Back to Journals » Infection and Drug Resistance » Volume 17
In vitro Activity of Ceftaroline Against Isolates of Gram-Positive Bacteria from Patients with Bloodstream Infections Collected as a Part of ATLAS Between 2017 and 2020
Authors Kempf M, Arhin FF, Kuraieva A, Utt E
Received 4 August 2023
Accepted for publication 18 January 2024
Published 31 January 2024 Volume 2024:17 Pages 343—354
DOI https://doi.org/10.2147/IDR.S423004
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Marie Kempf,1,2 Francis F Arhin,3 Alona Kuraieva,4 Eric Utt5
1Laboratory of Bacteriology, University Hospital Angers, Angers, France; 2INCIT, Inserm, CHU Angers, Univ Angers, Nantes Université, Angers, F-49000, France; 3Pfizer Inc, Kirkland, Quebec, Canada; 4Pfizer Inc, New York, NY, USA; 5Pfizer Inc, Groton, CT, USA
Correspondence: Eric Utt, Pfizer Inc, Gorton, CT, 06340, USA, Tel +1 860 9174808, Email [email protected]
Purpose: To assess the in vitro activity of ceftaroline and a panel of comparator agents against isolates of Gram-positive bacteria, including Staphylococcus aureus, Streptococcus pneumoniae, β-hemolytic streptococci, and coagulase-negative staphylococci (CoNS) from blood collected in Africa and Middle East (AfME), Asia Pacific (APAC), Europe, Latin America (LATAM), and North America from 2017 to 2020 as a part of the Antimicrobial Testing Leadership and Surveillance (ATLAS) program.
Methods: Susceptibility and minimum inhibitory concentration were determined using broth microdilution for all antimicrobial agents by a central reference laboratory according to the Clinical and Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines.
Results: Ceftaroline showed good activity (susceptibility ≥ 89.8%, MIC90 0.008– 2 mg/L) against all Gram-positive isolates tested. All isolates of methicillin-susceptible S. aureus, penicillin-susceptible S. pneumoniae, S. agalactiae, S. dysgalactiae, and S. pyogenes were susceptible to ceftaroline (MIC90 0.008– 0.25 mg/L). Ceftaroline susceptibility for MRSA isolates was 89.8% globally (MIC90 2 mg/L). Among the comparator agents, all isolates were susceptible to vancomycin, except S. epidermis (susceptibility, 99.9%). Among other agents, daptomycin, linezolid, and tigecycline showed potent activity (susceptibility ≥ 97.9%, MIC90 0.03– 2 mg/L) against all isolates tested.
Conclusion: Ceftaroline showed potent in vitro activity against global bloodstream isolates of Gram-positive bacteria collected between 2017 and 2020. Monitoring and surveillance of global as well as regional longitudinal trends of resistance rates among Gram-positive isolates causing bloodstream infections are important to limit the spread of AMR, establish stewardship measures, and manage and appropriately treat infections.
Keywords: ATLAS, bloodstream infections, ceftaroline, Gram-positive bacteria, surveillance
Introduction
Bloodstream infections (BSIs) are often difficult to treat and are associated with high morbidity, mortality, and social and economic burden.1,2 BSIs are responsible for 40% of community-acquired and hospital-acquired sepsis and about 20% of all intensive care unit (ICU)-acquired infections.3 Over 50% of all BSIs are caused by Gram-positive bacteria, including Staphylococcus aureus, coagulase-negative staphylococci (CoNS), and Streptococcus spp. including Streptococcus pneumoniae and β-hemolytic streptococci.1,3,4 A global SENTRY surveillance study assessing organisms causing BSIs from 1997 to 2016 reported that S. aureus accounted for 20.7%, S. epidermis 3.8%, and S. pneumoniae 2.8% of BSIs.5 Another study that assessed disability-adjusted-life-years (DALY) for infections caused by antibiotic-resistant bacteria in Europe reported a median DALY (per 100,000 population) percentage of 63.9 for methicillin-resistant S. aureus (MRSA), 49.1% for penicillin-resistant S. pneumoniae (PRSP), and 77.4% for penicillin-and-macrolide-resistant S. pneumoniae causing BSIs.6
Treatment and management of patients with BSIs involve accurate diagnosis to identify the infection source, choosing the appropriate antibacterial for treatment, duration of therapy, and effective source control.7 Furthermore, there is a lack of evidence on effective treatment options for Gram-positive BSI; current treatment approaches are mostly based on observational or non-randomized trials.7–9 Hence, treatment of BSIs, especially those caused by resistant strains of Gram-positive bacteria, is challenging.
