Back to Journals » International Journal of General Medicine » Volume 15
In-Hospital Outcomes and Recurrence of Infectious Spondylitis in Patients with and without Chronic Hemodialysis: A Nationwide Cohort Study
Authors Lu YA, Chen CY, Kuo G, Yen CL, Tian YC, Hsu HH
Received 8 November 2021
Accepted for publication 7 March 2022
Published 14 March 2022 Volume 2022:15 Pages 2991—3001
DOI https://doi.org/10.2147/IJGM.S348431
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yueh-An Lu, Chao-Yu Chen, George Kuo, Chieh-Li Yen, Ya-Chung Tian, Hsiang-Hao Hsu
Department of Nephrology, Kidney Research Center, Linkou Chang Gung Memorial Hospital, Chang Gung University, College of Medicine, Taoyuan, Taiwan
Correspondence: Hsiang-Hao Hsu, Department of Nephrology, Kidney Research Center, Linkou Chang Gung Memorial Hospital, No. 5 Fu-Shin Street, Kweishan, Taoyuan, 333, Taiwan, Tel +886-3-328-1200 ext. 8181, Fax +886-3-3282173, Email [email protected]
Purpose: The incidence of bloodstream infection among end-stage kidney disease (ESKD) patients on chronic hemodialysis (HD) was 26-fold higher than population controls, causing higher morbidity and costs. The aim of this investigation was to clarify the prognostic factors, in-hospital outcomes and recurrence of infectious spondylitis of patients with and without chronic HD.
Patients and Methods: This nationwide study analyzed 2592 patients who admitted for first-time infectious spondylitis between January 1, 2003, and December 31, 2015. Patients were classified into the chronic HD or the non-HD group. The logistic regression model and the general linear model were utilized to determine the impact of chronic HD on in-hospital mortality and recurrence. The Cox proportional hazard model was used to estimate the predictive factors of in-hospital mortality and recurrence.
Results: Compared to the non-HD group, patients in the chronic HD group had a higher risk of respiratory failure, sepsis, in-hospital mortality, longer hospital stay, and higher medical spending. Chronic HD was an independent risk factor for in-hospital mortality (hazard ratio 2.21, 95% confidence interval 1.34– 3.65, p=0.0019), but not for recurrence. Intravascular device implantation or revision was a prognosticator for the mortality of both groups and a predictor for recurrence of the non-HD group. Surgical treatment was associated with a decreased risk of recurrence, whereas treatment with CT-guided abscess drainage was associated with an increased risk of recurrence in both groups.
Conclusion: Patients with infectious spondylitis who were receiving chronic HD had a higher in-hospital mortality compared to those without HD. Intravascular device implantations or revision within 6 months was a significant predictor of in-hospital mortality and disease recurrence. Surgical treatment of infectious spondylitis had a lower risk of recurrence than those with CT-guided abscess drainage in both patient groups.
Keywords: infectious spondylitis, chronic hemodialysis, chronic kidney disease, end-stage kidney disease, outcome, recurrence, risk factor
Introduction
Infectious spondylitis is a rare disease that frequently occurs in the elderly population and those with depressed immunity. Since back pain is the most common initial presentation of infectious spondylitis, the diagnosis may be mistaken as degenerative spinal diseases that result in delayed treatment.1 Bloodstream infection with hematogenous spread of microorganisms is an important mechanism to develop infectious spondylitis. Pathogens may also achieve the vertebra through local invasion or venous/lymphatic flow after spinal surgery or a urinary tract infection (UTI). In the United States, the incidence of admission due to infectious spondylitis increased from 2.9/100,000 in 1998 to 5.4/100,000 in 2013.2 Those patients had higher short- and long-term mortality after admission and lower ability to return to work compared with the reference population.3,4
Patients with end-stage kidney disease (ESKD) have impaired both innate and adaptive immune system. Polymorphonuclear leukocytes and dendritic cell dysfunction with expansion of proinflammatory T cell and monocyte in patients with ESKD were associated with an increased susceptibility to bacterial and viral infections, poor vaccination responses and an increased risk of malignancies.5,6 The incidence of bloodstream infection among chronic HD patients was 26-fold higher than population controls.7 Using a central venous catheter as HD access, vascular operation, poor caring skill of vascular access and contamination of water purification system were risk factors for bacteremia, morbidity, and mortality in chronic HD patients.8 Previous studies indicated that patients on maintenance HD who suffered from infectious spondylitis had high in-hospital mortality and higher risk of methicillin-resistant staphylococcus aureus (MRSA) infection.9–11 MRSA infection and ESKD were considered to be related to a recurrence of infectious spondylitis.12 Patients with chronic HD should be looked on as a high-risk subgroup in infectious spondylitis.
