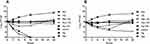Back to Journals » Infection and Drug Resistance » Volume 15
In vitro Antibacterial Activity and Resistance Prevention of Antimicrobial Combinations for Dihydropteroate Synthase-Carrying Stenotrophomonas maltophilia
Authors Zhao J, Huang Y, Li J, Zhang B, Dong Z , Wang D
Received 28 March 2022
Accepted for publication 3 June 2022
Published 13 June 2022 Volume 2022:15 Pages 3039—3046
DOI https://doi.org/10.2147/IDR.S368338
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Jin Zhao,1,* Yan Huang,1,* Jian Li,1,* Bo Zhang,1 Zhiwei Dong,2 Dong Wang1
1Department of Pulmonary and Critical Care Medicine, Air Force Medical Center, PLA, Beijing, 100142, People’s Republic of China; 2Department of General Surgery, Air Force Medical Center, PLA, Beijing, 100142, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dong Wang, Department of Pulmonary and Critical Care Medicine, Air Force Medical Center, PLA, No. 30 Fucheng Road, Haidian District, Beijing, 100142, People’s Republic of China, Tel +8613661256489, Fax +86 10-84671291-6202, Email [email protected] Zhiwei Dong, Department of General Surgery, Air Force Medical Center, PLA, No. 30 Fucheng Road, Haidian District, Beijing, 100142, People’s Republic of China, Tel +8618618261596, Fax +86 10-84671291-3106, Email [email protected]
Background: Stenotrophomonas maltophilia (S. maltophilia) is a multidrug-resistant gram-negative bacillus that is known to be an opportunistic pathogen, particularly in a hospital environment. The infection has a high morbidity and mortality. Sulfamethoxazole-trimethoprim (SXT) is the first-line agent recommended for its treatment. The global spread of dihydropteroate synthase (sul) genes has resulted in an increased resistance rate. However, the appropriate therapy for infections caused by sul-carrying S. maltophilia has not yet been established.
Objective: Our study aimed to identify the optimal antibiotic combinations that could both show high antibacterial activity against sul-carrying S. maltophilia and the ability to prevent the emergence of resistance at clinical dosage regimens.
Methods: Time-killing experiments and mutant prevention concentration (MPC) experiments were conducted to evaluate the antibacterial effect and ability to prevent resistance to minocycline, tigecycline, moxifloxacin, and ticarcillin/clavulanic acid (T/K), both alone and in combination, at clinically relevant antimicrobial concentrations.
Results: Minocycline, tigecycline, and T/K all exhibited bacteriostatic activity to sul-carrying S. maltophilia. The combination of minocycline plus T/K and tigecycline plus T/K neither enhanced the bactericidal ability nor prevented drug-resistant mutations. Moxifloxacin, at 2 mg/L, showed good bactericidal activity to most S. maltophilia, but bacterial regrowth at 24 h was observed in two strains. When combined with T/K, moxifloxacin showed good bactericidal activity in all moxifloxacin-sensitive strains. The concentrations of moxifloxacin alone were lower than most MPCs of the tested sul-carrying strains. When combined with T/K, the mean steady-state concentrations (MSC) of moxifloxacin could prevent 70% of resistance, and the peak concentration (Cmax) prevented 95% of resistance.
Conclusion: The combination of moxifloxacin and T/K can achieve a good in vitro bactericidal effect and prevent the emergence of resistance at clinical dosage regimens, and may be an optimal therapeutic strategy for S. maltophilia infections, especially for vulnerable immunocompromised and critically ill patients.
