Back to Journals » Journal of Inflammation Research » Volume 17
In-Depth Review of Loeffler Endocarditis: What Have We Learned?
Authors Su S , Liang L, Lü L, Li M, Zhang X, Jin Y, Wei W, Wan Z
Received 9 January 2024
Accepted for publication 19 March 2024
Published 28 March 2024 Volume 2024:17 Pages 1957—1969
DOI https://doi.org/10.2147/JIR.S458692
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Shitong Su,1,2 Lianjing Liang,1 Lin Lü,1 Mingfeng Li,3 Xiaoling Zhang,4 Yongmei Jin,5 Wei Wei,1 Zhi Wan6
1Department of Emergency Medicine, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China; 2Division of Head & Neck Tumor Multimodality Treatment, Cancer Center, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China; 3Department of Pathology, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China; 4Department of Cardiology, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China; 5Department of Laboratory Medicine, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China; 6Rare Diseases Center, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China
Correspondence: Wei Wei, Chest Pain center, Department of Emergency Medicine, West China Hospital, Sichuan University, No. 37, Guoxue Lane, Wuhou District, Chengdu, 610041, People’s Republic of China, Email [email protected] Zhi Wan, Rare Diseases Center, West China Hospital, Sichuan University, No. 37, Guoxue Lane, Wuhou District, Chengdu, 610041, People’s Republic of China, Email [email protected]
Abstract: Loeffler endocarditis, eosinophilic endocarditis or eosinophilic endomyocardial disease are conditions associated with hypereosinophilia and they affect the heart function. Loeffler endocarditis is a rare endomyocardial disorder thought to be caused by eosinophilic damage. The disorder is characterized by inflammatory infiltration, formation of thrombus within cardiovascular system, and ultimately fibrosis of the afflicted area. It can lead to multiple severe complications, including thromboembolic disease, thickening of fibrous tissue in the endocardium of ventricles, valve involvement, apical obliteration, and various heart disorders. Although early clinical intervention can lead to remission, the underlying mechanisms of the disorder remain unresolved. In the present article, we summarise the existing literature concerning Loeffler endocarditis based on PubMed, Web of Science, and other medical databases to conduct an in-depth review of the epidemiology, etiology, pathophysiological mechanisms, staging, diagnosis, treatment and prognosis of Loeffler endocarditis. Meanwhile, we provide novel patients data and clinical figures of Loeffler endocarditis to supplement the understanding of this cardiac disorder. The findings presented in this article provide a basis for further studies and can be used to improve management of the disorder.
Keywords: Loeffler endocarditis, hypereosinophilia, eosinophils damage
Introduction
Loeffler endocarditis is a rare endomyocardial disorder thought to be caused by eosinophilic damage.1–4 In 1936, Loeffler initially documented a case of progressive heart failure characterized by eosinophilia and thickening of the endocardium due to inflammation, which were recognized as manifestations of hypereosinophilic syndrome (HES).5 Loeffler endocarditis is caused by an elevated count of eosinophils, often associated with allergies, infections, malignancies, autoimmune events, parasite infection and Churg-Strauss syndrome, now termed eosinophilic granulomatosis with polyangiitis.6–10 The incidence of cardiac involvement in adults with HES presentations ranges from 40% to 50%.11,12 This form of endocarditis is characterized by high morbidity and mortality with limited effective treatments at the advanced stages.13 However, the published reviews on this endocardial disorder are limited and primarily rely on case reports and case series, thus they lack sufficient data and illustrations to elucidate the distinct traits of Loeffler endocarditis. Eosinophil infiltration into the heart results in cardiac damage as these cells undergo degranulation, releasing toxic granule proteins that contribute to heart damage,14 mainly caused by ribonucleases and eosinophils major basic protein.15 Loeffler endocarditis can cause certain complications, such as systemic thromboembolic events, progressive heart failure, arrhythmias, and sudden cardiac death.16–18 In this in-depth review, we discuss the epidemiology, etiology, pathophysiological mechanisms, staging, diagnosis, treatment and prognosis of Loeffler endocarditis. In addition, we share some relatively uncommon images obtained from patients with Loeffler endocarditis.
