics
Time-inhibition/killing kinetics monitoring was carried out according to the previous study.29 Briefly, the fungal suspensions were adjusted to reach ~105 CFU/mL in sterile RPMI 1640 medium, and then 100 μL of the fungal suspensions were transferred to 24-well plates containing 900 μL of a specific concentration of AMP-17 (1~8× MIC), and the plate was incubated at 35 °C with shaking (120 rpm). Then, the optical density (OD630 nm) of the culture was measured at a specific time. For the killing kinetics, 50 μL samples with different concentration were diluted serially and inoculated on SDA plates at specific times. Colony forming units (CFU) were counted after the culture had been at 35 °C for 48 h. FLC and AMB were used as positive controls. The assays were conducted in triplicate, with each experiment replicated twice.
Nucleotide Leakage
Nucleotide release assay was performed according to the method proposed by Lemos et al30 with minor modifications. Briefly, the fungal suspensions were adjusted to reach 1.0×106 CFU/mL in 10 mM PBS and incubated with AMP-17 (1~4× MIC). Cultures incubated with 10 mM PBS served as growth controls. Following incubation, cell suspensions at different time points (0, 1, 2, 4, 6, 8 h) were centrifuged at 10,000 × g for 10 min, and the supernatants were evaluated for OD at 260 nm in a spectrophotometer (Biotech, USA). The assays were conducted in triplicate, with each experiment replicated twice.
Flow Cytometry Analysis
The effect of AMP-17 on membrane permeability was detected by flow cytometry, according to a previously described method, with minor modifications.31 Briefly, C. neoformans cells were diluted to 1.0×106 CFU/mL. Cell suspensions were incubated for 6 h at 35 °C with 1, 2, and 4× MIC AMP-17. Subsequently, treated cells were incubated with 10 μM propidium iodide (PI; Sigma, US) for 15 min, followed by washing and resuspension in sterile PBS. The percentage of PI-positive cells was determined using the FACScan instrument (Beckman, USA). Results were obtained from two independent experiments.
Membrane Morphological Observation
Scanning Electron Microscopy (SEM)
The effect of AMP-17 on the morphology of planktonic cells of C. neoformans was analyzed by Scanning electron microscopy. Briefly, C. neoformans cells (1.0 × 106 CFU/mL) were seeded into 6-well plates and treated for 6 h at 35 °C with 1, 2, and 4× MIC AMP-17 before being collected by centrifugation (5000 rpm for 5 min). Subsequently, the samples of each group were washed thrice with 10 mM PBS and fixed with 1 mL 2.5% of glutaraldehyde at 4 °C overnight. Then, the fixed samples were washed thrice in PBS and dehydrated in a graded sequence of ethanol (50%, 70%, 90%, and 100%). Finally, the samples were observed under a Hitachi H-7650 SEM (Tokyo, Japan).
Confocal Laser Scanning Microscopy (CLSM)
C. neoformans cells (1.0 × 106 CFU/mL) were seeded into 6-well plates and treated with 1, 2, and 4× MIC AMP-17 for 6 h at 35 °C, and then stained with PI at a final concentration of 10 μM in the dark and incubated for 15 min. The stained cells were washed three times in 10 mM PBS before being resuspended in PBS. The samples were analyzed using CLSM (Olympus FV1000, Japan).
DNA Binding Assay
The DNA gel mobility shift assay was performed as described by Li et al32 with minor modifications. Briefly, Genomic DNA (50 ng) was mixed with 1, 2, 4× MIC AMP-17 in 10 μL, and the mixtures were incubated at 37 °C for 1 h. The products were detected by 1% agarose gel electrophoresis, and DNA migration was observed on the 4600SF type gel imaging analysis system (Tanon, China). The experiment was repeated at least three times.
Biofilm Formation
Biofilm was prepared according to the method proposed by Pierce et al.33 Briefly, 3–5 single colonies were inoculated into 5 mL SDB medium and grown to the logarithmic growth phase at 35 °C. After washing three times with 10 mM PBS and centrifugation at 3000×g for 5 min, the concentration of the fungal suspension was adjusted to 2×107 cells/mL using DMEM medium (containing 10% fetal bovine serum (FBS, Gibco)). The cell suspension (100 μL) was then transferred to pre-treated wells of polystyrene 96-well plates with FBS and cultured at 35 °C for 48 h with shaking to generate a biofilm. Subsequently, the plankton cells were discarded after being washed three times in PBS. True biofilm was defined as fungal cells that remained attached to the polystyrene surface. All assays were carried out in triplicates.