Ceftaroline, the active form of ceftaroline fosamil, is a cephalosporin with a broad in vitro activity against Gram-positive bacteria including MRSA and PRSP.10 Ceftaroline mediates its antibacterial activity by binding to penicillin-binding proteins to inhibit cell wall synthesis leading to cell lysis and death.10 Ceftaroline has been approved by the Food and Drug Administration and European Medicine Agency for acute bacterial skin and skin structure infections (ABSSSI)/complicated skin and skin structure infections (cSSTI) and community-acquired bacterial pneumonia (CAP) in both adult and pediatric patients.11,12 Ceftaroline has not been approved for treatment of BSIs by the EMA. However, ceftaroline has been approved for treatment of CAP with concurrent bacteremia caused by S. pneumoniae.11 Notably, ceftaroline has previously been used as a salvage therapy for BSI caused by MRSA.13,14 Interestingly, a retrospective observational study in the United States (US) assessing the use of ceftaroline outside of its approved indications over two years (2011–2013) showed that the most common off-label use of ceftaroline was for the treatment of BSIs.15 Furthermore, it was observed that ceftaroline was most frequently used following disease progression on prior antibiotic therapy.15 These reports indicate that ceftaroline could be effective for the treatment of BSIs.
Recently, there has been an emergence and spread of drug-resistant bacteria due to increase in the use of antibiotics.2,5 Considering the limited treatment options for BSI, especially those caused by resistant strains, it is important to monitor the resistance trends of these organisms to antimicrobials. There have been only limited number of studies assessing the activity of ceftaroline against Gram-positive bacteria causing BSI.16,17 Hence, to assess the activity of ceftaroline against a recent collection of Gram-positive bacteria from BSI, we conducted this in vitro study and tested ceftaroline and a panel of comparator agents against isolates of Gram-positive bacteria, including S. aureus, S. pneumoniae, β-hemolytic streptococci (S. agalactiae, S. dysgalactiae, and S. pyogenes), and coagulase-negative staphylococci (CoNS) from blood collected in Africa and Middle East (AfME), Asia Pacific (APAC), Europe, Latin America (LATAM), and North America from 2017 to 2020 as a part of the Antimicrobial Testing Leadership and Surveillance (ATLAS) program.18
Methods
Bacterial Isolates
Non-duplicate, clinically significant isolates (single isolate per patient) of S. aureus, S. pneumoniae, S. agalactiae, S. dysgalactiae, S. pyogenes, and CoNS, which included isolates of S. capitis, S. epidermis, S. haemolyticus, S. hominis, S. lugdunensis, S. saprophyticus, and S. simulans, independent of age, sex, previous antimicrobial use, or medical history were collected each year in different sites across regions worldwide (AfME, APAC, Europe, LATAM, and North America) from hospitalized patients with bloodstream infections between 2017 and 2020. Every year, each participating site was requested to collect a pre-defined number of isolates of selected species as a part of the ATLAS protocol. The isolates were identified at each site and shipped to a central reference laboratory (International Health Management Associates, Inc. Schaumburg, IL, USA) for species confirmation and antimicrobial susceptibility. Species confirmation was done using matrix-assisted laser desorption ionization time-of-flight spectrometry (Bruker Biotyper MALDI-TOF, Bruker Daltonics, Billerica, MA, USA).