Epidemiology and Etiology
Studies on Loeffler endocarditis are limited due to the few cases of the disease. Loeffler endocarditis has not been fully explored and its prevalence has not been accurately elucidated. Some scholars believe that eosinophilia is a risk factor for this uncommon cardiac condition. The prevalence of cardiac damage in adults with hypereosinophilia is 40% to 50%.11,12 Some studies report a higher frequency of cardiac damage in more than 50% of all patients with HES.19–21 Only a limited number of pediatric cases exhibiting cardiac involvement have been documented.7,22 Less than 10% of Loeffler endocarditis cases are observed in individuals under the age of 16.23 Factors that increase the risk of cardiac damage in patients diagnosed with HES include HLA-Bw44 positivity, thrombocytopenia, elevated vitamin B12 levels, splenomegaly, and the presence of FIP1L1-PDGFRA fusion tyrosine kinase.12,24,25 People who have lived in regions with high rate of parasite infections may have higher risk of cardiac damage resulted from secondary activation of eosinophilia.13 Eosinophilia can be classified into three sub-types including, primary, secondary and idiopathic. Idiopathic eosinophilia is a rare type without definite cause of the increased level of eosinophils, but this is the most common type in patients with Loeffler endocarditis.26–28
Pathophysiological Mechanisms
The pathophysiological mechanism of Loeffler endocarditis has not been fully elucidated. Some studies report that degranulated eosinophils release eosinophil cationic protein that attacks the endocardium. Moreover, the eosinophil cationic protein dysregulates blood coagulation leading to thrombus formation in the cardiovascular system, and eventually fibrosis.
Degranulation of Eosinophils
Eosinophils are types of white blood cells formed through differentiation of the bone marrow stem cell. Eosinophils occasionally infiltrate the cardiac system. Biopsy of Loeffler endocarditis unveiled the existence of degranulated eosinophils and eosinophil cationic protein within the endocardium, accompanied by activated eosinophils and hydrolytic enzymes in the myocardial interstitium.2 The cationic proteins are mediators of inflammation and are highly toxic to myocardial cells.14 Distinctive cardiac myocytolytic alterations, marked by disruptions at the intercellular junctions, become apparent when observed through electron microscope.29 Endomyocardial cells are highly susceptible to the effect of cationic proteins leading to apoptosis and necrosis.
Dysregulation of Blood Coagulation Homeostasis
Eosinophil cationic proteins combine with the anionic domain of coagulation factors inhibiting formation of coagulation regulatory protein complex. This complex prevents formation of thrombus by acting as anticoagulant. Moreover, eosinophil cationic proteins can activate coagulation factors and trigger vascular endothelial injury.30 As a result, substantial mural thrombi form within either the right or left ventricle, leading to a decrease in ventricular cavity dimensions. This reduction of ventricular cavity size and anticoagulant complex presents a potential trigger for systemic thromboembolic incidents and, eventually, contributes to the development of fibrosis.28,31,32
Staging of Loeffler Endocarditis
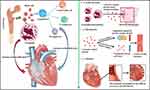
Loeffler endocarditis is classified into acute necrosis stage, thrombotic formation stage and fibrosis stage (Figure 1). Although the clinical features of the three stages is associated with their respective pathophysiological mechanism, they can overlap with each other during the disease development.13,33
Acute Necrosis Stage
The initial phase of this ailment is characterized as the acute necrotic stage. During this phase, endomyocardial apoptosis and necrosis are triggered by eosinophilia, facilitated by the degranulation and release of cationic proteins, alongside the presence of reactive oxygen species.11,16,34 Eosinophilic infiltration begins in this phase and is associated with inflammatory damage. The disease is typically asymptomatic during this stage. However, troponin levels may be elevated during the acute necrotic stage.35 Electrocardiogram conducted during the first stage may reveal sinus tachycardia, nonspecific ST changes, or atrioventricular block.36 Endomyocardial biopsy could reveal positive staining indicative of eosinophils and cationic protein presence.37 In a prior investigation, a 55-year-old male with biopsy-confirmed Loeffler endocarditis, diagnosed with the condition during the acute necrotic phase, was effectively treated using steroid therapy.38 These findings lay a basis for timely diagnosis and initiating treatment during the early stages of Loeffler endocarditis.
