Back to Journals » Drug Design, Development and Therapy » Volume 17
In vitro and in vivo Efficacies of Novel Harmine Derivatives in the Treatment of Cystic Echinococcosis
Authors Chen B, Yan M, Gao H, Ma Q, Li L, Lü G, Gong Y, Wen L, Xu S, Wang J, Zhao J
Received 27 April 2023
Accepted for publication 8 August 2023
Published 21 August 2023 Volume 2023:17 Pages 2441—2454
DOI https://doi.org/10.2147/DDDT.S419002
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Bei Chen,1,2,* Mingzhi Yan,1,2,* Huijing Gao,1,2 Qin Ma,3 Lihua Li,4 Guodong Lü,1,2 Yuehong Gong,1,2 Limei Wen,1,2 Shaoquan Xu,5 Jianhua Wang,1,2 Jun Zhao1,2
1First Affiliated Hospital of Xinjiang Medical University, Urumqi, Xinjiang, People’s Republic of China; 2State Key Laboratory of Pathogenesis, Prevention and Treatment of High Incidence Diseases in Central Asia, The First Affiliated Hospital of Xinjiang Medical University, Urumqi, Xinjiang, People’s Republic of China; 3HuaShiDan Pharmaceutical Company Limited, Urumqi, Xinjiang, People’s Republic of China; 4Xinjiang Urumqi Maternal and Child Health Hospital, Urumqi, Xinjiang, People’s Republic of China; 5College of Pharmacy, Xinjiang Medical University, Urumqi, Xinjiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jun Zhao; Jianhua Wang, First Affiliated Hospital of Xinjiang Medical University, State Key Laboratory of Pathogenesis, Prevention, and Treatment of Central Asian High Incidence Diseases, Urumqi, Xinjiang, 830011, People’s Republic of China, Email [email protected]; [email protected]
Introduction: Cystic echinococcosis (CE) is a chronic zoonotic parasitic disease caused by the larvae of the Echinococcus granulosus sensu lato (s.l.) cluster. The current existing drugs have limited therapeutic efficacy against cystic echinococcosis, and thus, there is an urgent need to develop new drugs.
Methods: In this study, 7 harmine (HM) derivatives were screened and the effects of HM derivatives on E. granulosus sensu stricto (s.s.) were evaluated by in vitro and mouse experiments. The safety of the HM derivatives was assessed by cytotoxicity assays, acute toxicity study in animals and subacute toxicity study.
Results: These results show that the HM derivatives H-2-168 and DH-004 exhibited more significant antiparasitic effects at an initial concentration of 40 μM. The results of further studies showed that H-2-168 and DH-004 had dose-dependent effects against protoscoleces and had satisfactory therapeutic outcomes in vivo. Electron microscopy observations demonstrated that H-2-168 and DH-004 caused severe disruption of the parasite ultrastructure. Notably, the results of the acute toxicity and subchronic toxicity studies showed that H-2-168 and DH-004 had significantly improved safety. In addition, we found that H-2-168 and DH-004 induced DNA damage in E. granulosus s.s., which may be the mechanism by which these drugs produce their therapeutic effects.
Discussion: Overall, the data from this work demonstrate that H-2-168 and DH-004 are highly effective candidate compounds with low toxicity for the treatment of CE and will provide a new therapeutic strategy for CE pharmacological treatment.
Keywords: harmine derivatives, β-carboline, Echinococcus granulosus sensu stricto, cystic echinococcosis, DNA damage
Graphical Abstract:
Introduction
Cystic echinococcosis (CE), a chronic zoonotic parasitic disease caused by infection with larvae of Echinococcus granulosus sensu lato (s.l.), is a global public health problem,1 as the incidence of CE ranges from 1/100,000 to 200/100,000 worldwide.2 China is an endemic region for CE, where it is mainly concentrated in western China. At least 1 million disability-adjusted life years and $3 billion in losses are caused by CE.3 Currently, surgery combined with drug therapy is the first choice for the treatment of this disease, but there are risks of infection and recurrence during surgery. Pharmacological treatment can effectively reduce the recurrence rate and improve the surgical cure rate.2 The drug recommended for treatment by the World Health Organization is albendazole, but this compound has poor absorption, low bioavailability, and low therapeutic efficacy.4 Unfortunately, to date, no new drugs are available. Therefore, there is an urgent need to develop new therapeutic drugs to treat CE.