Antimicrobial Susceptibility Testing
Susceptibility and minimum inhibitory concentrations (MIC) of isolates were determined using broth microdilution methodology for ceftaroline and a panel of comparator antimicrobial agents – ceftriaxone, daptomycin, erythromycin, levofloxacin, linezolid, minocycline, tigecycline, trimethoprim-sulfamethoxazole (TMP-SMX), and vancomycin. Antimicrobial susceptibility testing was performed according to Clinical and Laboratory Standards Institute (CLSI) guidelines.19 MICs were interpreted according to CLSI guidelines20 and version 13.0 of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoint tables.21 For tigecycline, no susceptibility breakpoints are defined per CLSI, hence FDA approved breakpoints were used.22 For S. aureus, EUCAST revised the clinical breakpoints for ceftaroline in 2017 to susceptible ≤1 mg/L and resistant >1 mg/L.23 Not all antimicrobials were tested in each year of the surveillance, hence varying numbers of isolates were recorded against the different antimicrobials. Methicillin resistance for each S. aureus isolate was determined using the oxacillin MIC method (MIC ≥ 4 mg/L confirmed methicillin resistance) per CLSI guidelines.20 S. pneumoniae were classified as penicillin-susceptible or -resistant per the CLSI MIC definitions for oral penicillin V (0.12–1 and ≥2 mg/mL).20 MIC breakpoints for ceftaroline in β-hemolytic streptococci (S. agalactiae, S. dysgalactiae, and S. pyogenes) have not been defined by EUCAST, specifying that the susceptibility to ceftaroline can be inferred from testing benzyl penicillin.21 For CoNS, both CLSI and EUCAST have not established breakpoints for ceftaroline, hence only MIC data have been presented.17
Results
Distribution of Isolates
A total of 16,104 isolates of Gram-positive bacteria from 319 sites in 60 countries including, S. aureus (N = 7,373, 306 sites), S. pneumoniae (N = 2,755, 253 sites), S. agalactiae (N = 801, 192 sites), S. dysgalactiae (N = 316, 136 sites), S. pyogenes (N = 704, 185 sites), and CoNS (N = 4,155, 275 sites) from blood were collected in different regions between 2017 and 2020 (Supplementary Table 1).
Among the S. aureus isolates, 69.5% (5,126/7,373) were MSSA, and 30.5% (2,247/7,373) were MRSA globally. Among the different regions, the highest proportion of MRSA isolates were collected in APAC (38.8%, 474/1223) and the lowest were collected in AfME (24.0%, 126/525; Table 1).
 |
Table 1 Distribution of Gram-Positive Isolates from Blood Collected Globally and Across Different Regions in 2017–2020 |
Among S. pneumoniae isolates, 76.1% were penicillin-susceptible Streptococcus pneumoniae (PSSP; 2,097/2,755) and 9.9%/4.1% (CLSI/EUCAST) were PRSP (CLSI, 273/2,755; EUCAST, 112/2755). Among the different regions, the highest proportion of PRSP isolates was collected in LATAM (CLSI, 22.1%, 45/204; EUCAST 10.8%, 22/204), and the lowest proportion was collected in North America (CLSI, 3.3%, 13/400; EUCAST, 1%, 4/400; Table 1).
Among the CoNS isolates collected, majority were identified as S. epidermis (56.0%, 2,326/4,155) and S. haemolyticus (38.9%, 1,615/4,155). The rest (5.1%) were identified as S. capitis (1.4%, 57/4,155), S. hominis (3.1%, 130/4,155), S. lugdunensis (0.3%, 13/4155), S. saprophyticus (0.3%, 12/4,155), and S. simulans (0.05%, 2/4,155).
Antimicrobial Activity of Ceftaroline and Comparators
S. aureus
Ceftaroline showed potent activity (susceptibility 96.8%, MIC90 1 mg/L) against all isolates of S. aureus globally. Among the comparators, all agents except erythromycin and levofloxacin showed good activity (susceptibility 93.4%–100.0%, MIC90 0.12–2 mg/L) against all S. aureus isolates. Notably, all the isolates were susceptible to linezolid (MIC90 2 mg/L) and vancomycin (MIC90 1 mg/L; Table 2).
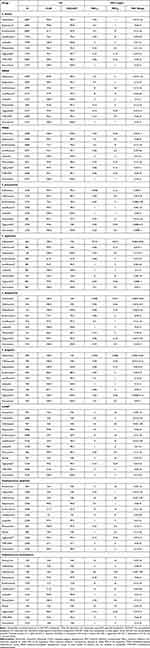 |
Table 2 Antimicrobial Activity of Ceftaroline and Comparators Against Gram-Positive Isolates from Blood Collected Globally in 2017–2020 |
 |
Table 3 Antimicrobial Activity of Ceftaroline and Comparators Against PRSP and PSSP Isolates from Blood Collected Globally in 2017–2020 |
For MRSA isolates, ceftaroline showed good activity (susceptibility 89.8%, MIC90 2 mg/L), globally. Among the comparators, all MRSA isolates, globally were susceptible to linezolid (MIC90 2 mg/L) and vancomycin (MIC90 1 mg/L) while daptomycin, tigecycline and TMP-SMX showed potent activity (susceptibility ≥ 95.6%, MIC90 ≤ 1 mg/L), and minocycline showed good activity (CLSI/EUCAST, susceptibility 92.8/87.2%, MIC90 2 mg/L; Table 2).