Studies that focused on the risk factors and outcomes between chronic HD and non-HD patients with infectious spondylitis were limited. The impact of intervention (surgery or CT-guided drainage) on the mortality and recurrence has not been addressed and recurrence among dialysis patients was rarely discussed in the literature. This study was designed to investigate the in-hospital outcomes and the recurrence of infectious spondylitis in a large-scale population. We used a nationwide database to access the chronic HD and the non-HD patients who were first-time admitted for infectious spondylitis. The aim of this investigation was to elucidate the prognostic factors, the in-hospital outcomes and the recurrence of infectious spondylitis of the patients with and without HD.
Patients and Methods
Data Source
This is a nationwide database study analyzing the National Health Insurance Research Database (NHIRD) of Taiwan. The NHIRD is derived from data collected as part of the National Health Insurance (NHI) program. NHI program has provided compulsory universal health insurance for approximately 99.6% of Taiwan’s population since 1995. The NHIRD releases diagnostic codes, procedure codes and charge codes in the form of encrypted datasets. It could be a valuable tool to understand the diseases in a specific cohort or to study the long-term outcomes of patients. In this work, we focused on patients with infectious spondylitis, especially those who receiving chronic HD. The Institutional Review Board of Chang Gung Memorial Hospital approved this study and waived the need for individual informed consent. The guidelines outlined in the Declaration of Helsinki were followed. The investigators followed the data protection and privacy regulations of NHIRD and all the analysis was conducted on-site analysis on specific servers.
Study Population
This study retrieved hospitalized patients who were first-time diagnosed with infectious spondylitis in NHIRD from January 1, 2003, to December 31, 2015 (n=2617). The hospitalization for first-time infectious spondylitis was defined as the index admission and the admission date was defined as the index date. The exclusion criteria were as follows: (1) patients with missing age or sex information (n=10); (2) patients with age less than 20 years on the index date (n=10); (3) patients who were receiving peritoneal dialysis when admission (n=5). A total of 2592 patients were included for analysis. Patients with ESKD who had received HD for at least 1 month before the index admission were classified as the chronic HD group (n=192); other patients were sorted out as the non-HD group (n=2400). A flow chart of the patient inclusion process is shown in Figure 1.
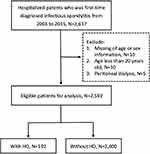 |
Figure 1 Flow chart of patient selection. |
Definition of Infectious Spondylitis, HD and Comorbid Variables
The diagnosis of infectious spondylitis was defined by using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code of 720.9 and reimbursement of intravenous antibiotics during the index admission. CKD was defined by the ICD-9-CM code of 585. HD was identified by reimbursement of HD procedure in the NHI system. The demographic results were registered from the NHIRD at the index date. The predisposing conditions were identified by certain diagnoses or procedures within 180 days before the index date for spinal surgery, infective endocarditis, and intravascular device implantations or revision; and within 180 days before the index date or in the index admission for cellulitis, UTI and pneumonia. Comorbidities were defined by claiming an ICD-9-CM diagnostic code in medical records at least twice before the index admission. Complications and treatments were defined by the diagnoses or the procedures concurrently during the index admission. Recurrence was defined as readmission for infectious spondylitis. All patients were traced from the index date to the date of death/recurrence or December 31, 2015.