Keywords: sulfamethoxazole-trimethoprim, dihydropteroate synthase, moxifloxacin, mutant prevention concentration, pharmacokinetic, Cmax
Introduction
Stenotrophomonas maltophilia (S. maltophilia) is a multidrug-resistant organism found in hospital settings that can cause respiratory, bloodstream, abdominal, and other severe hospital-acquired infections, with a high incidence and mortality rate.1–4 S. maltophilia exhibits intrinsic resistance to most commonly-used antimicrobial agents, and sulfamethoxazole-trimethoprim (SXT) is the first-line agent recommended for treatment.2,5,6 However, with the global spread of dihydropteroate synthase (sul) genes, SXT resistance has emerged, and there has been a rapid increase in its rates.7,8 Our previous epidemiological survey confirmed that the presence of sul genes was the predominant resistance mechanism for SXT in clinically-isolated S. maltophilia in China.9 S. maltophilia shows high susceptibility to tetracycline derivatives, including minocycline, doxycycline, and tigecycline, and these antimicrobials can be used as alternatives for the treatment of S. maltophilia infection, even for SXT-resistant strains.1,2,9 However, an in vitro study by Wei et al found that tetracycline derivatives exhibited bacteriostatic activity against S. maltophilia, which can only inhibit the proliferation of S. maltophilia instead of effectively killing the organisms.10 S. maltophilia infections typically occur in vulnerable, immunocompromised, and critically ill patients, whose immune system is usually impaired and cannot effectively kill pathogens.2,3,11 Therefore, the use of a sole antimicrobial agent may not provide adequate treatment. Moreover, S. maltophilia possesses a variety of intrinsic drug resistance mechanisms, such as efflux pumps. Long-term use of improper antimicrobial agents may result in the development of drug resistance, and combination therapy should be recommended for infections caused by sul-carrying S. maltophilia.1,6,10,12 However, there is limited data regarding which antibiotic combinations are the most effective.
The objectives of this study were to evaluate the in vitro antibacterial activity and ability to prevent drug-resistant mutations of different antimicrobial combinations against sul-carrying S. maltophilia. The concentrations of antimicrobial agents are chosen based on clinical pharmacokinetics to ensure that the therapeutic effects are evaluated in a clinically relevant manner.10,13
Materials and Methods
Bacterial Strains and Susceptibility Testing
Non-duplicated clinical S. maltophilia were collected from hospitalized patients at the Chinese PLA General Hospital and Air Force Medical Center from 2020 to 2021. These S. maltophilia strains were all isolated from respiratory tract specimens of patients with pulmonary infections. The identification of bacterial species was performed using a Vitek II bacterial identification system (bioMérieux, Marcy-l’Étoile, France) and further confirmed via a species-specific polymerase chain reaction.9,14,15 The detection of the sul genes (including sul1 and sul2 genes) was presented as follows: SUL1-F(GCTATTGGTCTCGGTGTCGC) and SUL1-B(GCATGATCTAACCCTCGGTCT) for sul1; SUL2-F(TTTCGGCATCGTCAACATAA) and SUL2-B(CCACGCGACAAGGCATA) for sul2. The PCR reaction volume system and cycling parameters were the same as in our previously published literature.15 Minimum inhibitory concentration (MIC) results for SXT, minocycline, tigecycline, ticarcillin/clavulanate (T/K), and moxifloxacin were obtained by the agar dilution method, and they were interpreted according to the breakpoints suggested by CLSI (2021), as previously described.9,16 Moxifloxacin and tigecycline have no published breakpoint criteria for S. maltophilia, so they were interpreted with reference to those of Enterobacteriaceae (susceptibility at 2 ug/mL, intermediate at 4 ug/mL, and resistance at 8 ug/mL), as in the study by Wei et al.10 Twenty sul-carrying bacterial strains susceptible to minocycline, tigecycline, moxifloxacin, and T/K were chosen for further in vitro experiments, and the carrying situation of sul genes and MICs are presented in Table S1.