All isolates of MSSA were susceptible to ceftaroline (MIC90 2 mg/L). Among the comparators, all agents except erythromycin showed potent activity (susceptibility ≥ 99.1%, MIC90 ≤ 2 mg/L), while levofloxacin showed good activity (susceptibility 93.1%, MIC90 0.5 mg/L; Table 2).
Among the different regions, ceftaroline showed slightly lower activity against all S. aureus (susceptibility 94.0%, MIC90 1 mg/L), and MRSA (susceptibility 84.5%, MIC90 2 mg/L) collected in APAC as compared to other regions (Supplementary Tables 2 and 3).
S. pneumoniae
Globally, ceftaroline showed potent activity (CLSI/EUCAST, susceptibility 99.9/99.6%, MIC90 0.12 mg/L) against all isolates of S. pneumoniae. Among the comparators, all agents except erythromycin and minocycline showed potent activity (susceptibility ≥ 96.4%, MIC90 ≤ 1 mg/L), against all isolates of S. pneumoniae. Additionally, all the isolates were susceptible to linezolid (MIC90 1 mg/L) and vancomycin (0.5 mg/L; Table 2).
Against PRSP isolates, ceftaroline showed potent activity (susceptibility 99.2%, MIC90 0.25 mg/L) per CLSI and good activity (susceptibility 91.4%, MIC90 0.25 mg/L) per EUCAST. Among the comparators, levofloxacin, linezolid, tigecycline, and vancomycin showed potent activity (susceptibility ≥ 95.5%, MIC90 ≤ 2 mg/L) against PRSP isolates, with all isolates being susceptible to linezolid and vancomycin (Table 3).
All isolates of PSSP were susceptible to ceftaroline (MIC90 0.015 mg/L). Among comparator agents, all PSSP isolates were susceptible to ceftriaxone (MIC90 0.015 mg/L), linezolid (MIC90 1 mg/L), tigecycline (MIC90 0.03 mg/L), and vancomycin (MIC90 0.25 mg/L), while levofloxacin showed potent activity (susceptibility 99.9%, MIC90 1 mg/L, Table 3).
Among the different regions, per EUCAST, ceftaroline showed lower activity against PRSP isolates collected in AfME (susceptibility 81.8%, MIC90 0.5 mg/L), Europe (susceptibility 88.6%, MIC90 0.5 mg/L), and APAC (susceptibility 91.7%, MIC90 0.25 mg/L) as compared to LATAM and North America (susceptibility 100%, MIC90 0.25 mg/L, Supplementary Table 4).
β-Hemolytic Streptococci
Globally, all isolates of S. agalactiae, S. dysgalactiae, and S. pyogenes were susceptible to ceftaroline (MIC90 ≤ 0.015 mg/L). Among comparator agents, all isolates of β-hemolytic streptococci were susceptible to daptomycin (MIC90 ≤ 0.5 mg/L), linezolid (MIC90 ≤ 1 mg/L), and vancomycin (MIC90 0.5 mg/L). Against ceftriaxone, all isolates of S. dysgalactiae (MIC90 0.06), and S. pyogenes (MIC90 0.03 mg/L) were susceptible, while isolates of S. agalactiae showed a susceptibility of 99.9% (800/801) with only one isolate being intermediate. Tigecycline showed potent activity (susceptibility ≥99.7%, MIC90 0.06–0.12 mg/L) against isolates of β-hemolytic streptococci with all isolates of S. pyogenes being susceptible (Table 2).
CoNS
Ceftaroline showed good activity (MIC90 2 mg/L) against all isolates of CoNS, globally. Among comparator agents, daptomycin, linezolid, minocycline, tigecycline, and vancomycin showed potent activity (susceptibility ≥ 96.7%, MIC90 0.25–2 mg/L) against isolates of CoNS (Table 2).