Thrombotic Formation Stage
The second phase is thrombotic formation stage. Eosinophil cationic protein inhibit the formation of coagulation regulatory protein complex and plays a role in progression of the disease by binding to endothelial thrombomodulin.11,16,19,34 In addition, damage of the vascular wall by eosinophils leads to a hypercoagulable state, resulting in thrombotic formation.11,39,40 Significantly, occurrence of systemic thromboembolic events secondary to the formation of intraventricular thrombi are major risk factors for morbidity and mortality within individuals affected by Loeffler endocarditis.41 Cerebral infarction, acute abdominal aortic obstruction, pulmonary embolism, and even sudden cardiac death can be caused by thrombus formation.18,28,31,32,42
Fibrosis Stage
The last phase of the disease is the fibrosis stage. This stage exhibits similar pathophysiology to restrictive cardiomyopathies.8,13,25 However, the pathological features observed during this stage are attributed to endocardial fibrosis induced by fibro-inflammatory processes and remodeling of granulation tissue, instead of amyloidosis, hemochromatosis, or non-caseating granulomas.8,13,43,44 Cardiac thrombus and afflicted endomyocardial tissue may be substituted by fibrosis changes. Previous studies reported Loeffler endocarditis patients exhibited heart failure caused by ventricular fibrosis.8,25 Findings in a previous review indicate that apical and ventricular obliteration associated with the thickening of fibrous endocardium of ventricles is observed during the third stage.13 An investigation involving 90 patients revealed that over half of the cases exhibited biventricular lesions, as opposed to being confined to one side of the heart.45 Involvement of the heart valves can lead to concurrent valvular regurgitation, frequently observed in the tricuspid or mitral valves, and irregular motion of the septum.19,46–48 In addition, the fibrosis can affect cardiac conduction leading to severe arrhythmia.8,19,49 Myocardial biopsy conducted during fibrosis stage shows low levels or absence of eosinophils.13
Diagnosis of Loeffler Endocarditis
The diagnosis criteria for this disease include occurrence of hypereosinophilia in the peripheral blood >1,500/mm3 on two separate occasions (>1 month apart),13,21,50 or prolonged hypereosinophilia, defined as the presence of elevated eosinophil levels (>1,500 eosinophils × 10^6/L) in peripheral blood for a duration exceeding 6 months associated with eosinophilic infiltration-related cardiac damage.4 A comprehensive diagnosis of Loeffler endocarditis should comprise analysis of peripheral blood, cardiac enzyme levels, liver and renal function tests, electrocardiogram (ECG), bone marrow test, abdominal and chest computerized tomography (CT), echocardiogram, cardiac magnetic resonance imaging (CMRI), and tissue biopsies.13 Endomyocardial biopsy is currently the gold standard for diagnosis of the condition.2,13
Clinical diagnosis
The clinical heterogeneity of Loeffler endocarditis limits understanding the pathogenesis of this disease. The clinical diagnosis of Loeffler endocarditis includes laboratory test, ECG and history of the patient to confirm nonspecific signs and symptoms observed during the early stages of Loeffler endocarditis. The initial manifestations of this disorder may comprise nonspecific cardiogenic symptoms, such as progressive heart failure, breathlessness, pulmonary edema, leg edema, pericarditis, pericardial effusions, chest pain, splenomegaly, and embolism associated with high levels of eosinophils in peripheral blood.8,18,19,25,50–52 Symptom onset is unpredictable, with a wide spectrum of manifestations ranging from mild presentations to severe and potentially fatal complications.18,19 Increased cardiac enzyme levels can be observed due cardiac damage caused by Loeffler endocarditis.13 Progressive heart failure is associated with elevated levels of brain natriuretic peptides (BNP).8,25,52 Frequent ECG findings include atrial enlargement, atrioventricular blocks, aberrant Q-wave, R-wave or T-wave patterns, diminished voltage, left ventricular hypertrophy, and non-specific ST changes.13,51,53 Comprehensive evaluation should meticulously rule out any alternate sources of systemic or cardiac dysfunction, through an in-depth assessment and exploration of potential factors. This includes investigating other possible causes, such as coronary artery disease, through the implementation of procedures like coronary angiography. Cardiac multimodality and systemic-associated evaluations should be conducted by the respective specialists.