Time-Kill Experiments
Six of the 20 candidate sul-carrying S. maltophilia were chosen randomly for the time-kill assays, and an overnight inoculum of approximately 106 colony-forming units (CFU)/mL was used. The drug concentrations used in the time-kill curves were lower than the mean steady-state concentrations (MSCs) of non-protein-bound drugs in humans to achieve a better simulation of the actual clinical conditions.10,17 The MSCs for minocycline (200 mg po 24 h) and moxifloxacin (400 mg, q24 h) were calculated based on the area under the antibiotic concentration-time curve (AUC) in serum or plasma over 24 h divided by 24 h (AUC0-24/24 h). The MSC of tigecycline (50 mg, q12 h) was based on the AUC in serum or plasma over 12 h divided by 12 h, and the MSC of T/K (3 g/0.1g, q8 h) was based on the AUC in serum or plasma over 8 h divided by 8 h.10,18 The pharmacokinetic parameters included in the time-kill experiments are shown in Table 1. The concentrations of each antimicrobial agent were as follows: minocycline, 2 mg/L; tigecycline, 0.25 mg/L; and moxifloxacin, 2 mg/L. As T/K is a compound preparation, of which ticarcillin is the antibacterial component and clavulanic acid is a β-lactamase inhibitor, 32/2 mg/L was used based on the MSC of ticarcillin. The antibacterial activities of minocycline, tigecycline, and moxifloxacin used alone and in combination with T/K were evaluated at 0, 3, 6, 12, and 24 h. Culture samples were serially diluted, spread on plates, and incubated at 35°C, and the resulting bacterial colonies were counted after 24 h. Bacteriostatic activity was defined as a decrease in bacterial concentration < 3 log10 compared to the bacterial concentration of the initial inoculum. Bactericidal activity was defined as a decrease in bacterial concentration ≥ 3 log10 compared with the initial inocula.10,23
 |
Table 1 Summary of the Pharmacokinetic Parameters and the Experimental Concentrations of the Antimicrobial Agents Used in the Time-Kill Experiments |
Determination of MPCs
The mutant prevention concentrations (MPCs) of minocycline, tigecycline, and moxifloxacin alone and in combination with T/K were determined in 20 candidate sul-carrying isolates using a modified agar dilution method, as previously described.24,25 In brief, approximately 0.3×1010 CFU/mL of bacterial cells were placed onto Mueller–Hinton Agar plates containing different concentrations of antimicrobial agents. Each drug concentration was included on at least four plates to ensure that the total cell number in the inoculum was > 1×1010. The plates were incubated at 35°C for 72 h. The MPC was defined as the lowest antibiotic concentration that prevented the visible growth of mutant colonies after 72 h.
Results
Time-Kill Experiments
The carrying situation of sul genes and MICs of the six strains chosen for the time-kill assays are presented in Table 2. Minocycline, tigecycline, and T/K exhibited bacteriostatic activity in all six sul-carrying S. maltophilia strains (Table 3). Minocycline plus T/K and tigecycline plus T/K combination therapies were not superior to minocycline or tigecycline monotherapy in terms of antimicrobial effects. Moxifloxacin at 2 mg/L showed good bactericidal activity in A1-A4 strains (Table 3, Figure 1A), but bacterial regrowth at 24 h was observed in A5 and A6 strains (Table 3, Figure 1B). When combined with T/K, the bactericidal activity of moxifloxacin was observed against all six S. maltophilia strains (Table 3, Figure 1).
 |
Table 2 The Carrying Situation of Dihydropteroate Synthase Genes and the Minimum Inhibitory Concentration of the Six Chosen Stenotrophomonas maltophilia Strains Used in the Time-Kill Experiment |
 |
Table 3 Change in Bacterial Concentrations at 24 h of the Six Sul-Carrying Stenotrophomonas maltophilia Strains |
Effectiveness of Antimicrobial Combinations for Resistance Prevention
The MPCs of moxifloxacin, minocycline, and tigecycline alone and in combination with T/K are presented in Table 4. The MSCs and peak concentration (Cmax) of the conventional clinical doses of moxifloxacin, minocycline, tigecycline, and T/K were all lower than the MPCs of all tested 20 strains of sul-carrying S. maltophilia and were within the mutant selection window (MSW). The ability to prevent resistance to minocycline and tigecycline was not significantly improved when these drugs were combined with T/K (Table 4). When moxifloxacin (400 mg q 24 h) was used, the MSC was 2 ug/mL, and the Cmax was 4.5 ug/mL (Table 2). The MSC was within the MSW in all 20 strains, while the Cmax could only prevent resistance of 10% of the strains. In combination with T/K, the MSC of moxifloxacin could prevent resistance of 70% of sul-carrying S. maltophilia strains, while the Cmax could prevent resistance of 95% of strains (Table 4).