Statistical Analysis
The demographic results were summarized using descriptive statistics. Continuous data were expressed as the mean ± standard deviation (SD) and categorical data were expressed as numbers or percentages (%) of each item. Differences between the chronic HD and non-HD groups were compared using the Student’s t-test, Mann–Whitney test, Chi-square test, and Fisher’s exact test. To determine the impact of chronic HD on in-hospital mortality and recurrence, we performed univariate logistic regression model and general linear model that adjusted the age, gender, predisposing condition in 6 months before admission, comorbid conditions, and hospital level. The Cox proportional hazard model was used to estimate the predictive factors of in-hospital mortality and recurrence in chronic HD and non-HD groups. The patient’s in-hospital survival of the two groups was generated according to the Kaplan–Meier method and compared by using the Log rank test. A p-value below 0.05 was considered statistically significant. This study used SAS software version 9.4 (SAS Institute, Cary, NC, USA) for data analysis.
Results
A total of 2592 patients who admitted for first-time infectious spondylitis between January 1, 2003, and December 31, 2015, were identified as being eligible for analysis. There were 192 patients classified as chronic HD group and 2400 patients classified as a non-HD group (Figure 1). Among the chronic HD group, 97.40% of the patients (n=187) had Catastrophic Illness Card (CIC) of ESKD, which were confirmed by two nephrologists for requiring long-term renal replacement therapy, before the index date. This result indicated that most of the cases in the chronic HD group were patients with ESKD on maintenance HD.
Patients in the chronic HD group were older; they had lower male-to-female ratio; they possessed a higher prevalence of diabetes mellitus (DM), hypertension, heart failure, coronary artery disease, prior myocardial infarction and prior stroke (Table 1). There was no difference in baseline characteristics in atrial fibrillation, chronic obstructive pulmonary disease, liver cirrhosis, malignancy, and hospital level. About the predisposing conditions in 6 months before admission, the chronic HD group had more intravascular device implantations or revision (31.25% vs 9.25%, p<0.0001) and pneumonia (26.56% vs 13.17%, p<0.0001) and less of UTI (17.19% vs 29.58%, p<0.0001). There was no difference in the proportion of spine surgery (3.65% vs 6.83%, p=0.0869), infective endocarditis (<5 cases vs 1.63%) and cellulitis (20.31% vs 17.96%, p=0.4153).
 |
Table 1 Demographic Characteristics of the Study Population |
Clinical consequences of the two groups are shown in Table 2. Compared to the non-HD group, the chronic HD group had a higher percentage of respiratory failure (8.33% vs 3.92%; odds ratio [OR] 2.23, 95% confidence interval [CI] 1.29–3.87), sepsis (35.42% vs 24.83%; OR 1.66, 95% CI 1.22–2.26), in-hospital mortality (13.02% vs 3.13%; OR 4.64, 95% CI 2.87–7.49), longer hospital stay (34.69±22.17 vs 30.44±19.23; OR 4.25) and higher medical expenditure (median 238,307 vs 152,111; OR 115,090.6). There was no difference in proportion of concurrent infective endocarditis (<5 cases vs 2.96%; OR 0.0811, 95% CI 0.0–1.24), new onset stroke (1.56% vs 1.54%; OR 1.01, 95% CI 0.31–3.32), new onset venous thrombosis (0.52% vs 0.50%; OR 1.04, 95% CI 0.13–8.06), surgical treatment (36.98% vs 35.88; OR 1.05, 95% CI 0.77–1.42) and CT-guided drainage of abscess (11.98% vs 10.67%; OR 1.14, 95% CI 0.72–1.80) between the two groups. During the follow-up period, 18.23% of the patients in the chronic HD group and 15.83% in the non-HD group developed a recurrence (OR 1.19, 95% CI 0.81–1.74, p=0.3841). The median of time from discharge to recurrence was 34 days and 37 days in the chronic HD group and non-HD group (OR −95.45, p=0.0690), respectively. Six patients in chronic HD group (17.14%) and 94 patients the in non-HD group (24.74%) received surgical treatment when recurrence (OR 0.79, 95% CI 0.34–1.83, p=0.5848).