Marrow Analysis
Loeffler endocarditis is a disorder linked to hypereosinophilia and defined by disrupted bone marrow development.4,42 Certain underlying causes, such as the lymphocytic and myeloproliferative diseases (malignancy, lymphoma and leukemia) can be detected relatively easily by bone marrow test. Myeloid disorders display clonal expansion of eosinophils, often accompanied by the presence of fusion genes, chromosomal rearrangements, or detectable gene mutations. Bone marrow biopsy can show the proportion of eosinophils and can be used for morphological analysis and molecular pathology. A prior study indicated that the existence of the FIP1L1-PDGFRA mutation is correlated with an elevated risk of cardiac involvement in cases of hypereosinophilia and is associated with a less favorable prognosis.54 A bone marrow examination was employed to diagnose a case of Loeffler endocarditis, revealing the presence of the FIP1L1-PDGFRA fusion gene in 82% of segmented nucleated cells.25 In a separate study, molecular assessments of bone marrow cells were conducted to diagnose a case of Loeffler endocarditis. The findings revealed lack of FIP1L1-PDGFRA fusion, PDGFRB mutation, as well as irregular myeloid maturation and concurrent lymphoproliferative disorder.38 The association of hypereosinophilia and B-lymphoblastic lymphoma was reported in a previous study.55 In our study, we present a bone marrow smear of Loeffler endocarditis showing significant eosinophilia without abnormal myeloid cells (Figure 2A), and the morphology of degranulated eosinophils (Figure 2B). Flow cytometry (FCM) showed the myeloid clonality and counts of eosinophils in Loeffler endocarditis (Figure 3).
 |
Figure 2 Bone marrow smear of Loeffler endocarditis. (A) Active myeloid proliferation, marked eosinophilia without abnormal myeloid cells (arrow); (B) Morphology of degranulated eosinophils (arrow). |
 |
Figure 3 Flow cytometry (FCM) of Loeffler endocarditis with significant myeloid hypereosinophilia. |
Characteristic Images
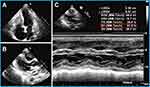
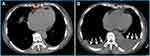
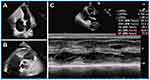
Cardiovascular imaging plays a key role in the diagnosis of Loeffler endocarditis.8,56,57 The choice of imaging techniques employed to diagnose Loeffler endocarditis evolves in tandem with the advancement of the disorder. Multimodal imaging is preferred due to its comprehensive and complementary nature. Echocardiography serves as a fundamental initial diagnostic tool, facilitating the assessment of cardiac function, intracardiac thrombosis, ventricular dimensions, hypertrophy, and valvular engagement.1,19,58,59 Echocardiography is effective for distinguishing typical thrombotic obliteration of the apex from apical hypertrophic cardiomyopathy, and it aids in evaluating the precise location, dimensions, and structure of thrombi.13 However, this method is does not discern the precise boundary between the endocardium and thrombus, potentially causing the oversight of affected regions and yielding false-negative results in biopsy findings.1 Loeffler endocarditis frequently impacts the lateral wall and valve apparatus, resulting in instances of mitral or tricuspid regurgitation. These occurrences can be identified through the utilization of echocardiography.13 In the case presented in our study, echocardiogram revealed that Loeffler endocarditis resulted in heart failure characterized by preserved ejection fraction (Figure 4). Further, left ventricular hypertrophy and complete apical obliteration (Figure 4A), right ventricular thrombus (Figure 4B), and 58% preserved ejection fraction (EF) (Figure 4C) were observed through echocardiography. The echocardiography features of Loeffler endocarditis may not typical thus providing insufficient details for diagnosis. The echocardiography approach results in nonspecific findings and may not enable differentiation of Loeffler endocarditis from other cardiomyopathies with similar manifestations.60,61 CMRI can be used to further assess the positive findings from echocardiography procedure. CMRI has high level of accuracy, as it can discern early alterations and comprehensively delineate the characteristics of the endocardium, as well as enable identification of thrombus presence across all phases of the disease.62 CMRI shows late gadolinium enhancement in the initial and third stages, which correlates with inflammatory damage and subsequent