Against S. epidermis, ceftaroline showed a higher activity (MIC90 1 mg/L) than against S. haemolyticus (MIC90 2 mg/L). Among the comparator agents, all isolates of S. haemolyticus were susceptible to vancomycin (MIC90 2 mg/L). Among the other comparator agents, daptomycin, minocycline, linezolid, tigecycline, and vancomycin (S. epidermis) showed potent activity (susceptibility ≥96.7%, MIC90 0.25–2 mg/L) against S. epidermis and S. haemolyticus isolates collected globally (Table 2).
Among the different geographical regions, ceftaroline showed sustained activity (MIC90 0.5–1 mg/L) across regions against S. epidermis isolates. Notably, a lower activity for ceftaroline was observed against S. haemolyticus isolates collected in APAC (MIC90 4 mg/L) compared to other regions (MIC90 2 mg/L; Supplementary Tables 2 and 3).
Discussion
The current study assessed the antimicrobial activity of ceftaroline and a panel of comparator agents against isolates of Gram-positive bacteria, including S. aureus, S. pneumoniae, S. agalactiae, S. dysgalactiae, S. pyogenes, and CoNS from blood collected globally and across different regions from 2017 to 2020. Overall, ceftaroline showed good activity with susceptibility ≥91.4% (for isolates in which breakpoints for ceftaroline are defined) and an MIC range of 0.004–16 mg/L against all Gram-positive isolates tested, except MRSA (susceptibility 89.8%, MIC90 2 mg/L). For MRSA, 4 isolates demonstrated MICs above the PK/PD breakpoints for ceftaroline of 8 mg/L (1 isolate from China and 2 isolates from Thailand showing an MIC and 1 isolate from South Korea showing an MIC of 16 mg/L; data not shown). Similarly, 5 isolates of S. haemolyticus (4 isolates from China and 1 isolate from Nigeria showing an MIC of 8 mg/L) and 1 isolate of S. epidermis (from Brazil showing an MIC of 32 mg/L) demonstrated MICs above the PK/PD breakpoints for ceftaroline (no data shown). Among the comparator agents, all isolates tested were susceptible to vancomycin and linezolid, except for linezolid activity against CoNS (susceptibility 98.7%, MIC90 2 mg/L). Among other agents, daptomycin, and tigecycline showed potent activity (susceptibility ≥ 99.1%, MIC90 0.03–0.25 mg/L) against all isolates tested.
In the current study, ceftaroline showed potent activity (susceptibility 96.8%, MIC90 1mg/L) against all S. aureus isolates collected globally. Two previous SENTRY studies assessing ceftaroline activity against Gram-positive isolates from BSI also reported similar findings against S. aureus, a 20-year study from 1997 to 2016 (susceptibility 96.2%, MIC90 1 mg/L) and a 10-year study from 2010 to 2019 (susceptibility 96.0%, MIC90 1 mg/L).5,16 Among the different regions in the current study, ceftaroline showed consistent activity (susceptibility 96.2%–98.4%, MIC90 1mg/L) against all S. aureus isolates in all the regions, except APAC, where it was slightly lower (susceptibility 94.0%, MIC90 1mg/L). The previously 10-year SENTRY study (2010–2019) also reported a lower susceptibility for ceftaroline in LATAM-APAC as compared to other regions (LATAM-APAC vs other regions, 91.1% vs 95.4%–98.1%).16 A similar trend of lower S. aureus susceptibility to ceftaroline in APAC (APAC vs other regions, 85.0% vs 91.3%–99.7%) was reported by a previous ATLAS study that assessed in vitro activity of ceftaroline against S. aureus isolates collected from various sources, including BSI from 2012 to 2017.24
All isolates of MSSA in the current study showed 100% susceptibility to ceftaroline (MIC90 0.25 mg/L). This observation is in line with the previous 10-year SENTRY study (2010–2019) and the ATLAS study (2012–2017; susceptibility > 99.9%, MIC90 0.25 mg/L in both studies).16,24 However, ceftaroline activity in the current study was lower (susceptibility 89.8%, MIC90 2 mg/L) against MRSA isolates as compared to the overall activity against S. aureus and MSSA. Among different regions, the activity of ceftaroline against MRSA in this study was lower in APAC as compared to other regions (susceptibility 84.5%, MIC90 2 mg/L vs susceptibility ≥87.6%, MIC90 1–2 mg/L). These observations are in line with that reported in the previous studies - 10-year SENTRY study (2010–2019) reporting a higher overall MIC90 of 2 mg/L for ceftaroline against MRSA compared to 0.25 mg/L against MSSA,16 and the ATLAS study (2012–2017) reporting an overall susceptibility of 89.3% and an MIC90 of 2 mg/L for MRSA and a lower susceptibility of 75.9% in APAC to ceftaroline as compared to the other regions (≥84.4%).24 Among the comparators, all agents except erythromycin and levofloxacin showed good activity (susceptibility ≥93.4%, MIC90 0.12–2 mg/L) against all S. aureus isolates. This data is in line with the previous studies, the 10-year SENTRY study (2010–2019) and the ATLAS study (2012–2017).16,24 While ceftaroline is active against S. aureus, there was lower activity against MRSA and against all isolates in APAC; this needs further surveillance for appropriate treatment and management of S. aureus infections.