The biofilm biomass was determined using crystal violet (CV; Solarbio, Beijing, China). The biofilms were fixed in absolute ethanol for 5~10 minutes before being allowed to dry. After that, each well was stained for 10 min with 0.5% CV, and the excess dye was removed with PBS. The bound CV was dissolved in 30% acetic acid, and the absorbance was measured at OD560 nm on a microtiter plate reader (Biotek Epoch 2, USA).
Effect of AMP-17 in Preventing C. neoformans Biofilm Formation
The effects of AMP-17 on C. neoformans biofilm formation were evaluated as described by Brilhante et al34 and Martinez et al,35 with some modifications. Briefly, the fungal suspensions were adjusted to 2.0×107 cells/mL in DMEM medium (Gibco, BRL Invitrogen, US), then 100 μL of the fungal suspensions were added to 96-well polystyrene plates containing 100 μL of AMP-17 at varied concentrations (8~64 μg/mL) and incubated for 48 h at 35 °C. Subsequently, the plate was washed thrice with sterile PBS, and the biofilm was quantified by the 2, 3-Bis-(2-Methoxy-4-Nitro-5-Sulfophenyl)-2H-Tetrazolium-5-Carboxanilide (XTT; BBI, Shanghai, China) reduction assay and biofilm inhibitory concentration. The metabolic activity that inhibited 80% biofilm formation was the lowest biofilm inhibitory concentration (BIC80) of AMP-17 compared to the negative control. The experiment was performed in triplicate and repeated twice.
Effect of AMP-17 on Preformed Biofilm of C. neoformans
The effects of AMP-17 against pre-biofilms of C. neoformans were analyzed as described by Kumari et al16 with some modifications. For the pre-biofilm assay, the biofilm was made as described in the previous section. Thereafter, serially double-diluted AMP-17 (32~256 μg/mL) was added into the wells of pre-washed and pre-biofilms, and the plate was incubated at 35 °C for 48 h. Subsequently, the pre-biofilm was quantified by the XTT reduction assay and biofilm-eradicating concentration. The metabolic activity that eradicated 80% of the pre-biofilm was the lowest biofilm eradication concentration (BEC80) of AMP-17, compared to the negative control. The experiment was carried out in triplicate and repeated twice.
Optical Microscopy Analysis
The effect of AMP-17 against the structure of pre-biofilms was observed by inverting OM, as previously described,36 with slight modifications. Briefly, AMP-17 was added to a pre-biofilm 24-well plate containing sterile polylysine-treated coverslips for 6 h, after which the supernatant was aspirated and washed with sterile PBS to remove the unattached cells. The samples were then stained with 0.5% (w/v) CV for 10 min and washed three times with sterile PBS before being observed with an inverted OM (Nikon, Japan).
Confocal Laser Scanning Microscope Analysis
To evaluate the eradication effect of AMP-17 on pre-biofilms by CLSM, as previously described,37,38 with minor modifications. Briefly, A sterile polylysine-treated cover glass was used as the carrier, and the biofilm was grown for 48 h and treated with AMP-17 for 6 h, after which the supernatant was aspirated and washed thrice with sterile PBS, followed by staining with SYTO 9 (10 μM) and PI (10 μM) according to the manufacturer’s instructions. Stained biofilms on the culture plate were observed using a CLSM (Nikon A1+, Japan). The earliest and latest disappearance of fluorescence from the bottom of the biofilm to the surface was recorded layer by layer along the Z-axis, and the corresponding biofilm thickness was calculated accordingly; each layer was 1 μm. The resulting images stacks were quantified using ImageJ (USA) and then rendered in three-dimensional mode using image analysis software (NIS-Elements AR 5.21.00, Japan).
Statistical Analysis
All data were analyzed using GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA). Differences were analyzed by one-way ANOVA using Dunnett’s analysis. Data are expressed as mean ± standard deviation (Mean ± SD), and P < 0.05 was considered statistically significant.
Results
Antifungal Activity of AMP-17 Against C. neoformans in vitro
MIC values were evaluated the antifungal activity of AMP-17 against planktonic cells of C. neoformans. The results showed that AMP-17 had high antifungal activity against 18 strains of C. neoformans, with MIC values ranging from 4 to 16 μg/mL (Table 1). Similarly, FLC and AMB also displayed significant antifungal activity against C. neoformans planktonic cells, with MIC values of 2 to 8 μg/mL and 0.25 to 0.5 μg/mL, respectively (Table 1). Notably, AMP-17, FLC, and AMB exhibited the most significant in vitro antifungal effects against C. neoformans IFM56769, with MIC values of 4, 2, and 0.25 μg/mL, respectively.