fibrosis, respectively. Additionally, intracardiac thrombus is evident during the second stage.16,49,51,52,63 Furthermore, CMRI can also be used to investigate endomyocardial inflammatory alterations and edema, detectable through an elevated T2 signal13 In a previous instance, CMRI was utilized for diagnosis of a case of Loeffler endocarditis, revealing the presence of a right ventricular mass consisting of thrombus alongside extensive endomyocardial fibrosis.18 Additionally, CMRI revealed complete obliteration of the left ventricular apex caused by a mass exhibiting a density comparable to that of the myocardium.64 CMRI is effective in distinguishing Loeffler endocarditis from other cardiomyopathies, such as myocardial apical tumors or apical hypertrophic cardiomyopathy.64 Furthermore, CMRI can serve as a tool for monitoring the progression of Loeffler endocarditis by assessing the augmented accumulation of fibrotic tissue, diastolic or systolic dysfunction, and changes related to thrombosis.13 Chest CT is an initial diagnostic approach when evaluating a patient with suspected Loeffler endocarditis. The role of CT evolves throughout the various stages of the disease. CT is performed to identify intracardiac thrombotic lesions and to delineate the subendomyocardial thrombus during the second stage by revealing an isodense signal intensity of the endocardium and the thrombus.65 In the third stage, CT can also be used to precisely identify the regions of replacement fibrosis, which are characterized by ventricular mass (Figure 5A). These features in the regions of replacement fibrosis indicate endomyocardial pathologic remodeling.65,66 Furthermore, CT is employed to evaluate extracardiac involvement in cases of Loeffler endocarditis,67 such as sarcoidosis, pulmonary fibrosis, pulmonary edema, and unilateral or bilateral pleural effusion (Figure 5B). Notably, CT is not effective for examination of the acute necrotic stage.65 These findings show that different imaging approaches are suitable for different stages of the disease, thus multimodality imaging approach should be used for diagnosis of Loeffler endocarditis.
Histopathological Biopsy
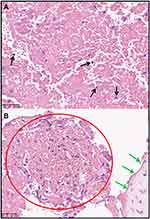
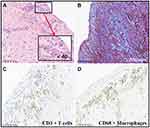
The definitive and established method for diagnosis of Loeffler endocarditis is through the utilization of endomyocardial biopsy, which serves as the gold standard. There are few histopathological images of Loeffler endocarditis in published studies because it is risky to perform the operation.13 The histopathological features are correlated with the stages of Loeffler endocarditis. Inflammatory necrosis caused by eosinophilic infiltration can be observed under a microscope (Figure 6A). However, most studies do not present microscopy images of the second stage characterized by intracardiac thrombus (Figure 6B). Endomyocardial biopsy can be used to distinguish the boundary between thrombus and endocardium. Notably, the third stage of endomyocardial fibrosis is always nonspecific because an eosinophilic damage may take long to develop into fibrotic disease (Figure 7A).51,63 A previous study reported absence of endomyocardial eosinophilic infiltration during the third stage.68 Masson staining (Figure 7B) and immunohistochemical method (Figure 7C–D) are useful methods to confirm fibrosis and inflammatory repair of endomyocardial eosinophilic damage.32
Treatment and Prognosis of Loeffler Endocarditis
Loeffler endocarditis is a form or the cardiac manifestation of the hypereosinophilia syndrome. The aim of treatment of this disease is to decrease the levels of eosinophils and to prevent further organ damage and systemic thromboembolic events. The choice of treatment is dictated by the disease stage and the degree of cardiac/extracardiac damage. Corticosteroids are the most frequently used drugs applied through a stepwise approach.13,38,69,70 After corticosteroids, the treatment regimen may involve cytotoxic agents such as hydroxyurea, azathioprine, or tyrosine kinase inhibitors, as well as mepolizumab, an immunomodulatory agent directed towards eosinophils. In cases that prove resistant to conventional treatments, interferon-alpha can be considered as an alternative approach.13,38,50,71,72 Samples obtained from individuals with Loeffler endocarditis should undergo testing using fluorescence in situ hybridization (FISH) to identify the presence of the FIP1L1-PDGRFA gene fusion. This