In the current study, globally, ceftaroline showed potent activity against all isolates of S. pneumoniae, (CLSI/EUCAST, susceptibility 99.9%/99.6%, MIC90 0.12 mg/L). A previous study assessing the activity of ceftaroline and its comparators against S. pneumoniae isolates from CAP collected in Europe, Asia, and LATAM from 2015 to 2017 also reported potent activity of ceftaroline against all S. pneumoniae (CLSI, susceptibility 99.9%, MIC90 0.12 mg/L) isolates.25 Globally, all isolates of PSSP in the current study were susceptible to ceftaroline (MIC90 0.015 mg/L). However, for PRSP isolates, while ceftaroline demonstrated potent activity per CLSI (susceptibility 99.2%, MIC90 0.25 mg/L), the activity was lower per EUCAST (susceptibility 91.4%, MIC90 0.25 mg/L). Across the different regions, ceftaroline activity was sustained per CLSI (susceptibility ≥98.4%, MIC90 0.25 mg/L). However, per EUCAST, while all PRSP isolates in LATAM and North America were susceptible to ceftaroline (MIC90 0.25), lower activity was observed in APAC (susceptibility 91.7%, MIC90 0.25 mg/L), Europe (susceptibility 88.6%, MIC90 0.5 mg/L), and AfME (susceptibility 81.8%, MIC90 0.5 mg/L). Interestingly, a previous AWARE study that assessed activity of ceftaroline against S. pneumoniae isolates (per EUCAST) from CAP collected in AfME, Asia, Europe, Oceania, and LATAM from 2015 to 2016 reported 100% susceptibility for PRSP isolates collected in LATAM and Oceania (MIC90 0.25 mg/L) and potent but comparatively lower activity in Europe (susceptibility 94.6%, MIC90 0.25 mg/L) and further lower activity in AfME (susceptibility 86.8%, MIC90 0.5 mg/L) and Asia (susceptibility 77.4%, MIC90 1 mg/L).26 Among the comparators, all agents except ceftriaxone, erythromycin, and minocycline demonstrated potent activity (susceptibility ≥ 96.4%, MIC90 0.03–1 mg/L) against all PRSP isolates, globally.
In our study, all isolates of β-hemolytic streptococci globally were susceptible to ceftaroline (S. agalactiae and S. dysgalactiae: MIC90 0.015 mg/L; S. pyogenes: MIC90 0.008 mg/L). These data are in line with a previous ATLAS study that assessed activity of ceftaroline and its comparators against isolates from SSTI collected in AfME, APAC, Europe, and LATAM from 2015 to 2017 (S. agalactiae: MIC90 0.015–0.03 mg/L; S. dysgalactiae: MIC90 0.008–0.015 mg/L; S. pyogenes: MIC90 0.008 mg/L).27
Against CoNS isolates, ceftaroline had an MIC90 of 2 mg/L, globally in this study. A previous AWARE study that evaluated the activity of ceftaroline against CoNS collected from various sources in the US from 2013 to 2014 reported an MIC90 of 0.5 mg/L against isolates from BSIs.17 However, ceftaroline had an MIC90 of 0.5 mg/L against S. epidermis and 2 mg/L against isolates of S. haemolyticus collected in North America, which is in line with the previous AWARE study conducted in the US.17
This study has certain limitations. A pre-defined number of isolates were collected each year from the centers, and hence, the results of the study cannot be interpreted as prevalence of infection or used for general epidemiological assessment. Clinical and epidemiological information about the patient population were not available for the isolates collected in this study. Information such as the infection source, type, and history antimicrobial use could add value and insight to the interpretation of the susceptibility results. Not all antimicrobials were tested every year and the number of participating centers in each region varied between the years.