Peganum harmala L., a plant in the Zingiberaceae family, grows spontaneously in semiarid conditions, steppe areas and sandy soils and has long been used in traditional medicine.5 Its main constituent is harmine (HM, 7-methoxy-1-methyl-9H-pyrido[3,4-b] indole), a tricyclic β-carboline alkaloid with a wide range of pharmacological properties, including antitumor,6 antidepressant,7 vasorelaxant,8 and antiparasitic effects.9 Our recent study found that HM has anti-E. granulosus s.l. efficacy in vitro.10 However, it is noteworthy that HM has severe neurotoxicity, including tremor, convulsions, excitation, and neural inhibition, which limits its clinical application and development.11
Making modifications to the chemical structure of a drug is an effective strategy to improve the therapeutic effect of the drug while reducing its toxicity. Generally, the active monomeric components of natural products are selected as lead compounds, and their associated chemical structures are modified to obtain derivatives of the natural product active ingredients for clinical application.12,13 Studies have reported the application of HM derivatives for antitumor purposes, demonstrating the feasibility of HM structural modification.6,14,15 Furthermore, HM derivatives have shown higher activity and lower neurotoxicity than HM.16 Previously, our research team synthesized an HM derivative, DH-330, to evaluate its therapeutic effect on CE.17 In the present study, the chemical structure of HM was further modified and optimized with the aim of screening for efficient derivatives (Figure 1). Moreover, with both in vitro and in vivo experiments, we evaluated the therapeutic effects of the derivative on CE and its toxic effects to the host with the expectation that promising new compounds to treat CE would be found. Additionally, the ability of the HM derivatives to cause DNA damage was also explored, providing a theoretical basis for the subsequent exploration of their possible mechanisms of action.
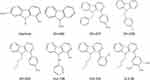 |
Figure 1 Chemical structures of the harmine derivatives studied in this work. |
Materials and Methods
Ethics Statement
Kunming mice (license number: SYXK (Xin) 2018-0003), aged 8 weeks, were purchased from the Experimental Animal Center of Xinjiang Medical University. All protocols involving animals were approved by the Animal Welfare and Committee of First Affiliated Hospital of Xinjiang Medical University (IACUC-20170420-04) and conformed to the Guidelines for the Care and Use of Animals in Xinjiang Medical University.
Materials
HM and its derivatives (purity > 98%) were synthesized by Xinjiang Huashidan Pharmaceutical Co., Ltd. Albendazole (ABZ, purity > 98%) was purchased from Sigma-Aldrich (St. Louis, USA).
Parasite Collection and Culture
Protoscoleces (PSCs) were isolated from the liver cysts of naturally infected sheep slaughtered in a slaughterhouse in Urumqi, Xinjiang, China. These sheep were originally planned for routine slaughter. For parasite collection and culture, methods were as described previously.18 Briefly, the collected PSCs were washed twice with PBS (Biological Industries, Kibbutz Beit, HaEmek, Israel), digested with 1% pepsin for 30 min and washed twice with PBS. Then, the PSCs were transferred to 25 cm2 cell culture flasks containing RPMI 1640 medium (Gibco, Grand Island, NY, USA) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin (HyClone, South Logan, UT, USA) and 10% fetal bovine serum (Gibco, Grand Island, NY, USA). The viability of the parasites was assessed by 1% eosin staining and was required to be > 98%. PSCs that met the requirements were incubated in an incubator at 37 °C and 5% CO2.
In vitro Drug Treatment of E. granulosus s.s. PSCs
Before the start of the experiment, PSCs were cultured for 3 days and tested for viability (> 98%). PSCs were treated in vitro in 96-well plates containing 200 PSCs per well. All drugs were prepared in dimethyl sulfoxide (DMSO) as 20 mM stock solutions and added to the wells to a final concentration of 40 μM for the initial screening. The screened drugs DH-330, H-2-168 and DH-004 were used at a concentration gradient of 40, 80, 120, 160, 200 and 240 μM. PSCs cultured in medium containing 1% DMSO were used as negative controls. ABZ and HM (both 120 μM) were used as positive controls, and the viability of PSCs was detected by a 1% eosin staining assay after 48 h of in vitro treatment (PSCs that turned red after eosin staining were considered dead while surviving PSCs were colorless).19 Each experiment was repeated at least three times. PSCs were collected for observation by scanning electron microscopy (SEM) (LEO1430VP, LEO company, Germany) after drug intervention, and the experiments were performed as previously described.20
Drug Treatment of E. granulosus s.s.-Infected Mice