 |
Table 1 MIC, BIC80 and BEC80 of AMP-17 Against Cryptococcus neoformans |
Growth Inhibition and Killing Kinetics
Growth inhibition kinetics of AMP-17 were evaluated against C. neoformans at different time intervals. 1~8× MIC AMP-17 showed a significant inhibitory effect on the growth curve of the tested fungi (Figure 1A). With increasing time, the inhibitory effect of low concentrations of AMP-17 on the fungal growth curve weakened; however, it still exhibited a significant inhibitory effect when compared to the control. Notably, the effectiveness of 2 and 8× MIC AMP-17 in inhibiting C. neoformans growth was comparable to that of 2× and 4× MIC FLC and AMB, respectively. The above data indicated that the growth of C. neoformans cells was inhibited by AMP-17 during all tested times. Furthermore, the time-killing kinetics of AMP-17 was performed on C. neoformans at concentrations of 1~8× MIC. As shown in Figure 1B, AMP-17 displayed concentration-dependent killing. The high concentration of AMP-17 (8× MIC) did not completely eliminate the colonies after 6 h of treatment, but still displayed in 2.9-log CFU reductions in C. neoformans. Subsequently, the number of CFU decreased with increasing time. However, the number of CFU increased slightly after FLC (2 × MIC) and AMP-17 (2 × MIC) treatments, but all CFU numbers decreased compared to the control (P < 0.05). The killing kinetics of AMB (4× MIC) were demonstrated by eradicating the fungal cells in 3 h.
AMP-17 Exerted Antifungal Effects by Disrupting Fungal Membrane
The disruption of the cell membrane has been reported as a common or typical mode of action for some AMPs. Increasing experiments were carried out in this study to gain insight into the antifungal mechanism of AMP-17. To begin, the most sensitive strain (IFM56769) for AMP-17, FLC, and AMB was chosen based on the above MIC data, and AMP-17 damage to the membrane of C. neoformans was determined by measuring 260 nm of absorbing material. The OD260 nm of the AMP-17-treated cultures showed a dose and time-dependent increase (Figure 2) compared to the negative control. The above results suggest that AMP-17 may target the outer membrane of C. neoformans, causing intracellular material to leak out and increasing the maximum absorbance at 260 nm.
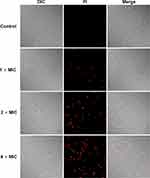
To further confirm the damage of AMP-17 to C. neoformans membranes, C. neoformans cells treated with AMP-17 were observed by flow cytometry, CLSM, and SEM images. Flow cytometry analysis showed that the percentage of PI-positive cells was 0.76% in the absence of AMP-17. The cells were shown to be more damaged after treatment with AMP-17 at concentrations of 1× MIC, 2× MIC, and 4× MIC, with 7.24%, 11.42%, and 22.88% PI fluorescence signals, respectively (Figures 3 and S1). The above data suggested that AMP-17 may have played a significant role in degrading the membrane permeability of C. neoformans. Additionally, CLSM images revealed that after treatment with AMP-17 in C. neoformans for 6 h, the red fluorescence (dead fungus) gradually increased with increasing AMP-17 concentration, implying that PI can penetrate the cell membrane and bind to DNA/RNA, resulting in characteristic red fluorescence, whereas the PBS control group showed no red fluorescence (Figure 4). Furthermore, SEM images showed that untreated C. neoformans cells were round or oval, with a smooth cell surface and distinct cell borders (Figure 5A). However, AMP-17 caused cells severe damage, including large differences in cell size, sunken surfaces, irregularities in shape, ruptured, shrunk, and leaked cytoplasmic contents (Figure 5B–D). These results suggested that AMP-17 may damage C. neoformans cells by disrupting the cell membrane.
DNA-Binding Property Analysis
It has been reported that the antifungal effect of AMPs is not restricted to membrane perforation; it can interact with intracellular targets (such as DNA). Thus, this study explored the possibility of intracellular targets by detecting the DNA binding properties of AMP-17. The results showed that AMP-17 was incapable of binding to genomic DNA, even though it was at a high concentration and treated with the DNA for 1 h (Figure S2), indicating that DNA is not one of the targets that causes the antifungal activity of AMP-17.
Effect of AMP-17 Against C. neoformans Biofilm Formation and Preformed Biofilm
The potential and efficacy of AMP-17 against C. neoformans biofilm formation and pre-biofilm was determined in terms of BIC80 and BEC80 respectively. The results showed that AMP-17 (BIC80: 16~32 μg/mL; BEC80: 64~128 μg/mL), FLC (BIC80: 128~256 μg/mL; BEC80: > 512 μg/mL) and AMB (BIC80: 2~8 μg/mL; BEC80: 32~64 μg/mL) could inhibit the biofilm formation and eradicated the pre-biofilm of C. neoformans (Table 1). Among them, the therapeutic effect of AMB is the most significant, followed by AMP-17, and finally FLC. Furthermore, biofilm-associated Cryptococcus cells were significantly more resistant to AMP-17, FLC and AMB than planktonic cells.