Conclusions
Overall, ceftaroline showed good in vitro activity against global isolates of Gram-positive bacteria, including S. aureus, S. pneumoniae, S. pyogenes, S. agalactiae, S. dysgalactiae, and CoNS, tested in this study (2017–2020). Among the comparators, linezolid, vancomycin, daptomycin, and tigecycline showed potent activity against all isolates. In the recent years, with increase in use of antimicrobials and spread of drug resistance among Gram-positive bacteria, it has become challenging to manage the treatment of drug-resistant BSIs, especially those caused by S. aureus and, particularly, MRSA, where the mortality rates are high. Hence, monitoring and surveillance of global as well as regional longitudinal trends of resistance rates among Gram-positive isolates causing BSIs are important to limit the spread of AMR and establish appropriate stewardship measures. Despite the study limitations, data presented in the study provide crucial insights into the in vitro activity of ceftaroline, which may be considered as a good treatment option for BSIs caused by these Gram-positive organisms.
Acknowledgments
Under the direction of the authors, Arjun Krishnakumar (Ph.D., CMPP™), an employee of Pfizer drafted the initial version of the manuscript, edited subsequent versions, and prepared the manuscript for submission. Editorial support was provided by Sweta Samantaray (PhD), an employee of Pfizer.
Funding
This study was sponsored by Pfizer Inc.
Disclosure
AK and EU are current employees of Pfizer and hold stock/stock options with Pfizer. FFA was an employee of Pfizer when this study was conducted. MK has received research support from Pfizer previously, and she also reports personal fees from bioMérieux, personal fees, and non-financial support from MSD, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Dunbar SA, Gardner C, Das S. Diagnosis and management of bloodstream infections with rapid, multiplexed molecular assays. Front Cell Infect Microbiol. 2022;12:859935. doi:10.3389/fcimb.2022.859935
2. Zhu Q, Yue Y, Zhu L, et al. Epidemiology and microbiology of gram-positive bloodstream infections in a tertiary-care hospital in Beijing, China: a 6-year retrospective study. Antimicrob Resist Infect Control. 2018;7(1):107. doi:10.1186/s13756-018-0398-x
3. Timsit JF, Ruppe E, Barbier F, Tabah A, Bassetti M. Bloodstream infections in critically ill patients: an expert statement. Intensive Care Med. 2020;46(2):266–284. doi:10.1007/s00134-020-05950-6
4. Duan X, Zhang R, Zhang X, Ding X, Sun T. Identification of prognostic factors in patients with streptococcus bloodstream infection. Front Med Lausanne. 2022;9:832007. doi:10.3389/fmed.2022.832007
5. Diekema DJ, Hsueh PR, Mendes RE, et al. The microbiology of bloodstream infection: 20-year trends from the SENTRY antimicrobial surveillance program. Antimi Agen Chem. 2019; 63(7):1.
6. Cassini A, Hogberg LD, Plachouras D, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European economic area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19(1):56–66. doi:10.1016/S1473-3099(18)30605-4
7. Giannella M, Bartoletti M, Gatti M, Viale P. Advances in the therapy of bacterial bloodstream infections. Clin Microbiol Infect. 2020;26(2):158–167. doi:10.1016/j.cmi.2019.11.001
8. Doernberg SB, Lodise TP, Thaden JT, et al. Gram-positive bacterial infections: research priorities, accomplishments, and future directions of the antibacterial resistance leadership group. Clin Infect Dis. 2017;64(suppl_1):S24–S29. doi:10.1093/cid/ciw828
9. Quinn NJ, Sebaaly JC, Patel BA, Weinrib DA, Anderson WE, Roshdy DG. Effectiveness of oral antibiotics for definitive therapy of non-staphylococcal gram-positive bacterial bloodstream infections. Ther Adv Infect Dis. 2019;6:2049936119863013. doi:10.1177/2049936119863013
10. Shirley DA, Heil EL, Johnson JK. Ceftaroline fosamil: a brief clinical review. Infect Dis Ther. 2013;2(2):95–110. doi:10.1007/s40121-013-0010-x
11. TEFLARO® (ceftaroline fosamil) for injection, for intravenous use. 2022. Available from: https://www.rxabbvie.com/pdf/teflaro_pi.pdf.