The E. granulosus s.s.-infected mouse model was established as described previously21 by intraperitoneally infecting 8-week-old female Kunming mice with 50 microcysts (microcysts produced from E. granulosus s.s. PSCs pre-cultured in vitro for 2 months). For the microcyst preparation, as previously described,22 briefly, PSCs (>98% viability) that had undergone 1% pepsin digestion were cultured in culture flasks containing RPMI 1640 medium [medium containing 20% (v/v) fetal bovine serum, 0.45% (w/v) yeast extract, 0.4% (w/v) glucose, 100 IU/mL of penicillin, and 100 μg/mL streptomycin]. The culture flasks were stored in an incubator at 37°C, 5%CO2 and the medium was changed every 3 days. At 6 months after infection, the mice were examined for lesions by B-mode ultrasound. The successfully infected mice were randomly divided into 12 groups of 6 female mice each, and each group was randomly divided as follows: (1) control group, given 0.5% carboxymethyl cellulose (CMC-Na) [CMC-Na dissolved in dissolved saline and configured as 0.5% (w/v) CMC-Na]; (2) ABZ group, given 50 mg/kg/day ABZ suspension; (3) HM group, given 50 mg/kg/day HM suspension; (4) DH-330 groups (low, medium and high), given 25, 50 or 100 mg/kg/day DH-330 suspensions; (5) DH-004 groups (low, medium and high), given 25, 50 or 100 mg/kg/day DH-004 suspension; and (6) H-2-168 groups (low, medium and high), given 25, 50 or 100 mg/kg/day H-2-168 suspension. Oral administration was carried out according to the dose of 0.1 mL/10 g. ABZ, HM and their derivatives were formulated in 0.5% CMC-Na, respectively, and administered by oral gavage daily for 28 days. At the end of the treatment, mice were anesthetized with isoflurane to collect blood and euthanized by cervical dislocation to prevent pain. The cysts in the peritoneal cavities of the mice were isolated and weighed, and the rate of cyst weight reduction was calculated as follows: (mean cyst weight in the control group - mean cyst weight in the treatment group)/mean cyst weight in the control group × 100%.23 Randomly selected treated mouse cysts were observed by transmission electron microscopy (TEM) (JEM1230, JEOL company, Japan) as described previously.19
MTT Assay to Evaluate the Cytotoxicity of H-2-168 and DH-004
PC12 cells (purchased from Nanjing KGI Biotechnology Development Co., Ltd.) were cultured in RPMI 1640 medium (supplemented with 10% horse serum, 5% fetal bovine serum and 1% penicillin/streptomycin), and cells in the logarithmic growth phase were taken for the experiment. Cells were inoculated in 96-well plates at 1×104 cells per well and incubated at 37 °C and 5% CO2 in a cell culture incubator for 12 h. HM, H-2-168 and DH-004 at final concentrations of 6.25, 12.5, 25, 50, 100 and 200 μM were added in triplicate to the wells containing culture medium. An equal volume of DMSO solvent was used as a control. After incubation in a cell incubator for 48 h, the supernatant was removed, and 90 μL of fresh medium (without serum) and 10 μL of MTT solution (AMRESCO, Solon, OH, USA) were added to each well. Cells were incubated in the incubator for 4 h. The supernatant was removed and 110 μL of formazan lysate was added to each well, and after shaking for 10 min, and the absorbance was measured at 570 nm. The inhibition rate was calculated according to the following formula: [1-(ODtreated-ODblank)/(ODcontrol-ODblank)] × 100%.
Hoechst 33342 Staining Assay to Detect PC12 Cell Apoptosis
PC12 cells were inoculated at 1×105 per well in 6-well plates and incubated at 37 °C in a 5% CO2 incubator until cell fusion reached 70%~80%. PC12 cells were divided into a DMSO control group, 200 μM HM group, 200 μM H-2-168 group and 200 μM DH-004 group. After 48 h of incubation, the culture medium was aspirated and discarded, 1 mL of Hoechst 33342 (Beyotime, Shanghai, China) staining solution was added, and incubation was continued in the incubator. Thirty minutes later, the staining solution was discarded, and the cells were washed three times with PBS and finally observed under an inverted fluorescence microscope.
Acute Toxicity Study
The acute toxicity study was performed according to Organization for Economic Co-operation and Development Guideline No. 423 (OECD Guideline No. 423).24 Ten Kunming mice were included in each group (5 females and 5 males). All of the mice in the HM, DH-004 and H-2-168 groups received a single dose of 2000 mg/kg orally. The dose was increased if the animals survived and decreased if they died. The animals were continuously observed for changes in general behavior for 4 h after administration. The surviving animals were observed for 14 days.
Subacute Toxicity Study
The subacute toxicity study was conducted with reference to OECD Guideline No. 407.25 Based on the results of the acute toxicity study, a specific study was designed for the HM and HM derivative drug doses. Sixty Kunming mice were randomly divided into 10 groups of 6 animals each (3 female and 3 male): (1) control group, given 0.5% CMC-Na [CMC-Na dissolved in dissolved saline and configured as 0.5% (w/v) CMC-Na]; (2) HM groups (low, medium and high), given 25, 50 or 100 mg/kg/day HM suspension; (3) DH-004 groups (low, medium and high), given 50, 100 or 200 mg/kg/day DH-330 suspension; and (4) H-2-168 groups (low, medium and high), given 50, 100 or 200 mg/kg/day H-2-168 suspension. After 30 days of continuous oral drug treatment, blood was obtained from the retro-orbital sinus of anesthetized mice. The numbers of red blood cells (RBCs), white blood cells (WBCs), neutrophils (Neus), monocytes (Mons), eosinophils (Eoss), lymphocytes (LYMs), and platelets (PLTs) and hemoglobin (HGB) content were measured in the mouse peripheral blood with an automated blood cell analyzer (BC-20, Mindray, Shenzhen, China). After peripheral blood coagulation, serum was separated by centrifugation (3000×g, 10 min) for the determination of aspartate aminotransferase (AST), alanine aminotransferase (ALT), urea nitrogen (UREA), alkaline phosphatase (ALP), and creatinine (CREA). Liver, kidney and brain tissues from euthanized mice were fixed in 10% formaldehyde solution for 24 h, routinely dehydrated and then paraffin-embedded, sectioned and stained with HE for histopathological examination.