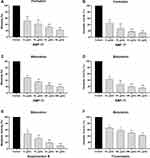
The biomass and metabolic activity of C. neoformans biofilms formation have gradually decreased with the increase of AMP-17 concentration. Compared with the negative control, biomass and metabolic activity were reduced by 78% and 86% at concentrations of AMP-17 of 64 μg/mL, respectively (Figure 6A and B). In addition, mature biofilms of C. neoformans also reduced significantly in biomass and metabolic activity after exposure to AMP-17. Biomass reductions of AMP-17 at 256 μg/mL reached 80%, while reductions in metabolic activity reached 85%, compared to the untreated control group (Figure 6C and D). Exposure of mature Cryptococcus biofilms to AMB resulted in a 90% reduction in metabolic activity at a concentration of 64 μg/mL (Figure 6E). However, Exposure to FLC only resulted in a 58% reduction in metabolic activity at a concentration of 512 μg/mL (Figure 6F).
Analyzing the Changes in Biofilm Cells Morphology After AMP-17 Treatment
To corroborate the anti-biofilm activity of AMP-17, OM and CLSM images were used to visualize the pre-biofilms after treatment with AMP-17. OM images showed the presence of many extracellular matrix (ECM), micro and macro-colonies in the untreated biofilm, and the yeast cells aggregated into clusters to form multilayered membrane-like structures (Figure S3A). After treatment with AMP-17 (1/2, 1, and 2× BEC80) for 6 h, the biofilm structure was destroyed and the cells were loosely distributed (Figure S3B–D). In addition, CLSM images found that a large number of biofilms aggregated in the negative control, mainly living fungi that emit green fluorescence (Figure 7A), with a biofilm thickness of 15 μm (Figure 7E). The number of sessile cells was reduced and a dense biofilm could not be produced after treatment with AMP-17 (1/2, 1, and 2 BEC80) for 6 h (Figure 7B–D), with biofilm thicknesses of 10 μm, 9 μm, and 7 μm, respectively (Figure 7E). Meanwhile, the number of dead fungi gradually increased, and many fungi in the field of vision emitted red fluorescence. The statistical analysis of at least three biofilm images of each group displayed a high proportion of dead fungus (PI intensity) in the AMP-17 treated biofilms (35.01%, 41.02%, and 49.29%) as compared to the control group (20.83%) (Figure 7F). The above results indicated that AMP-17 had the potential ability to eradicate the pre-biofilm of C. neoformans.
Discussion
Cryptococcus neoformans is one of the common human fungal pathogens in clinically impaired individuals, and cryptococcal meningitis caused by it remains a significant cause of morbidity and mortality amongst patients living with human immunodeficiency virus (HIV).39,40 In addition, the emergence of drug-resistant strains and their capacity to establish recalcitrant biofilms in medical settings is a major concern associated with managing cryptococcosis.41 Furthermore, C. neoformans biofilms are resistant to antifungal agents and host defense mechanisms, resulting in high morbidity and mortality.42 Therefore, it has become indispensable to identify or develop a novel class of agents that are natural and directed against targets that do not impart selective pressure or promote drug resistance. Recently, some AMPs have demonstrated antifungal and anti-biofilm activity, also called anti-biofilm peptides.43–45 This study discovered that AMP-17 has effective antifungal activity against C. neoformans planktonic cells and biofilm, suggesting that AMP-17 shows potential as a novel antifungal agent.