assessment serves as a crucial tool for guiding the appropriate management of the disease.13,24,38,54 The significance of this test lies in its ability to determine the presence of the FIP1L1-PDGRFA gene fusion, which in turn informs treatment decisions, especially considering the effectiveness of imatinib in individuals harboring this fusion. The efficacy of such drugs has been substantiated, particularly in the initial phases of Loeffler endocarditis.24,54 A previous study documented significant ventricular remodeling in Loeffler endocarditis patients treated solely with prednisone and warfarin, as evidenced by a favorable outcome over a follow-up period of 7 years.47 We prefer to believe that it is not reverse remodeling but a clinical manifestation of damage repair and ventricular compensation. Presently, medical therapeutic options are limited in the advanced stage of the disease. During these stages, most patients manifest heart failure due to restrictive changes. Management of this condition typically involves a combination of diuretics, beta-adrenergic blockers, digoxin, ACE inhibitors (ACEI), angiotensin receptor blockers (ARB), aldosterone antagonists, and corticosteroids. In addition, there should be a common standard, such as NYHA scoring or EF value, to reflect the efficacy of medications in these patients with heart failure manifestation. However, the assessment and management processes remain center-specific. In our study, a case of Loeffler endocarditis (Figure 8A–B) with heart failure was successfully treated with diuretics, ACEI and corticosteroids indicated by 77.5% EF (Figure 8C). This is the same patient who has been showed in Figure 4. The cause of the improved symptoms is controversial for the patient. We thought that an EF of 58% to 77.5% may be the intuitive cause of improved symptoms after diuretics, ACEI and corticosteroids. However, expert considered that it is typically diastolic heart failure with preserved EF. Therefore, the EF is not helpful unless it was previously abnormally low. Simultaneously, we should search the evidence of systolic heart failure for the patient. In addition, this expert suspect that the diuretics and corticosteroids are what the patient responded to otherwise. We believe that these viewpoints are valuable to conduct our clinical pratices. Furthermore, adequate anticoagulant therapy is important for patients with high risk of thromboembolic events. The risk of fatal heart failure and cardiac arrhythmias also increases when fibrosis ensues due to the restrictive cardiomyopathy and circulation system damage. Biopsy-proven endomyocardial fibrosis commonly represents a chronic cardiac damage with no effective treatment.11,13,51 Although, a previous study reported rapid remission of mitral regurgitation in a patient with Loeffler endocarditis 10 days after treatment with prednisolone.2 Valve replacement is an effective treatment approach for cases of Loeffler endocarditis with valve damage. However, valve replacements are also at higher risk in these patients and may not last as long as in other indications, such as rheumatic or congenital valve disorders for replacement. Endocardial stripping and heart transplant are the primary treatments approaches at this stage, but they are associated with high operative mortality.
A positive prognosis is closely associated with the early detection of the disease. However, a substantial number of patients are diagnosed at advanced stages, often following the manifestation of severe symptoms associated with cardiac or multi-organ impairment. Limited treatment options are available for patients presenting with fibrosis and the 2-year mortality rate for this group is 30–50%,38 the 1-year survival rate is 70–80% and the 10-year survival rate is 30%.73
Conclusion
Loeffler endocarditis represents a rare disease linked to hypereosinophilia, characterized by an unfavorable prognosis and high mortality rates. Timely diagnosis and prompt intervention play a pivotal role in enhancing patient outcome. The utilization of diverse diagnostic methods is crucial for diagnosis and staging of Loeffler endocarditis. Concomitant heart failure is the most common clinical course, necessitating a special comment as bridge to understand the efficacy of standard heart failure medications. Simultaneously, a comprehensive and multifaceted therapeutic approach is imperative for effective management patients with this condition.