Comet Assay to Detect DNA Damage in E. granulosus s.s. PSCs After Drug Treatment
Prior to the start of the experiment, PSCs were added to 96-well plates at a density of approximately 200 PSCs per well, with three replicate wells per group. The experiment was divided into a treatment group, 1% DMSO group, 100 μM DH-004 group and 100 μM H-2-168 group. After 48 h of drug treatment, the PSCs were collected and rinsed with PBS three times. The comet assay was performed as described previously.10 Briefly, approximately 200 PSCs were mixed with 0.75% low melting point agarose and precoated on slides containing 0.6% agarose. Electrophoresis was performed for 30 min in 1×Tris-borate-EDTA buffer under ambient conditions at room temperature (electrophoresis conditions: 25 V and 300 mA). The slides were soaked in Tris-HCl (pH 7.5) for 15 min, stained with PI for 20 min, and observed under an inverted fluorescence microscope.
Statistical Analysis
No formal sample size calculations were performed; sample sizes were determined based on previous experimental experience and preliminary experiments. The data were analyzed using IBM SPSS Statistics 20 software. The chi-square test and one-way analysis of variance (ANOVA) were used to determine statistical significance between the control group and each treatment group. P values < 0.05, 0.01 and 0.001 were considered to indicate statistical significance.
Results
Effect of Drug Treatment on the Viability and Ultrastructure of E. granulosus s.s. PSCs in vitro
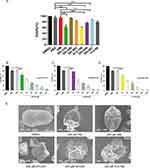
To assess the effect of the synthesized HM derivatives on E. granulosus s.s. in vitro, the viability of PSCs was measured by 1% eosin staining after 48 h of incubation with 40 µM HM derivatives, using 40 μΜ HM and ABZ as positive controls. The antiparasitic effect of DH-330 was reported previously, and this compound was also used as a positive control in this experiment. As shown in Figure 2A, after 48 h of incubation, the viabilities of the PSCs treated with the HM derivatives DH-208, DH-082, H-2-168, DH-004, DH-007, DH-330, and H-2-98 were 93.12 ± 1.01%, 83.10 ± 1.93%, 59.05 ± 2.86%, 76.33 ± 1.82%, 85.76 ± 3.02%, 62.78 ± 5.90% and 80.18 ± 3.90%, respectively, while the viabilities of the PSCs in the HM and ABZ groups remained at 94.73 ± 4.52% and 94.84 ± 5.00%, respectively. PSC viability in the DMSO control group remained at 100% throughout the experimental period. Among the 7 HM derivatives, H-2-168, DH-004 and DH-330 were particularly effective in vitro. Therefore, H-2-168, DH-004 and DH-330 were screened for further study, which included incubation with PSCs at compound concentrations of 40, 80, 120, 160, 200 and 240 μM for 48 h. The results showed that the three derivatives had a dose-dependent effect on PSCs. At a concentration of 120 μM, H-2-168, DH-004 and DH-330 were more effective than ABZ and HM in vitro. Notably, at a concentration of 200 μM, H-2-168, DH-004 and DH-330 killed more than 90% of the PSCs (Figure 2B–D). The LC50 values of H-2-168, DH-004 and DH-330 were 57.00 ± 2.00, 66.58 ± 3.09 and 61.25 ± 5.29 μM, respectively.
To investigate the effects of the HM derivatives on the PSC ultrastructure, SEM was used to observe any changes (Figure 2E). After 48 h of incubation in vitro, the morphological structures of the PSCs were full and intact in the blank and 1% DMSO groups. The 240 μM H-2-168, DH-004 and DH-330 groups showed severe disruption of the PSC structure, with wrinkled bodies and the loss of hooks and microtriches.
Evaluation of the Therapeutic Effects of the HM Derivatives on E. granulosus s.s.-Infected Mice
To study the in vivo therapeutic effect of the derivatives, healthy female Kunming mice infected intraperitoneally with PSCs were treated by pharmacological incubation for 4 weeks. At the end of treatment, mice were cervically dislocated under isoflurane anesthesia, then dissected and the weight of each cyst was measured. The cyst weights from the mice in the ABZ, HM and HM derivative groups were significantly reduced compared with that in the control group (P < 0.001). The cyst weights from the mice in the 50 mg/kg H-2-168 (4.75 ± 1.31 g), 100 mg/kg H-2-168 (4.30 ± 0.99 g) and DH-004 (4.72 ± 1.44 g) groups were significantly lower than those in the 50 mg/kg HM (6.53 ± 1.56 g) and ABZ (6.76 ± 1. 22 g) groups (P < 0.05). However, there was no difference in the effect of DH-330 compared with ABZ and HM at the doses used in this experiment (P > 0.05) (Figure 3A).