In the present study, planktonic susceptibility tests against 18 fungal strains indicated that AMP-17, FLC, and AMB had significant in vitro antifungal activity against C. neoformans. Among them, AMP-17, FLC and AMB showed the most significant activity against C. neoformans IFM56769 with MIC80 of 4, 2 and 0.25 μg/mL, respectively. This phenomenon suggested that AMP-17 had different antifungal activities against strains of the same genus, which might be due to differences in the fungal cell wall. Glycoproteins and polysaccharides (dextran and chitin) make up the majority of fungal cell walls, but there are other components that vary by fungal species.46 Furthermore, the inhibitory and killing effects of AMP-17 were also observed in the current study. The results showed that AMP-17 exhibited inhibitory activity against the growth of C. neoformans planktonic cells, and there was a correlation between the concentration of AMP-17 and antifungal activity. The growth of C. neoformans cells was almost fully inhibited by 4 and 8× MIC AMP-17 during all the time of incubation. Meanwhile, the 2× and 4× MIC of AMP-17 inhibited C. neoformans growth with comparable efficiency to the 2× and 4× MIC of the clinical drugs FLC and AMB, respectively. In addition, the time-kill results showed that 4× and 8× MIC AMP-17 did not completely eliminate CFU after 6 h of treatment. However, it still influences a 2.1 and 2.9-log reduction in CFU of C. neoformans, respectively. In contrast, FLC (2× MIC) also failed to completely eliminate colonies, leading to only a 1.6-log reduction in C. neoformans CFU after 6 h of treatment, whereas AMB (4 × MIC) completely eradicated fungal cells within 3 h. The mechanisms by which AMPs kill microorganisms vary case by case and are largely unknown. However, several proposed mechanisms for killing fungi share some common features. For example, most AMPs can disrupt the integrity of the fungal cell membrane and wall, and to achieve this, interaction between AMP molecules and fungal membrane and wall components is necessary.47,48 Through monitoring nucleotide leakage in C. neoformans, we found that AMP-17 led to an increase in nucleotide release, suggesting an increase in fungal cell permeability likely caused by disturbance of the fungal cell wall and membrane. Most α-helical antifungal peptides have been reported to exert antifungal activity by interfering with membrane permeability.31 PI, a membrane-impermeable dye, was used to detect cells that had permeable membranes. PI enters into cells and binds to nucleic acids, fluorescing red. Flow cytometry analysis found that AMP-17 treatment significantly increased membrane permeability. Furthermore, CLSM images revealed a significant increase in the number of PI stained cells in C. neoformans cells treated with AMP-17, suggesting that AMP-17 may exert its antifungal effect by destroying the integrity of fungal cell membranes. Moreover, SEM images testified severe damage to C. neoformans cells treated with AMP-17, such as cell deformation, depression, shrinkage, and rupture, indicating that AMP-17 may be the result of direct interaction with cell wall and cell membrane components. However, membrane penetration was not the only way that AMPs killed microorganisms. Some reports indicate that AMPs can exert anti-fungal activity through multiple pathways (such as DNA, RNA, and protein).48,49 Thus, the ability of AMP-17 to bind fungal DNA was evaluated in this study. The data indicated that DNA was not one of the targets that causes the antifungal activity of AMP-17.
It has been reported that cryptococcal biofilms are less sensitive to antifungal agents and to various antimicrobial molecules produced by the immune system,20 and it is able to form biofilms on glass surfaces and in individual wells of polystyrene plates.20,50,51 The formation of C. neoformans biofilm mainly follows the following events: surface attachment of fungi, formation of micro-colonies, and matrix production.17,52 However, the development of biofilm depends on the release of capsular polysaccharides to solid surfaces to produce an extracellular polysaccharide matrix (EPM).17 Glucuronoxylomannan (GXM) is the main component of polysaccharide capsules, which play a central role in the formation of biofilm and its pathogenesis.17,53 The release of C. neoformans GXM is necessary for adhesion to solid supports and the subsequent formation of biofilms had been confirmed.17 However, it is difficult to eradicate the infection with antifungal agents if the biofilm is established. Biofilms constitute a physical barrier that prevents the effective penetration of antifungal agents. Compared with planktonic cells, biofilms confer higher antifungal activity to microorganisms that form biofilms.54,55 In the current study, we investigated that AMP-17 exhibited effective anti-biofilm activity against C. neoformans. AMP-17 effectively reduced the biomass and metabolic activity of the biofilm formation and pre-biofilm stages at concentrations of 8~64 μg/mL and 32~256 μg/mL, respectively. Furthermore, AMB significantly reduced the metabolic activity of pre-biofilm at concentrations ranging from 8 to 64 g/mL. However, compared to AMP-17, increasing concentrations of the positive control (fluconazole) resulted in a significant increase in biofilm biomass and metabolic activity. This phenomenon confirms that Cryptococcus biofilms are less sensitive to antifungal agents and various antimicrobial molecules produced by the immune system, as reported by Martinez et al20 and Brilhante et al.34
From the analysis of OM images, it was shown that due to the increase in the amount of extracellular material surrounding the cells, the microstructure of C. neoformans biofilm became more complex and formed a dense structure closely attached to the plastic support. After AMP- 17 was treated for 6h, C. neoformans failed to form a dense biofilm, and the biofilm was easily detached, and the cells were decreased, damaged or collapsed. The CLSM results further quantified the thickness of the biofilm and the ratio of live/dead fungi, and the results found that after 48 h, the biofilm formed on the coverslip was thick and dense, mainly composed of live fungi with a thickness of approximately 15 μm. After treatment with AMP-17, the number of dead fungi gradually increased, and the biofilm became significantly thinner. Thus, the above findings indicated that AMP-17 had potential eradication ability against the pre-biofilm of C. neoformans.