 |
Table 2 Clinical Characteristics and Consequences |
The Cox proportional hazard model showed that chronic HD was an independent risk factor for in-hospital mortality (hazard ratio [HR] 2.21, 95% CI 1.34–3.65, p=0.0019) (Table 3). The in-hospital survival of the two groups was shown in Figure 2 (P of Log rank test <0.0001). Incident chronic HD had no significant association with the recurrence rate of infectious spondylitis during the follow-up period (HR 1.37, 95% CI 0.95–1.97, p=0.0974).
 |
Table 3 Risk Factor Analysis of in-Hospital Mortality and Recurrence by Chronic HD |
 |
Figure 2 Kaplan–Meier survival curves of in-hospital mortality of in patients with infectious spondylitis (chronic HD vs non-HD). Abbreviaiton: HD, hemodialysis. |
Results of characteristics associated with in-hospital mortality are shown in Table 4. In the chronic HD group, a higher in-hospital mortality was linked with a predisposing condition of intravascular device implantations or revision (HR 4.68, 95% CI 1.68–13.00), an underlying DM (HR 4.23, 95% CI 1.27–14.16), hypertension (HR 11.12, 95% CI 3.50–35.31) or prior myocardial infarction (HR 7.00, 95% CI 1.51–32.50). A predisposing condition of cellulitis (HR 0.20, 95% CI 0.04–0.99) and pneumonia (HR 0.21, 95% CI 0.05–0.90) were protective factors in the chronic HD group. In the non-HD group, older age (HR 1.02, 95% CI 1.00–1.04), a predisposing condition of intravascular device implantations or revision (HR 2.76, 95% CI 1.42–5.36), an underlying disease of DM (HR 2.23, 95% CI 1.30–3.84), hypertension (HR 8.76, 95% CI 5.20–14.74) or prior myocardial infarction (HR 2.32, 95% CI 1.01–5.31), respiratory failure (HR 3.21, 95% CI 1.79–5.75) and sepsis (HR 3.32, 95% CI 1.98–5.55) were associated with higher in-hospital mortality. A predisposing condition of UTI (HR 0.54, 95% CI 0.31–0.96) was a protective factor of in-hospital mortality in the non-HD group. It is worth to note that surgical intervention and CT-guided drainage did not increased post-operative mortality in patients of spondylodiscitis with HD.
 |
Table 4 Correlation of Characters with in-Hospital Mortality |
Results of characteristics associated with recurrence are shown in Table 5. CT-guided drainage of abscess was associated with an increased risk of recurrence (HR 3.04, 95% CI 1.05–8.79) whereas hospitalized in a medical center (HR 0.37, 95% CI 0.16–0.85) and surgical treatment (HR 0.09, 95% CI 0.02–0.34) were associated with a decreased risk of recurrence in the chronic HD group. In non-HD group, a predisposing condition of intravascular device implantations or revision (HR 1.44, 95% CI 1.04–1.99), an underlying disease of chronic obstructive pulmonary disease (HR 1.46, 95% CI 1.10–1.95), sepsis (HR 1.45, 95% CI 1.15–1.83) and CT-guided drainage of abscess (HR 1.49, 95% CI 1.12–2.00) were associated with an increased risk of recurrence. Older age (HR 0.99, 95% CI 0.98–1.00), an underlying disease of hypertension (OR 0.51, 95% CI 0.32–0.81) or coronary artery disease (OR 0.66, 95% CI 0.47–0.93) and surgical treatment (HR 0.72, 95% CI 0.57–0.90) were protective factors, respectively.