To assess the effects of the HM derivatives on the ultrastructure of mouse cysts in vivo, TEM was used. As shown in Figure 3B, the cysts in the model group had normal structures including a laminated layer, a germinal layer, neatly arranged microtriches and clear nuclei. The cysts in the ABZ (50 mg/kg), HM (50 mg/kg) and DH-330 (50 mg/kg) groups had fewer microtriches and an unclear germinal layer structure but clear nuclei. At a dose of 50 mg/kg, mice in the H-2-168 and DH-004 groups showed fewer microtriches, the presence of vesicles and lipid droplet-like structures, a disrupted cell structure, and damaged nuclei (Figure 3B).
In vitro Toxicity Assessment of H-2-168 and DH-004 in PC12 Cells
Previous studies have shown that HM is neurotoxic, thus limiting its clinical use.26 The neurotoxicity of DH-004 and H-2-168 to PC12 cells was assessed by MTT assay. The derivatives were administered at concentrations of 6.25, 12.5, 25, 50, 100, and 200 μM for 48 h of treatment (Figure 4). The results showed that HM and its derivatives exhibited cytotoxicity in a dose-dependent manner. When the concentration reached 6.25 μM, 29.05 ± 3.10%, 3.16 ± 1.37% and 2.17 ± 2.23% of the PC12 cells were inhibited by HM, DH-004 and H-168, respectively. When the concentration of each was increased to 200 μM, PC12 cell viability inhibition was 87.02 ± 5.65%, 58.05 ± 5.39% and 34.28 ± 1.40% for HM, DH-004 and H-2-168, respectively. The IC50 values of H-2-168 and DH-004 in PC12 cells were 351.23 μM and 147.04 μM, respectively, which were higher than that of HM (17.97 μM).
Effects of H-2-168 and DH-004 on the Apoptosis of PC12 Cells
To further examine the apoptotic effects of H-2-168 and DH-004 on PC12 cells, the nuclei were observed under an inverted fluorescence microscope after Hoechst 33258 staining. The nuclei in the DMSO control group were uniform in size and brightness, with smooth and intact edges. In contrast, in the 200 µM HM group, a reduced number of cells with varying morphological sizes and significantly denser granules or bright blue fluorescence was observed in the cells. Derivatives H-2-168 and DH-004 showed less of an effect on PC12 cell morphology than HM at the same concentration (Figure 5).
Acute Toxicity Study to Evaluate the Safety of H-2-168 and DH-004
To further assess the safety of H-2-168 and DH-004, their acute toxicity to mice was evaluated in vivo. As shown in Table 1, the Dm of HM and its derivatives was 2000 mg/kg, and the Dn was 99.8 mg/kg. The LD50 and its 95% confidence interval were 446.80 mg/kg (329.49~605.88 mg/kg) in mice in the oral HM group. The oral derivatives DH-004 and H-2-168 had LD50 values and 95% confidence intervals of 1107.16 mg/kg (869.84~1432.29 mg/kg) and 1425.86 mg/kg (1119.92~2022.07 mg/kg) in mice, respectively.
 |
Table 1 The Results from the Acute Toxicity Test in Mice. (n=10) |
Subchronic Toxicity Study to Evaluate the Safety of H-2-168 and DH-004
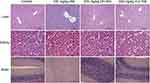
Table 2 shows the values of certain biochemical parameters in mice at the end of drug treatment. The WBC (P < 0.01), Neu (P < 0.01) and Lym (P < 0.05) levels in the 100 mg/kg HM group were significantly higher than those in the control group, while the routine blood indices in the mice in the DH-004 and H-2-168 groups did not change significantly compared to the control (P > 0.05). The pathological results showed chronic inflammatory cell infiltration in the portal area of the liver tissue in the 100 mg/kg HM group, as well as a loose cellular arrangement, swelling and deformation of brain tissue at this dose. No significant pathological changes were observed in the organs of the mice treated with each dose of derivatives H-2-168 and DH-004 (Figure 6).
 |
Table 2 Effects of HM and Its Derivatives on the Biochemical Parameters of Healthy Kunming Mice After 30 Days of Oral Administration (n=6) |
Effects of H-2-168 and DH-004 on DNA Damage in E. granulosus s.s. PSCs
To investigate the possible mechanism by which H-2-168 and DH-004 kill E. granulosus s.s. PSCs, the comet assay was used to assess the extent of DNA damage to PSCs caused by H-2-168 and DH-004. As shown in Figure 7, under the same electrophoretic conditions, the nuclei in the DMSO group were compact and did not show tails. However, the number of comets increased in the H-2-168 and DH-004 groups compared to the DMSO group.
 |
Figure 7 Effects of HM and its derivatives on DNA damage in E. granulosus s.s. PSCs. Images of DNA damage in PSCs detected by the comet assay. Bar = 500 μm. |
Discussion
Screening active compounds from natural medicinal plants has gained importance in the field of parasitic infections.20,27 The discovery of artemisinin and its use in malaria is seen as strong evidence of this approach.28 Previous work demonstrated that HM exhibits anti-E. granulosus s.s. effects, but its toxicity is not negligible.10 Compound derivatization techniques are a way to increase efficacy and reduce toxicity.29,30 In the present study, we screened 7 HM derivatives and found that the HM derivatives H-2-168 and DH-004 are promising therapeutic compounds for the treatment of E. granulosus s.s. infections and that the induction of E. granulosus s.s. DNA damage may be responsible for their efficacy.