 |
Table 4 The Ability of Antimicrobial Agents to Prevent the Occurrence of Resistance at Clinical Dosage Regimens Against Sul-Carrying S. maltophilia |
Discussion
SXT is the first-line agent recommended for the treatment of S. maltophilia infections.2,5 The global spread of sul genes has increased the rates of resistance to SXT in recent years. However, there is no consensus regarding the treatment of sul-carrying S. maltophilia.8,9 Therefore, we evaluated the in vitro antibacterial activity of different antimicrobial combinations against sul-carrying S. maltophilia by a time-kill experiment and investigated their ability to curb the emergence of resistance by an MPC experiment. We found that the commonly used alternative drugs, minocycline and tigecycline, showed bacteriostatic effects and could not effectively prevent resistance, even after being combined with TK. Moxifloxacin showed bactericidal effects against most strains, but bacterial regrowth at 24 h was observed in several strains. When moxifloxacin was combined with TK at a clinically relevant concentration, it not only showed good bactericidal effects but also inhibited the occurrence of resistance.
S. maltophilia, even sul-carrying S. maltophilia, exhibits high sensitivity to tetracycline derivatives, such as minocycline and tigecycline.1,2,9 However, both minocycline and tigecycline showed bacteriostatic activity instead of bactericidal activity towards S. maltophilia,4,10,26 and there was no increase in antibacterial activity in combination with T/K. We further evaluated the ability of these drugs to prevent resistance using an MPC experiment. MPC is the concentration threshold above which no single-step drug-resistant mutant strains can be selected.25 MSW is the concentration range from the MIC to the MPC. When bacteria grow within the MSW concentration for prolonged periods, there is an enrichment of drug-resistant strains.24,27 The MSC and Cmax values of minocycline, tigecycline, and T/K at clinically relevant doses were lower than the MPC values and were within the MSW of all sul-carrying S. maltophilia strains, suggesting that long-term single-agent therapy with these drugs may result in enrichment of strains with resistant mutations. When used in combination with T/K, minocycline and tigecycline could still prevent the occurrence of resistance. Based on these results, even in combination with T/K, clinically utilized minocycline or tigecycline dosing regimens may fail to achieve the expected effect against S. maltophilia infection in patients with hematological malignancies, prolonged neutropenia, or receiving broad-spectrum antimicrobial therapy.11,28
The new fluoroquinolones, such as moxifloxacin and levofloxacin, have good in vitro activity and safety and are thus widely used in the treatment of pulmonary infections, hence being called respiratory quinolones. Based on observational evidence, levofloxacin and moxifloxacin are reasonable alternatives to SXT for the treatment of bloodstream and lower respiratory tract infections caused by S maltophilia.10,29–31 It is reported that sul-carrying S. maltophilia were more susceptible to moxifloxacin than levofloxacin in in vitro experiments, and moxifloxacin can inhibit the biofilm they form on the surface of the respiratory tract or intubation tubes.9,32–34 Therefore, moxifloxacin has a high clinical value as a therapeutic option for sul-carrying S. maltophilia. Based on clinical pharmacokinetics, the antibacterial activity and the ability to prevent resistance to moxifloxacin (400 mg q 24 h) in MSC (2 ug/mL) and Cmax (4.5 ug/mL) concentration were evaluated. Moxifloxacin, at 2 ug/mL, showed bactericidal effects against most strains, but bacterial regrowth at 24 h was also observed in several strains, as a previous study reported.35 The MPCs of most tested strains were higher than 4.5 ug/mL, and long-term application of single-agent moxifloxacin may result in the selective enrichment of resistant mutants and lead to treatment failure. It is reported that overuse of fluoroquinolones worldwide has resulted in a higher resistance rate among many bacterial pathogens, including S. maltophilia36,37 Moreover, overexpression of the SmeDEF efflux pump is the common drug resistance mechanism for S. maltophilia to quinolones, doxycycline, and tigecycline,15 and improper long-term use of quinolones may lead to extensive resistance through hyperexpression of SmeDEF efflux pumps. In combination with T/K, moxifloxacin exhibited bactericidal effects against all tested sul-carrying S. maltophilia strains and reduced the MPCs of most tested S. maltophilia to below 2 ug/mL, indicating that the combination can effectively prevent the emergence of resistance. Based on in vitro experiments, moxifloxacin (400 mg q 24h) combined with T/K (3 g/0.1 g q 8 h) may be an optimal therapeutic option for sul-carrying S. maltophilia infections. The serum concentration of moxifloxacin after intravenous administration was 31% higher than that after oral administration.35,38 Therefore, intravenous administration of moxifloxacin is first recommended.