12. Pfizer. Zinforo 600 mg powder for concentrate for solution for infusion: summary of product characteristics. 2022. Available from: https://www.ema.europa.eu/en/documents/product-information/zinforo-epar-product-information_en.pdf.
13. Sakoulas G, Moise PA, Casapao AM, et al. Antimicrobial salvage therapy for persistent staphylococcal bacteremia using daptomycin plus ceftaroline. Clin Ther. 2014;36(10):1317–1333. doi:10.1016/j.clinthera.2014.05.061
14. Tattevin P, Boutoille D, Vitrat V, et al. Salvage treatment of methicillin-resistant staphylococcal endocarditis with ceftaroline: a multicentre observational study. J Antimicrob Chemother. 2014;69(7):2010–2013. doi:10.1093/jac/dku085
15. Casapao AM, Davis SL, Barr VO, et al. Large retrospective evaluation of the effectiveness and safety of ceftaroline fosamil therapy. Antimi Agen Chem. 2014;58(5):2541–2546. doi:10.1128/AAC.02371-13
16. Sader HS, Carvalhaes CG, Mendes RE. Ceftaroline activity against Staphylococcus aureus isolated from patients with infective endocarditis, worldwide (2010–2019). Int J Infect Dis. 2021;102:524–528. doi:10.1016/j.ijid.2020.11.130
17. Sader HS, Farrell DJ, Flamm RK, Streit JM, Mendes RE, Jones RN. Antimicrobial activity of ceftaroline and comparator agents when tested against numerous species of coagulase-negative Staphylococcus causing infection in US hospitals. Diagn Microbiol Infect Dis. 2016;85(1):80–84. doi:10.1016/j.diagmicrobio.2016.01.010
18. Antimicrobial Testing Leadership and Surveillance (ATLAS). 2022. Available from: https://atlas-surveillance.com/.
19. CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Wayne, PA: Clinical and Laboratory Standards Institute; 2018.
20. CLSI. Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute; 2022.
21. EUCAST. Breakpoint tables for interpretation of MICs and zone diameters; 2023.
22. Pfizer. Tygacil. Package insert. 2022. Available from: https://www.pfizer.com/products/product-detail/tygacil.
23. EUCAST. Ceftaroline S. aureus breakpoints revised: addendum (July 2017) to EUCAST breakpoint tables v. 7.1. 2023. Available from: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Addenda/Addendum_2017-07-14.pdf.
24. Zhang Z, Chen M, Yu Y, Liu B, Liu Y. In vitro activity of ceftaroline And comparators against staphylococcus aureus isolates: results from 6 years of the atlas program (2012 To 2017). Infect Drug Resist. 2019;12:3349–3358. doi:10.2147/IDR.S226649
25. Sader HS, Flamm RK, Streit JM, Carvalhaes CG, Mendes RE. Antimicrobial activity of ceftaroline and comparator agents tested against organisms isolated from patients with community-acquired bacterial pneumonia in Europe, Asia, and Latin America. Int J Infect Dis. 2018;77:82–86. doi:10.1016/j.ijid.2018.10.004
26. Bae IG, Stone GG. Activity of ceftaroline against pathogens associated with community-acquired pneumonia collected as part of the AWARE surveillance program, 2015–2016. Diagn Microbiol Infect Dis. 2019;95(3):114843. doi:10.1016/j.diagmicrobio.2019.05.015
27. Pierard D, Stone GG. In vitro activity of ceftaroline and comparators against bacterial isolates collected globally from patients with skin infections. J Glob Antimicrob Resist. 2021;26:4–10. doi:10.1016/j.jgar.2021.04.020
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