HM is an effective compound that can kill E. granulosus s.s.10 We previously synthesized derivatives of HM, and among them, DH-330 showed antiparasitic effects superior to those of HM, suggesting that structural modification of HM for the treatment of CE is feasible.17 To further improve the therapeutic effect, systematic structural modifications were suggested by computer-aided drug molecular design techniques at five sites in the HM structure, including positions 1, 2, 3, 7, and 9. Thirty-two types of structures were designed, and 1037 novel compounds were obtained. It was found that the antitumor effects of HM were associated with compounds with modifications at the 7 and 9 positions, but a methoxy substituent at the 7 position may induce neurotoxicity.31 In addition, drawing on the previously reported HM derivative DH-330 substituted at the 1 and 9 positions, we finally selected 7 derivatives for further screening.
Preliminary drug screening of the selected derivatives showed that only DH-208 had a poor therapeutic effect in vitro, which was presumably related to the type and position of the chemically modified group.32,33 The remaining derivatives were superior to HM and ABZ in vitro. Notably, at a concentration of 40 μM, H-2-168 was superior to the previously reported DH-330 and DH-004. H-2-168, DH-004 and DH-330 were then tested at a gradient of concentrations and exhibited a dose-dependent effect on PSCs. At a concentration of 120 μM, the in vitro antiparasitic effects of H-2-168, DH-330 and DH-004 were significantly better than those of ABZ and HM. The changes in parasite ultrastructure observed by SEM were consistent with this result. Currently, although drugs for CE have been widely developed and have shown significant parasiticidal effects in vitro, the in vivo effects are unsatisfactory. Therefore, we further evaluated the therapeutic efficacy of H-2-168 and DH-004 in E. granulosus s.s.-infected mice. At the same dosage (50 mg/kg/day), the treatment effect of H-2-168 was significantly better than that of ABZ and HM. At a dosage of 100 mg/kg/day, the treatment effect of DH-004 was also better than that of ABZ and HM. The TEM results further demonstrated that after treatment with 50 mg/kg H-2-168, DH-330 and DH-004, the ultrastructure of the mouse vesicles was disrupted to different degrees. These results suggest that H-2-168 has a significant effect to treat CE both in vitro and in vivo.
Despite its multiple pharmacological effects, the neurotoxicity of HM has hindered its clinical use.33 Therefore, it is crucial to evaluate the neurotoxicity of HM derivatives. The cytotoxicity results showed that the IC50 of H-2-168 (351.23 μM) was significantly higher than that of HM (17.97 μM) and DH-004 (147.04 μM), which may be related to substitution at the 7 positions. Furthermore, the Hoechst 33342 staining data showed that H-2-168 and DH-004 caused less apoptosis of PC12 cells than HM, further demonstrating the lower neurotoxicity of H-2-168 and DH-004. Notably, H-2-168 (LD50 = 1425.86 mg/kg) and DH-004 (LD50 = 1107.16 mg/kg) also showed better safety than HM (LD50 = 572.04 mg/kg) in an acute toxicity study in mice. In addition, the results of the subchronic toxicity study showed that the number of inflammatory cells significantly increased in the liver and serum of mice in the HM group compared to the control group, which may cause an inflammatory response in mice. However, H-2-168 and DH-004 had no significant effects on the organs and serum of mice. Therefore, the above study demonstrated that H-2-168 and DH-004 had improved effects to treat CE along with decreased toxicity.
According to previous reports, HM interferes with DNA replication and synthesis and inhibits DNA topoisomerase through DNA binding and causes DNA damage.34 Thus, it is reasonable to speculate that HM derivatives may have similar pharmacological mechanisms. The comet assay is a traditional and sensitive technique for detecting DNA damage in single cells.35,36 Recently, this technique has been applied to detect the extent of DNA damage in E. granulosus s.s.37 In the present study, a distinctly comet-like tail was observed in E. granulosus s.s. after incubation with H-2-168 and DH-004 in vitro, which demonstrates that H-2-168 and DH-004 caused DNA damage in E. granulosus s.s. Gong et al17 found that EgTopo2a was a target of DH-330 through which this compound could induce DNA damage in E. granulosus s.s. Further research will be performed to more precisely identify the drug targets of H-2-168.