Our study had some limitations. First, although the pharmacokinetic parameters of antimicrobial agents in humans were taken into consideration, the antibiotic concentrations used in the experiments were constant, which cannot simulate the dynamic drug concentration changes in the human body. In addition, the immune system plays an important role in defending against bacterial infections, but its effect was not considered in our experiment. Further in vitro pharmacokinetic/pharmacodynamic and animal studies are still needed to fully evaluate the efficacy of this drug combination.
Conclusion
Minocycline, tigecycline, and T/K all exhibited bacteriostatic activity against sul-carrying S. maltophilia, and long-term single-agent therapy with these drugs may result in the enrichment of strains with resistant mutations. The combination of moxifloxacin and T/K can achieve good in vitro bactericidal effects and prevent the emergence of resistance at clinical dosage regimens and may be an optimal therapeutic strategy for sul-carrying S. maltophilia infection, especially for vulnerable immunocompromised and critically ill patients.
Abbreviations
S. maltophilia, Stenotrophomonas maltophilia; SXT, Sulfamethoxazole-trimethoprim; sul, dihydropteroate synthase; MSC, mean steady-state concentrations; Cmax, peak concentration; CFU, colony-forming units; MIC, minimum inhibitory concentration; MPC, mutant prevention concentration; Min, minocycline; Tig, tigecycline; Mox, moxifloxacin; T/K, ticarcillin/clavulanic acid; AUC, area under the antibiotic concentration-time curve.
Data Sharing Statement
The data used to support the findings of this study are available from Dong Wang upon request through [email protected].
Funding
This study was supported by the “Young doctor boost Project” (21ZTO17).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chang YT, Lin CY, Chen YH, Hsueh PR. Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front Microbiol. 2015;6:893. doi:10.3389/fmicb.2015.00893
2. Looney WJ, Narita M, Mühlemann K. Stenotrophomonas maltophilia: an emerging opportunist human pathogen. Lancet Infect Dis. 2009;9(5):312–323. doi:10.1016/S1473-3099(09)70083-0
3. Falagas ME, Kastoris AC, Vouloumanou EK, Rafailidis PI, Kapaskelis AM, Dimopoulos G. Attributable mortality of Stenotrophomonas maltophilia infections: a systematic review of the literature. Future Microbiol. 2009;4(9):1103–1109. doi:10.2217/fmb.09.84
4. Fratoni AJ, Nicolau DP, Kuti JL. Minocycline pharmacodynamics against Stenotrophomonas maltophilia in the neutropenic murine infection model: implications for susceptibility breakpoints. J Antimicrob Chemother. 2022;77(4):1052–1060. doi:10.1093/jac/dkac018
5. Abbott IJ, Slavin MA, Turnidge JD, Thursky KA, Worth LJ. Stenotrophomonas maltophilia: emerging disease patterns and challenges for treatment. Expert Rev Anti-Infect Ther. 2011;9(4):471–488. doi:10.1586/eri.11.24
6. Pollini S, Rossolini GM, Pallecchi L, Andelkovic MV. Antimicrobial treatment of Stenotrophomonas maltophilia invasive infections: systematic review. Antibiotics. 2019;31(6):297–306.