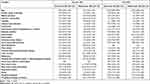 |
Table 5 Correlation of Characters with Recurrence |
Discussion
This nationwide cohort study analyzed 2592 patients who were first-time admitted for infectious spondylitis from 2003 to 2015. Compared to the non-HD group, chronic HD patients with infectious spondylitis were prone to develop respiratory failure and sepsis. The in-hospital mortality was 13.02% in the chronic HD group and 3.13% in the non-HD group, respectively. As hypothesized, incident chronic HD was associated with an increased risk of in-hospital mortality in patients with infectious spondylitis. Nevertheless, our results showed no significant association between incident chronic HD and disease recurrence. Also, surgical intervention did not increase mortality in patients of spondylodiscitis with HD, which differed from a previous research work.13
The non-HD group was male-predominant whereas the chronic HD group had similar case numbers of males and females.1,9,14 The difference in the sex composition might indicate that there were different risk factors of developing infectious spondylitis in patients with and without chronic HD. The study listed the frequently mentioned predisposing conditions: spinal surgery, infective endocarditis, cellulitis, UTI, pneumonia, and intravascular device implantations or revision. It was notable that 31.25% of the patients in the chronic HD group had received intravascular device implantations or revisions within 6 months before admission. The vascular manipulation might result in transient bacteremia and increase the risk of developing infectious spondylitis. The study also showed that intravascular device implantations or revisions were associated with an increased risk of in-hospital mortality in both groups and recurrence in the non-HD group. This result was compatible with a previous study, which stated that using a central venous catheter was a significant risk factor for progression and mortality of infectious spondylitis.15 As a bloodstream infection, risk of concurrent infectious endocarditis in patients with intravascular device-associated infectious spondylitis was high.16 These results suggested the critical role of a vascular manipulation on the pathophysiology and outcomes of infectious spondylitis, especially in chronic HD patients.
Guideline of Infectious Diseases Society of America suggests a total duration of 6 weeks of parenteral or highly bioavailable oral antimicrobial therapy as the standard treatment.17 Surgical intervention of infection is recommended in the presence of progressive neurologic deficits, progressive deformity and spinal instability. Some studies showed that a percutaneous aspiration and drainage might be an effective treatment alternative to open surgery in patients with abscess.18,19 We had similar results that a surgical treatment or a CT-guided drainage of abscess were not associated with in-hospital mortality. Nevertheless, our results showed that a surgical treatment was a protective factor of recurrence, whereas a CT-guided drainage of abscess was a risk factor for recurrence in both the chronic HD and non-HD groups. A surgery allows microbiological confirmation, histopathologic analysis, adequate debridement, spine stabilization and better pain control.20,21 Minimal spinal surgery with or without endoscopic equipment was an effective treatment with lower intra-operative risk.22,23 To our knowledge, this was the first study warranting the association between treatment choice for surgery/CT-guided drainage and recurrence. Aggressive treatment with effective antibiotic and surgical debridement may be beneficial in selective patients. Hypothesis in this area needs further study.
CKD and ESKD are associated with an increased risk of morbidity and mortality in patients with infectious diseases.24 Chronic HD-related immune dysfunction is the key node to the susceptible bloodstream infection. Factors associated with impaired immune system in EKDS include uremic toxins, fluid overload, multivitamin deficiency, catheter and biocompatible membrane and medications.25 Other reported risk factors of mortality were old age, male sex, continuous use of glucocorticoids, drug-resistant Staphylococcus infection, diabetes mellitus, congestive heart, cerebrovascular disease, and liver disease.2,26 MRSA was the most frequent pathogen of infectious spondylitis in patients with chronic HD.27 The incidence of MRSA is 2.5-fold than the non-HD patients.10 MRSA infection was associated with poor clinical outcome and recurrence in infectious spondylitis.11,12 The predictive analysis of in-hospital mortality and recurrence showed that DM, hypertension and post myocardial infarction were major risk factors for mortality, whereas surgical treatment and CT-guided drainage of abscess were associated with recurrence. This might suggest that patient’s in-hospital survival is mostly related to their underlying diseases and a recurrent depends on pathogenesis and treatment process.
This study has several limitations. Since the etiologic microorganism was not available in the NHIRD, we were unable to analyze the impact of pathogen on patient’s outcome and recurrence. Our previous study that used a propensity-score matching method to analyze patients with and without chronic HD in a 300 patient cohort might provide some reference of the microbiological data.10 The exact time of symptom onset, sequela after discharge and life quality post infection were not clearly known. Patients with infectious spondylitis usually have gradually onset of symptoms and vague disease onset date. The impact of chronic HD on patient’s sequela and life quality after discharge needs further study.