Data Sharing Statement
All data and material generated or analyzed during this study are included in this published study.
Ethics Approval and Consent
All methods were performed in accordance with the relevant guidelines and regulations, and approved by the Ethics Committee on Biomedical Research, West China Hospital of Sichuan University (Number: 2019-755).
Consent for Publication
All authors have approved the manuscript for submission and publication.
Funding
This study was supported by funding of Science & Technology Department of Sichuan Province (Numbers: 2021YJ0135).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Caruso S, Marrone G, Gentile G, et al. Case 305: Loeffler endocarditis. Radiology. 2022;304(3):736–742. doi:10.1148/radiol.210453
2. Inoue T, Watanabe C, Ayukawa H, et al. Biopsy-proven Loeffler endocarditis successfully treated with steroids. Circulation. 2015;131(8):e353–4. doi:10.1161/CIRCULATIONAHA.114.012976
3. Langwieser N, von Olshausen G, Rischpler C, et al. Confirmation of diagnosis and graduation of inflammatory activity of Loeffler endocarditis by hybrid positron emission tomography/magnetic resonance imaging. Eur Heart J. 2014;35(36):2496. doi:10.1093/eurheartj/ehu148
4. Benezet-Mazuecos J, de la Fuente A, Marcos‐Alberca P, et al. Loeffler endocarditis: what have we learned? Am J Hematol. 2007;82(10):861–862. doi:10.1002/ajh.20957
5. Loeffler W. Endocarditis parietalis fibroplastica mit blut eosinophilia. Schweiz Med Wochenschr. 1936;65:817–820.
6. Bain BJ. Eosinophilia-Idiopathic or not? N Engl J Med. 1999;341(15):1141–1143. doi:10.1056/NEJM199910073411509
7. Dzelebdzic S, Sasaki N, Welch E, et al. hypereosinophilic syndrome with advanced-stage Loeffler Endocarditis. Case. 2022;6(4):191–195. doi:10.1016/j.case.2022.02.004
8. Lupu S, Pop M, Mitre A, et al. Loeffler endocarditis causing heart failure with preserved ejection fraction (HFpEF): characteristic images and diagnostic pathway. Diagnostics. 2022;12(9):doi:10.3390/diagnostics12092157
9. Kuenzli E, Neumayr A, Chaney M, et al. Toxocariasis-associated cardiac diseases-A systematic review of the literature. Acta Trop. 2016;154:107–120. doi:10.1016/j.actatropica.2015.11.003
10. Latt H, Mantilla B, San D, et al. Loeffler endocarditis and associated parasitosis: a diagnostic challenge. Cureus. 2020;12(5):e8152. doi:10.7759/cureus.8152
11. Ogbogu PU, Rosing DR, Horne MK, et al. Cardiovascular manifestations of hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2007;27(3):457–475. doi:10.1016/j.iac.2007.07.001
12. Weller PF, Bubley GJ. The idiopathic hypereosinophilic syndrome. Blood. 1994;83(10):2759–2779. doi:10.1182/blood.V83.10.2759.2759
13. Salih M, Ibrahim R, Tirunagiri D, et al. Loeffler’s endocarditis and hypereosinophilic syndrome. Cardiol Rev. 2021;29(3):150–155. doi:10.1097/CRD.0000000000000324
14. Hogan SP, Rosenberg HF, Moqbel R, et al. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38(5):709–750. doi:10.1111/j.1365-2222.2008.02958.x
15. Fauci AS. NIH conference. The idiopathic hypereosinophilic syndrome. clinical, pathophysiologic, and therapeutic considerations. Ann Intern Med. 1982;97(1):78–92. doi:10.7326/0003-4819-97-1-78
16. Mankad R, Bonnichsen C, Mankad S, et al. Hypereosinophilic syndrome: cardiac diagnosis and management. Heart. 2016;102(2):100–106. doi:10.1136/heartjnl-2015-307959
17. Zhao Y, Jiang P, Chen X, et al. Case report: different clinical manifestations of the rare Loeffler endocarditis. Front Cardiovasc Med. 2022;9:970446. doi:10.3389/fcvm.2022.970446
18. Chao BH, Cline‐Parhamovich K, Grizzard JD, et al. Fatal Loeffler’s endocarditis due to hypereosinophilic syndrome. Am J Hematol. 2007;82(10):920–923. doi:10.1002/ajh.20933
19. Baltazares-Lipp ME, Soto-González JI, Aboitiz-Rivera CM, et al. Hypereosinophilic syndrome: a case of fatal Loeffler endocarditis. Case Rep Cardiol. 2016;2016:2359532.