7. Toleman MA, Bennett PM, Bennett DM, Jones RN, Walsh TR. Global emergence of trimethoprim/sulfamethoxazole resistance in Stenotrophomonas maltophilia mediated by acquisition of sul genes. Emerg Infect Dis. 2007;13(4):559–565. doi:10.3201/eid1304.061378
8. Al-Jasser AM. Stenotrophomonas maltophilia resistant to trimethoprim-sulfamethoxazole: an increasing problem. Ann Clin Microbiol Antimicrob. 2006;5:23. doi:10.1186/1476-0711-5-23
9. Zhao J, Xing Y, Liu W, et al. Surveillance of dihydropteroate synthase genes in Stenotrophomonas maltophilia by LAMP: implications for infection control and initial therapy. Front Microbiol. 2016;7:1723. doi:10.3389/fmicb.2016.01723
10. Wei C, Ni W, Cai X, Zhao J, Cui J. Evaluation of trimethoprim/sulfamethoxazole (SXT), minocycline, tigecycline, moxifloxacin, and ceftazidime alone and in combinations for SXT-susceptible and SXT-resistant Stenotrophomonas maltophilia by in vitro time-kill experiments. PLoS One. 2016;11(3):e0152132. doi:10.1371/journal.pone.0152132
11. Garazi M, Singer C, Tai J, Ginocchio CC. Bloodstream infections caused by Stenotrophomonas maltophilia: a seven-year review. J Hosp Infect. 2012;81(2):114–118. doi:10.1016/j.jhin.2012.02.008
12. Dizbay M, Shah MD, Coe KE, et al. Efficacy of combination therapy versus monotherapy in the treatment of Stenotrophomonas maltophilia pneumonia. Turk J Med Sci. 2019;74(7):2055–2059.
13. Drlica K, Zhao X. Mutant selection window hypothesis updated. Clin Infect Dis. 2007;44(5):681–688. doi:10.1086/511642
14. Whitby PW, Carter KB, Burns JL, Royall JA, LiPuma JJ, Stull TL. Identification and detection of Stenotrophomonas maltophilia by rRNA-directed PCR. J Clin Microbiol. 2000;38(12):4305–4309. doi:10.1128/JCM.38.12.4305-4309.2000
15. Zhao J, Liu Y, Liu Y, et al. Frequency and genetic determinants of tigecycline resistance in clinically isolated Stenotrophomonas maltophilia in Beijing, China. Front Microbiol. 2018;9:549. doi:10.3389/fmicb.2018.00549
16. CLSI. Performance Standards for Antimicrobial Susceptibility Testing.
17. Tängdén T, Hickman RA, Forsberg P, Lagerbäck P, Giske CG, Cars O. Evaluation of double- and triple-antibiotic combinations for VIM- and NDM-producing Klebsiella pneumoniae by in vitro time-kill experiments. Antimicrob Agents Chemother. 2014;58(3):1757–1762. doi:10.1128/AAC.00741-13
18. Meagher AK, Ambrose PG, Grasela TH, Ellis-Grosse EJ. Pharmacokinetic/pharmacodynamic profile for tigecycline-a new glycylcycline antimicrobial agent. Diagn Microbiol Infect Dis. 2005;52(3):165–171. doi:10.1016/j.diagmicrobio.2005.05.006
19. Rodvold KA, Neuhauser M. Pharmacokinetics and pharmacodynamics of fluoroquinolones. Pharmacotherapy. 2001;21(10 Pt 2):233S–252S. doi:10.1592/phco.21.16.233s.33992
20. Guglielmo BJ, Flaherty JF, Batman R, Barriere SL, Gambertoglio JG. Comparative pharmacokinetics of low- and high-dose ticarcillin. Antimicrob Agents Chemother. 1986;30(3):359–360. doi:10.1128/AAC.30.3.359
21. Jaresko GS, Barriere SL, Johnson BL
22. Burgess DS, Frei CR. Comparison of beta-lactam regimens for the treatment of gram-negative pulmonary infections in the intensive care unit based on pharmacokinetics/pharmacodynamics. J Antimicrob Chemother. 2005;56(5):893–898. doi:10.1093/jac/dki335
23. Pillai SK, Moellering RC, Eliopoulos GM. Antimicrobial combinations. In: Lorian V, editor. Antibiotics in Laboratory Medicine.