In conclusion, patients with infectious spondylitis who were receiving chronic HD had higher in-hospital mortality compared to those without HD. There was no difference in recurrence between the two study groups. Intravascular device implantations or revision within 6 months was a significant predictor of in-hospital mortality and disease recurrence. Surgical intervention was not associated with increased in-hospital mortality. Surgical treatment of infectious spondylitis had a lower risk of recurrence than those with CT-guided abscess drainage in both patient groups.
Acknowledgments
The authors would like to thank the statistical assistance and wish to acknowledge the support of the Maintenance Project of the Center for Big Data Analytics and Statistics (Grant CLRPG3D0044) at Chang Gung Memorial Hospital for study design and monitor, data analysis and interpretation. The authors also would like to thank Research Grant from Linkou Chang-Gung Memorial Hospital for the support of the maintenance project (Grant CMRPG3G1811).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chang WS, Ho MW, Lin PC, et al. Clinical characteristics, treatments, and outcomes of hematogenous pyogenic vertebral osteomyelitis, 12-year experience from a tertiary hospital in central Taiwan. J Microbiol Immunol Infect. 2018;51(2):235–242. doi:10.1016/j.jmii.2017.08.002
2. Issa K, Diebo BG, Faloon M, et al. The epidemiology of vertebral osteomyelitis in the United States from 1998 to 2013. Clin Spine Surg. 2018;31(2):E102–E108. doi:10.1097/BSD.0000000000000597
3. Kehrer M, Hallas J, Baelum J, Jensen TG, Pedersen C, Lassen AT. Reduced ability to work both before and after infectious spondylodiscitis in working-age patients. Infect Dis (Lond). 2017;49(2):95–103. doi:10.1080/23744235.2016.1217348
4. Kehrer M, Pedersen C, Jensen TG, Hallas J, Lassen AT. Increased short- and long-term mortality among patients with infectious spondylodiscitis compared with a reference population. Spine J. 2015;15(6):1233–1240. doi:10.1016/j.spinee.2015.02.021
5. Betjes MG. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol. 2013;9(5):255–265. doi:10.1038/nrneph.2013.44
6. Cohen G. Immune dysfunction in uremia 2020. Toxins (Basel). 2020;12(7):439. doi:10.3390/toxins12070439
7. Skov Dalgaard L, Norgaard M, Jespersen B, et al. Risk and prognosis of bloodstream infections among patients on chronic hemodialysis: a population-based cohort study. PLoS One. 2015;10(4):e0124547. doi:10.1371/journal.pone.0124547
8. Katneni R, Hedayati SS. Central venous catheter-related bacteremia in chronic hemodialysis patients: epidemiology and evidence-based management. Nat Clin Pract Nephrol. 2007;3(5):256–266. doi:10.1038/ncpneph0447
9. Lu YA, Sun WC, Kuo G, et al. Epidemiology and outcomes of infectious spondylodiscitis in hemodialysis patients. Spine (Phila Pa 1976). 2018;43(12):869–876. doi:10.1097/BRS.0000000000002443
10. Kuo G, Sun WC, Lu YA, et al. Chronic dialysis patients with infectious spondylodiscitis have poorer outcomes than non-dialysis populations. Ther Clin Risk Manag. 2018;14:257–263. doi:10.2147/TCRM.S153546
11. Ascione T, Balato G, Di Donato SL, et al. Clinical and microbiological outcomes in haematogenous spondylodiscitis treated conservatively. Eur Spine J. 2017;26(Suppl 4):489–495. doi:10.1007/s00586-017-5036-4