Conclusion
In summary, this study showed that AMP-17 had antifungal activity against planktonic cells and biofilm of C. neoformans. Furthermore, AMP-17 acted not only on the cell membrane but also on the intracellular level, indicating that the antimicrobial peptide may have a multi-target mechanism that results in fungus death. Therefore, these results indicated a promising treatment strategy for Cryptococcus-related infections, as well as a theoretical foundation for the investigation of novel AMP mechanisms. However, this study was restricted to in vitro experiments. Further research should be more validated in vivo, which might be one strategy to treat associated cryptococcosis.
Acknowledgments
This work was supported by the National Natural Science Foundation of China [grant numbers: 81760647, 82060381] and Key Technologies R&D Program for Science and Technology Department of Guizhou Province ([2019]2823).
Author Contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Prof. Dr. Guo Guo reports a licensed patent ZL 2016 10428119. 8. The authors report no conflicts of interest in this work.
References
1. Zavala S, Baddley JW. Cryptococcosis. Semin Respir Crit Care Med. 2020;41(1):69–79. doi:10.1055/s-0039-3400280
2. Coelho C, Bocca AL, Casadevall A. The intracellular life of Cryptococcus neoformans. Annu Rev Pathol. 2014;9(1):219–238. doi:10.1146/annurev-pathol-012513-104653
3. Kronstad JW, Attarian R, Cadieux B, et al. Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nat Rev Microbiol. 2011;9(3):193–203. doi:10.1038/nrmicro2522
4. Shorman M, Evans D, Gibson C, et al. Cases of disseminated cryptococcosis in intravenous drug abusers without HIV infection: a new risk factor? Med Mycol Case Rep. 2016;14:17–19. doi:10.1016/j.mmcr.2016.12.003
5. Williamson PR, Jarvis JN, Panackal AA, et al. Cryptococcal meningitis epidemiology, immunology, diagnosis and therapy. Nat Rev Neurol. 2017;13(1):13–24. doi:10.1038/nrneurol.2016.167
6. Santos-Gandelman J, Machado-Silva A. Drug development for cryptococcosis treatment: what can patents tell us? Mem Inst Oswaldo Cruz. 2019;114:e180391. doi:10.1590/0074-02760180391
7. Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. 2017;17(8):873–881. doi:10.1016/S1473-3099(17)30243-8
8. Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(3):291–322. doi:10.1086/649858
9. Garvey EP, Sharp AD, Warn PA, et al. The novel fungal CYP51 inhibitor VT-1598 is efficacious alone and in combination with liposomal amphotericin B in a murine model of cryptococcal meningitis. J Antimicrob Chemoth. 2018;73(10):2815–2822. doi:10.1093/jac/dky242
10. Loyse A, Thangaraj H, Easterbrook P, et al. Cryptococcal meningitis: improving access to essential antifungal medicines in resource-poor countries. Lancet Infect Dis. 2013;13(7):629–637. doi:10.1016/S1473-3099(13)70078-1
11. Kong Q, Cao Z, Lv N, et al. Minocycline and fluconazole have a synergistic effect against Cryptococcus neoformans both in vitro and in vivo. Front Microbiol. 2020;11:836. doi:10.3389/fmicb.2020.00836
12. May RC, Stone NR, Wiesner DL, et al. Cryptococcus: from environmental saprophyte to global pathogen. Nat Rev Microbiol. 2016;14(2):106–117. doi:10.1038/nrmicro.2015.6
13. Datta A, Yadav V, Ghosh A, et al. Mode of action of a designed antimicrobial peptide: high potency against Cryptococcus neoformans. Biophys J. 2016;111(8):1724–1737. doi:10.1016/j.bpj.2016.08.032
14. Mayer FL, Kronstad JW. Disarming fungal pathogens: Bacillus safensis inhibits virulence factor production and biofilm formation by Cryptococcus neoformans and Candida albicans. mBio. 2017;8(5):e01537–17. doi:10.1128/mBio.01537-17
15. Ramage G, Williams C. Chapter two–the clinical importance of fungal biofilms. Adv Appl Microbiol. 2013;84(4):27–83. doi:10.1016/B978-0-12-407673-0.00002-3
16. Kumari P, Mishra R, Arora N, et al. Antifungal and anti-biofilm activity of essential oil active components against Cryptococcus neoformans and Cryptococcus laurentii. Front Microbiol. 2017;8:2161. doi:10.3389/fmicb.2017.02161