Although the present trial was designed to address the limitations of previous studies, there are some limitations of the current study. HM has previously been reported to have a variety of toxic side effects,11 and although cytotoxicity, acute toxicity, and subchronic toxicity studies were conducted in the present study to address the toxicity of HM derivatives H-2-168 and DH-004, a comprehensive analysis of all possible adverse events was beyond the scope of the present study. CE is a disease that requires long-term treatment; therefore, long-term studies and monitoring are still needed regarding the therapeutic evaluation of HM derivatives H-2-168 and DH-004. In addition, in this study, we found that HM derivatives H-2-168 and DH-004 can induce the occurrence of DNA damage in the PSCs, but the regulatory mechanism is still unknown, and we will further carry out more in-depth studies on the potential targets of H-2-168 and DH-004, to clarify the pharmacological mechanism and to provide a basis for the development of novel drugs.
In conclusion, this study systematically synthesized and screened HM derivatives against E. granulosus s.s. In particular, H-2-168 and DH-004 are considered novel drug candidates with high efficiency and low toxicity and provide a new strategy for the treatment of CE with drugs.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (82160700, 82260722), the Open Project of State Key Laboratory of Pathogenesis, Prevention and Treatment of High Incidence Diseases in Central Asia Fund (SKL-HIDCA-2022-26, SKL-HIDCA-2022-BC3, SKL-HIDCA-2022-9) and Natural Science Foundation of Xinjiang Uygur Autonomous Region (2021D01D15). We thank Haiyan Ren of the Department of Electron Microscopy of Xinjiang Medical University for providing technical support with the use of electron microscopes. We thank American Journal Experts (AJE) for their assistance with language editing.
Author Contributions
All authors contributed to the study design, execution, acquisition of data, data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Cadavid Restrepo AM, Yang YR, McManus DP, et al. The landscape epidemiology of echinococcoses. Infect Dis Poverty. 2016;5(1):13. doi:10.1186/s40249-016-0109-x
2. Wen H, Vuitton L, Tuxun T, et al. Echinococcosis: advances in the 21st century. Clin Microbiol Rev. 2019;32(2):e00075–18. doi:10.1128/cmr.00075-18
3. Agudelo Higuita NI, Brunetti E, McCloskey C, Kraft CS. Cystic echinococcosis. J Clin Microbiol. 2016;54(3):518–523. doi:10.1128/jcm.02420-15
4. Horton J. Albendazole for the treatment of echinococcosis. Fundam Clin Pharmacol. 2003;17(2):205–212. doi:10.1046/j.1472-8206.2003.00171.x
5. Moloudizargari M, Mikaili P, Aghajanshakeri S, Asghari MH, Shayegh J. Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacogn Rev. 2013;7(14):199–212. doi:10.4103/0973-7847.120524
6. Zhang XF, Sun RQ, Jia YF, et al. Synthesis and mechanisms of action of novel harmine derivatives as potential antitumor agents. Sci Rep. 2016;6:33204. doi:10.1038/srep33204
7. Liu F, Wu J, Gong Y, et al. Harmine produces antidepressant-like effects via restoration of astrocytic functions. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79(Pt B):258–267. doi:10.1016/j.pnpbp.2017.06.012
8. Berrougui H, Martín-Cordero C, Khalil A, et al. Vasorelaxant effects of harmine and harmaline extracted from Peganum harmala L. seeds in isolated rat aorta. Pharmacol Res. 2006;54(2):150–157. doi:10.1016/j.phrs.2006.04.001
9. Shahinas D, Macmullin G, Benedict C, Crandall I, Pillai DR. Harmine is a potent antimalarial targeting Hsp90 and synergizes with chloroquine and artemisinin. Antimicrob Agents Chemother. 2012;56(8):4207–4213. doi:10.1128/aac.00328-12
10. Lu S, Wen L, Gong Y, et al. In vitro effects of harmine against Echinococcus granulosus protoscoleces by stimulating DNA damage. Exp Parasitol. 2021;226–227:108121. doi:10.1016/j.exppara.2021.108121
11. Li Z, Chen L, He C, et al. Improving anti-tumor outcomes for colorectal cancer therapy through in situ thermosensitive gel loading harmine. Am J Transl Res. 2020;12(5):1658–1671.