12. Park KH, Cho OH, Lee JH, et al. Optimal duration of antibiotic therapy in patients with hematogenous vertebral osteomyelitis at low risk and high risk of recurrence. Clin Infect Dis. 2016;62(10):1262–1269. doi:10.1093/cid/ciw098
13. Chen LH, Fu TS, Kao YH, et al. Surgical treatment of infectious spondylitis in patients undergoing hemodialysis therapy. Eur Spine J. 2010;19(12):2223–2228. doi:10.1007/s00586-010-1501-z
14. Gupta A, Kowalski TJ, Osmon DR, et al. Long-term outcome of pyogenic vertebral osteomyelitis: a cohort study of 260 patients. Open Forum Infect Dis. 2014;1(3):ofu107. doi:10.1093/ofid/ofu107
15. Cobo Sanchez JL, Gandara Revuelta M, Cuadrado Mantecon ME, Sainz Alonso RA, Sanchez Cano MS. Infectious spondylodiscitis in patients with central venous catheters for haemodialysis: a retrospective study. J Ren Care. 2012;38(3):147–150. doi:10.1111/j.1755-6686.2012.00312.x
16. Renz N, Haupenthal J, Schuetz MA, Trampuz A. Hematogenous vertebral osteomyelitis associated with intravascular device-associated infections - A retrospective cohort study. Diagn Microbiol Infect Dis. 2017;88(1):75–81. doi:10.1016/j.diagmicrobio.2017.01.020
17. Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015;61(6):e26–46. doi:10.1093/cid/civ482
18. Matsubara T, Yamada K, Sato K, Gotoh M, Nagata K, Shiba N. Clinical outcomes of percutaneous suction aspiration and drainage for the treatment of infective spondylodiscitis with paravertebral or epidural abscess. Spine J. 2018;18(9):1558–1569. doi:10.1016/j.spinee.2018.02.020
19. Griffith-Jones W, Nasto LA, Pola E, Stokes OM, Mehdian H. Percutaneous suction and irrigation for the treatment of recalcitrant pyogenic spondylodiscitis. J Orthop Traumatol. 2018;19(1):10. doi:10.1186/s10195-018-0496-9
20. Martin-Alonso J, Delgado-Lopez PD, Castilla-Diez JM, et al. [Role of surgery in spontaneous spondylodiscitis: experience in 83 consecutive patients]. Neurocirugia (Astur). 2018;29(2):64–78. Spanish.
21. Miller JA, Achey RL, Derakhshan A, Lubelski D, Benzel EC, Mroz TE. Neurologic complications, reoperation, and clinical outcomes after surgery for vertebral osteomyelitis. Spine (Phila Pa 1976). 2016;41(4):E197–204. doi:10.1097/BRS.0000000000001157
22. Lee CY, Huang TJ, Li YY, Cheng CC, Wu MH. Comparison of minimal access and traditional anterior spinal surgery in managing infectious spondylitis: a minimum 2-year follow-up. Spine J. 2014;14(7):1099–1105. doi:10.1016/j.spinee.2013.07.470
23. Yang SC, Chen WJ, Chen HS, Kao YH, Yu SW, Tu YK. Extended indications of percutaneous endoscopic lavage and drainage for the treatment of lumbar infectious spondylitis. Eur Spine J. 2014;23(4):846–853. doi:10.1007/s00586-013-3157-y
24. Cheikh Hassan HI, Tang M, Djurdjev O, Langsford D, Sood MM, Levin A. Infection in advanced chronic kidney disease leads to increased risk of cardiovascular events, end-stage kidney disease and mortality. Kidney Int. 2016;90(4):897–904. doi:10.1016/j.kint.2016.07.013
25. Sharif MR, Chitsazian Z, Moosavian M, et al. Immune disorders in hemodialysis patients. Iran J Kidney Dis. 2015;9(2):84–96.
26. Kokabu T, Takahata M, Ishiguro N, Iwasaki N. Long-term prognosis of hematogenous vertebral osteomyelitis: mortality, quality of life, and pain. J Orthop Sci. 2017;22(5):822–827. doi:10.1016/j.jos.2017.05.017
27. Madhavan K, Chieng LO, Armstrong VL, Wang MY. Spondylodiscitis in end-stage renal disease: a systematic review. J Neurosurg Spine. 2019;1–9. doi:10.3171/2018.9.SPINE18824
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