17. Martinez LR, Casadevall A. Specific antibody can prevent fungal biofilm formation and this effect correlates with protective efficacy. Infect Immun. 2005;73(10):6350–6362. doi:10.1128/IAI.73.10.6350-6362.2005
18. Casadevall A, Steenbergen JN, Nosanchuk JD. ‘Ready made’ virulence and ‘dual use’ virulence factors in pathogenic environmental fungi–the Cryptococcus neoformans paradigm. Curr Opin Microbiol. 2003;6(4):332–337. doi:10.1016/s1369-5274(03)00082-1
19. Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108. doi:10.1038/nrmicro821
20. Martinez LR, Casadevall A. Cryptococcus neoformans cells in biofilms are less susceptible than planktonic cells to antimicrobial molecules produced by the innate immune system. Infect Immun. 2006;74(11):6118–6123. doi:10.1128/IAI.00995-06
21. Jhong JH, Chi YH, Li WC, et al. dbAMP: an integrated resource for exploring antimicrobial peptides with functional activities and physicochemical properties on transcriptome and proteome data. Nucleic Acids Res. 2019;47(D1):D285–D297. doi:10.1093/nar/gky1030
22. Fjell CD, Hiss JA, Hancock RE, et al. Designing antimicrobial peptides: form follows function [published correction appears in Nat Rev Drug Discov. 2012 Feb;11(2):168]. Nat Rev Drug Discov. 2011;11(1):37–51. doi:10.1038/nrd3591
23. van der Does AM, Hiemstra PS, Mookherjee N. Antimicrobial host defence peptides: immunomodulatory functions and translational prospects. Adv Exp Med Biol. 2019;1117:149–171. doi:10.1007/978-981-13-3588-4_10
24. Xiu JF, Wang T, Wang Y, et al. Histological observation and expression patterns of antimicrobial peptides during fungal infection in Musca domestica (Diptera: muscidae) larvae. Braz Arch Biol Technol. 2016;59:1–13. doi:10.1590/1678-4324-2016160147
25. Guo G, Tao R, Li Y, et al. Identification and characterization of a novel antimicrobial protein from the housefly Musca domestica. Biochem Biophys Res Commun. 2017;490(3):746–752. doi:10.1016/j.bbrc.2017.06.112
26. Yang LB, Guo G, Zhao XY, et al. Antifungal activity and physicochemical properties of a novel antimicrobial protein AMP-17 from Musca domestica. Pol J Microbiol. 2019;68(3):383–390. doi:10.33073/pjm-2019-041
27. Ma H, Zhao X, Yang L, et al. Antimicrobial peptide AMP-17 affects Candida albicans by disrupting its cell wall and cell membrane integrity. Infect Drug Resist. 2020;13:2509–2520. doi:10.2147/IDR.S250278
28. CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard-Third Edition. M27-A3 Ed. (CLSI, Ed.). Wayne, PA: Clinical and Laboratory Standards Institute; 2008.
29. Seyedjavadi SS, Khani S, Eslamifar A, et al. The antifungal peptide MCh-AMP1 derived from Matricaria chamomilla inhibits Candida albicans growth via inducing ROS generation and altering fungal cell membrane permeability. Front Microbiol. 2020;10:3150. doi:10.3389/fmicb.2019.03150
30. Lemos ASO, Florêncio JR, Pinto NCC, et al. Antifungal activity of the natural coumarin scopoletin against planktonic cells and biofilms from a multidrug-resistant Candida tropicalis strain. Front Microbiol. 2020;11:1525. doi:10.3389/fmicb.2020.01525
31. Yang Y, Wang C, Gao N, et al. A novel dual-targeted α-Helical peptide with potent antifungal activity against fluconazole-resistant Candida albicans clinical isolates. Front Microbiol. 2020;11:548620. doi:10.3389/fmicb.2020.548620
32. Li L, Sun J, Xia S, et al. Mechanism of antifungal activity of antimicrobial peptide APP, a cell-penetrating peptide derivative, against Candida albicans: intracellular DNA binding and cell cycle arrest. Appl Microbiol Biotechnol. 2016;100(7):3245–3253. doi:10.1007/s00253-015-7265-y
33. Pierce CG, Uppuluri P, Tristan AR, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3(9):1494–1500. doi:10.1038/nport.2008.141
34. Brilhante RSN, Gotay WJP, Pereira VS, et al. Antifungal activity of promethazine and chlorpromazine against planktonic cells and biofilms of Cryptococcus neoformans/Cryptococcus gattii complex species. Med Mycol. 2020;58(7):906–912. doi:10.1093/mmy/myz140