12. Potapov VA, Ishigeev RS, Shkurchenko IV, Zinchenko SV, Amosova SV. Natural compounds and their structural analogs in regio- and stereoselective synthesis of new families of water-soluble 2H,3H-[1,3]thia- and -Selenazolo[3,2-a]pyridin-4-ium heterocycles by annulation reactions. Molecules. 2020;25(2):376. doi:10.3390/molecules25020376
13. Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79(3):629–661. doi:10.1021/acs.jnatprod.5b01055
14. Frédérick R, Bruyère C, Vancraeynest C, et al. Novel trisubstituted harmine derivatives with original in vitro anticancer activity. J Med Chem. 2012;55(14):6489–6501. doi:10.1021/jm300542e
15. Meinguet C, Bruyère C, Frédérick R, et al. 3D-QSAR, design, synthesis and characterization of trisubstituted harmine derivatives with in vitro antiproliferative properties. Eur J Med Chem. 2015;94:45–55. doi:10.1016/j.ejmech.2015.02.044
16. Zhang L, Li D, Yu S. Pharmacological effects of harmine and its derivatives: a review. Arch Pharm Res. 2020;43(12):1259–1275. doi:10.1007/s12272-020-01283-6
17. Gong Y, Lv S, Tian C, et al. Effect of harmine and its derivatives against echinococcus granulosus and comparison of DNA damage targets. J Biomed Nanotechnol. 2020;16(6):827–841. doi:10.1166/jbn.2020.2940
18. Wen LM, Lü GD, Zhao J, et al. Molecular cloning and characterization of ribosomal protein RPS9 in Echinococcus granulosus. J Parasitol. 2017;103(6):699–707. doi:10.1645/16-164
19. Amahong K, Yan M, Li J, et al. EgGLUT1 is crucial for the viability of Echinococcus granulosus sensu stricto metacestode: a new therapeutic target? Front Cell Infect Microbiol. 2021;11:747739. doi:10.3389/fcimb.2021.747739
20. Yan M, Li J, Liu H, et al. In vitro efficacy of Capparis spinosa extraction against larvae viability of Echinococcus granulosus sensu stricto. J Vet Med Sci. 2022;84(3):465–472. doi:10.1292/jvms.21-0609
21. Wang W, Li J, Yao J, et al. In vitro and in vivo efficacies of novel carbazole aminoalcohols in the treatment of cystic echinococcosis. J Antimicrob Chemother. 2017;72(11):3122–3130. doi:10.1093/jac/dkx250
22. Zhang WB, Jones MK, Li J, McManus DP. Echinococcus granulosus: pre-culture of protoscoleces in vitro significantly increases development and viability of secondary hydatid cysts in mice. Exp Parasitol. 2005;110(1):88–90. doi:10.1016/j.exppara.2005.02.003
23. Loos JA, Dávila VA, Rodrígues CR, et al. Metformin exhibits preventive and therapeutic efficacy against experimental cystic echinococcosis. PLoS Negl Trop Dis. 2017;11(2):e0005370. doi:10.1371/journal.pntd.0005370
24. Guideline O, Testing FOR, Chemicals OF. Acute oral toxicity – fixed dose procedure; 2001.
25. OECD. OECD Guidelines for the Testing of Chemicals; 2004.
26. Sun Q, Liu C, Jiang K, et al. A preliminary study on the neurotoxic mechanism of harmine in Caenorhabditis elegans. Comp Biochem Physiol C Toxicol Pharmacol. 2021;245:109038. doi:10.1016/j.cbpc.2021.109038
27. Alvi MA, Khan S, Ali RMA, et al. Herbal medicines against hydatid disease: a systematic review (2000–2021). Life. 2022;12(5):676. doi:10.3390/life12050676
28. Ma N, Zhang Z, Liao F, Jiang T, Tu Y. The birth of artemisinin. Pharmacol Ther. 2020;216:107658. doi:10.1016/j.pharmthera.2020.107658
29. Marinović M, Perković I, Fontinha D, et al. Novel harmicines with improved potency against plasmodium. Molecules. 2020;25(19):4376. doi:10.3390/molecules25194376
30. Marinović M, Poje G, Perković I, et al. Further investigation of harmicines as novel antiplasmodial agents: synthesis, structure-activity relationship and insight into the mechanism of action. Eur J Med Chem. 2021;224:113687. doi:10.1016/j.ejmech.2021.113687
31. Li S, Wang A, Gu F, et al. Novel harmine derivatives for tumor targeted therapy. Oncotarget. 2015;6(11):8988–9001. doi:10.18632/oncotarget.3276
32. Perković I, Raić-Malić S, Fontinha D, et al. Harmicines - harmine and cinnamic acid hybrids as novel antiplasmodial hits. Eur J Med Chem. 2020;187:111927. doi:10.1016/j.ejmech.2019.111927
33. Dai J, Dan W, Ren S, Shang C, Wang J. Design, synthesis and biological evaluations of quaternization harman analogues as potential antibacterial agents. Eur J Med Chem. 2018;160:23–36. doi:10.1016/j.ejmech.2018.10.012
34. Shu B, Zhang J, Jiang Z, Cui G, Veeran S, Zhong G. Harmine induced apoptosis in Spodoptera frugiperda Sf9 cells by activating the endogenous apoptotic pathways and inhibiting DNA topoisomerase I activity. Pestic Biochem Physiol. 2019;155:26–35. doi:10.1016/j.pestbp.2019.01.002
35. Ostling O, Johanson KJ. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem Biophys Res Commun. 1984;123(1):291–298. doi:10.1016/0006-291x(84)90411-x
36. Tice RR, Agurell E, Anderson D, et al. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 2000;35(3):206–221. doi:10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j
37. Li YF, Wen LM, Zhao J, et al. In vitro and in vivo efficacy of DNA damage repair inhibitor veliparib in combination with artesunate against Echinococcus granulosus. Dis Markers. 2020;2020:8259820. doi:10.1155/2020/8259820
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.