24. Ni W, Cui J, Liang B, et al. In vitro effects of tigecycline in combination with colistin (polymyxin E) and sulbactam against multidrug-resistant Acinetobacter baumannii. J Antibiot. 2013;66(12):705–708. doi:10.1038/ja.2013.84
25. Zhao X, Drlica K. Restricting the selection of antibiotic-resistant mutants: a general strategy derived from fluoroquinolone studies. Clin Infect Dis. 2001;33(suppl 3):S147–S156. doi:10.1086/321841
26. Wei C, Ni W, Cai X, Cui J, Monte A. Carlo pharmacokinetic/pharmacodynamic simulation to evaluate the efficacy of minocycline, tigecycline, moxifloxacin, and levofloxacin in the treatment of hospital-acquired pneumonia caused by Stenotrophomonas maltophilia. Infect Dis. 2015;47(12):846–851. doi:10.3109/23744235.2015.1064542
27. Drlica K. The mutant selection window and antimicrobial resistance. J Antimicrob Chemother. 2003;52(1):11–17. doi:10.1093/jac/dkg269
28. Cho SY, Lee DG, Choi SM, et al. Stenotrophomonas maltophilia bloodstream infection in patients with hematologic malignancies: a retrospective study and in vitro activities of antimicrobial combinations. BMC Infect Dis. 2015;15:69. doi:10.1186/s12879-015-0801-7
29. Sarzynski SH, Warner S, Sun J, et al. Trimethoprim-sulfamethoxazole versus levofloxacin for Stenotrophomonas maltophilia infections: a retrospective comparative effectiveness study of electronic health records from 154 US hospitals. Open Forum Infect Dis. 2022;9(2):ofab644. doi:10.1093/ofid/ofab644
30. Watson L, Esterly J, Jensen AO, Postelnick M, Aguirre A, McLaughlin M. Sulfamethoxazole/trimethoprim versus fluoroquinolones for the treatment of Stenotrophomonas maltophilia bloodstream infections. J Glob Antimicrob Resist. 2018;12:104–106. doi:10.1016/j.jgar.2017.09.015
31. Wang YL, Scipione MR, Dubrovskaya Y, Papadopoulos J. Monotherapy with fluoroquinolone or trimethoprim-sulfamethoxazole for treatment of Stenotrophomonas maltophilia infections. Antimicrob Agents Chemother. 2014;58(1):176–182. doi:10.1128/AAC.01324-13
32. Wang A, Wang Q, Kudinha T, Xiao S, Zhuo C. Effects of fluoroquinolones and azithromycin on biofilm formation of Stenotrophomonas maltophilia. Sci Rep Sci Rep. 2016;6:29701. doi:10.1038/srep29701
33. Pompilio A, Catavitello C, Picciani C, et al. Subinhibitory concentrations of moxifloxacin decrease adhesion and biofilm formation of Stenotrophomonas maltophilia from cystic fibrosis. J Med Microbiol. 2010;59(1):76–81. doi:10.1099/jmm.0.011981-0
34. Di Bonaventura G, Spedicato I, D’Antonio D, Robuffo I, Piccolomini R. Biofilm formation by Stenotrophomonas maltophilia: modulation by quinolones, trimethoprim-sulfamethoxazole, and ceftazidime. Antimicrob Agents Chemother. 2004;48(1):151–160. doi:10.1128/AAC.48.1.151-160.2004
35. Giamarellos-Bourboulis EJ, Karnesis L, Galani I, Giamarellou H. In vitro killing effect of moxifloxacin on clinical isolates of Stenotrophomonas maltophilia resistant to trimethoprim-sulfamethoxazole. Antimicrob Agents Chemother. 2002;46(12):3997–3999. doi:10.1128/AAC.46.12.3997-3999.2002
36. Chang YT, Lin CY, Lu PL, et al. Stenotrophomonas maltophilia bloodstream infection: comparison between community-onset and hospital-acquired infections. J Microbiol Immunol Infect. 2014;47(1):28–35. doi:10.1016/j.jmii.2012.08.014
37. Pien CJ, Kuo HY, Chang SW, et al. Risk factors for levofloxacin resistance in Stenotrophomonas maltophilia from respiratory tract in a regional hospital. J Microbiol Immunol Infect. 2015;48(3):291–295. doi:10.1016/j.jmii.2013.09.005
38. Stass H, Kubitza D. Pharmacokinetics and elimination of moxifloxacin after oral and intravenous administration in man. J Antimicrob Chemother. 1999;43(supplB):83–90. doi:10.1093/jac/43.suppl_2.83
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.