35. Martinez LR, Casadevall A. Cryptococcus neoformans biofilm formation depends on surface support and carbon source and reduces fungal cell susceptibility to heat, cold, and UV light. Appl Environ Microbiol. 2007;73(14):4592–4601. doi:10.1128/AEM.02506-06
36. Quinteros MA, Galera ILD, Tolosa J, et al. Novel antifungal activity of oligostyrylbenzenes compounds on Candida tropicalis biofilms. Med Mycol. 2021;59(3):244–252. doi:10.1093/mmy/myaa046
37. Ravi S, Pierce C, Witt C, et al. Biofilm formation by Cryptococcus neoformans under distinct environmental conditions. Mycopathologia. 2009;167(6):307–314. doi:10.1007/s11046-008-9180-6
38. Liu W, Wu Z, Mao C, et al. Antimicrobial peptide Cec4 eradicates the bacteria of clinical carbapenem-resistant Acinetobacter baumannii biofilm. Front Microbiol. 2020;11:1532. doi:10.3389/fmicb.2020.01532
39. Spec A, Powderly WG. Cryptococcal meningitis in AIDS. Handb Clin Neurol. 2018;152:139–150. doi:10.1016/B978-0-444-63849-6.00011-6
40. Lawrence DS, Boyer-Chammard T, Jarvis JN. Emerging concepts in HIV-associated cryptococcal meningitis. Curr Opin Infect Dis. 2019;32(1):16–23. doi:10.1097/QCO.0000000000000514
41. Smith KD, Achan B, Hullsiek KH, et al. Increased antifungal drug resistance in clinical isolates of Cryptococcus neoformans in Uganda. Antimicrob Agents Chemother. 2015;59(12):7197–7204. doi:10.1128/AAC.01299-15
42. Martinez LR, Casadevall A. Biofilm formation by Cryptococcus neoformans. Microbiol Spectr. 2015;3(3). doi:10.1128/microbiolspec.MB-0006-2014
43. Raman N, Lee MR, Rodríguez López AL, et al. Antifungal activity of a β-peptide in synthetic urine media: toward materials-based approaches to reducing catheter-associated urinary tract fungal infections. Acta Biomater. 2016;43:240–250. doi:10.1016/j.actbio.2016.07.016
44. Ma L, Ye X, Sun P, et al. Antimicrobial and antibiofilm activity of the EeCentrocin 1 derived peptide EC1-17KV via membrane disruption. EBioMedicine. 2020;55:102775. doi:10.1016/j.ebiom.2020.102775
45. Yang Y, Chen F, Chen HY, et al. A Novel antimicrobial peptide scyreprocin from mud crab Scylla paramamosain showing potent antifungal and anti-biofilm activity. Front Microbiol. 2020;11:1589. doi:10.3389/fmicb.2020.01589
46. Tan L, Bai L, Wang L, et al. Antifungal activity of spider venom-derived peptide lycosin-I against Candida tropicalis. Microbiol Res. 2018;216:120–128. doi:10.1016/j.micres.2018.08.012
47. Cortés JCG, Curto MÁ, Carvalho VSD, et al. The fungal cell wall as a target for the development of new antifungal therapies. Biotechnol Adv. 2019;37(6):107352. doi:10.1016/j.biotechadv.2019.02.008
48. van der Weerden NL, Bleackley MR, Anderson MA. Properties and mechanisms of action of naturally occurring antifungal peptides. Cell Mol Life Sci. 2013;70(19):3545–3570. doi:10.1007/s00018-013-1260-1
49. Le CF, Fang CM, Sekaran SD. Intracellular targeting mechanisms by antimicrobial peptides. Antimicrob Agents Chemother. 2017;61(4):e02340–16. doi:10.1128/AAC.02340-16
50. Tavares ER, Gionco B, Morguette AEB, et al. Phenotypic characteristics and transcriptome profile of Cryptococcus gattii biofilm. Sci Rep. 2019;9(1):6438. doi:10.1038/s41598-019-42896-2
51. Korem M, Kagan S, Polacheck I. The effect of novel heterocyclic compounds on cryptococcal biofilm. J Fungi. 2017;3(3):42. doi:10.3390/jof3030042
52. Martinez LR, Casadevall A. Susceptibility of Cryptococcus neoformans biofilms to antifungal agents in vitro. Antimicrob Agents Chemother. 2006;50(3):1021–1033. doi:10.1128/AAC.50.3.1021-1033.2006
53. Vecchiarelli A. Immunoregulation by capsular components of Cryptococcus neoformans. Med Mycol. 2000;38(6):407–417. doi:10.1080/mmy.38.6.407.417
54. Al-Fattani MA, Douglas LJ. Penetration of candida biofilms by antifungal agents. Antimicrob Agents Chemother. 2004;48(9):3291–3297. doi:10.1128/AAC.48.9.3291-3297.2004
55. Chandra J, Kuhn DM, Mukherjee PK, et al. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001;183(18):5385–5394. doi:10.1128/JB.183.18.5385-5394.2001
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.