20. Wang S, Wang A, Guo B, et al. Loeffler endocarditis with multiple cerebral embolism. J Stroke Cerebrovasc Dis. 2014;23:1709–1712. doi:10.1016/j.jstrokecerebrovasdis.2013.10.023
21. Schooley RT, Flaum MA, Gralnick HR, et al. A clinicopathologic correlation of the idiopathic hypereosinophilic syndrome. II. clinical manifestations. Blood. 1981;58:1021–1026. doi:10.1182/blood.V58.5.1021.1021
22. Mansour M, Rahal M, Chammas E, et al. Cardiac involvement in hypereosinophilic syndrome. Ann Pediatr Cardiol. 2018;11:217–218. doi:10.4103/apc.APC_168_17
23. Brambatti M, Matassini MV, Adler ED, et al. Eosinophilic myocarditis: characteristics, treatment, and outcomes. J Am Coll Cardiol. 2017;70(19):2363–2375. doi:10.1016/j.jacc.2017.09.023
24. Cools J, DeAngelo DJ, Gotlib J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic Syndrome. N Engl J Med. 2003;348(13):1201–1214. doi:10.1056/NEJMoa025217
25. Gao M, Zhang W, Zhao W, et al. Loeffler endocarditis as a rare cause of heart failure with preserved ejection fraction: a case report and review of literature. Medicine. 2018;97(11):e0079. doi:10.1097/MD.0000000000010079
26. Allderdice C, Marcu C, Kabirdas D, et al. Intracardiac thrombus in leukemia: role of cardiac magnetic resonance imaging in eosinophilic myocarditis. CASE. 2018;2(3):114–117. doi:10.1016/j.case.2017.12.003
27. Zhou Y, Huang D, Ge J, et al. Loeffler endocarditis in idiopathic hypereosinophilic syndrome demonstrated by magnetic resonance imaging effectively treated by corticosteroids. Acta Cardiol Sin. 2019;35(5):542–545. doi:10.6515/ACS.201909_35(5).20190505A
28. Lin CH, Chang W-N, Chua S, et al. Idiopathic hypereosinophilia syndrome with Loeffler endocarditis, embolic cerebral infarction, and left hydranencephaly: a case report. Acta Neurol Taiwan. 2009;18(3):207–212.
29. Hayashi S, Isobe M, Okubo Y, et al. Improvement of eosinophilic heart disease after steroid therapy: successful demonstration by endomyocardial biopsied specimens. Heart Vessels. 1999;14:104–108. doi:10.1007/BF02481750
30. Sano K, Kobayashi M, Sakaguchi N, et al. A rat model of hypereosinophilic syndrome. Pathol Int. 2001;51(2):82–88. doi:10.1046/j.1440-1827.2001.01175.x
31. Gottdiener JS, Maron BJ, Schooley RT, et al. Two-dimensional echocardiographic assessment of the idiopathic hypereosinophilic syndrome. Anatomic basis of mitral regurgitation and peripheral embolization. Circulation. 1983;67:572–578. doi:10.1161/01.CIR.67.3.572
32. Bohne M, Bohnen S, Voigt H-C, et al. Systemic thrombo-embolic events in a middle-aged male with Loeffler endocarditis without peripheral eosinophilia-a case report. BMC Cardiovasc Disord. 2022;22(1):541. doi:10.1186/s12872-022-02911-3
33. Kim NK, Kim C-Y, Kim JH, et al. A hypereosinophilic syndrome with cardiac involvement from thrombotic stage to fibrotic stage. J Cardiovasc Ultrasound. 2015;23(2):100–102. doi:10.4250/jcu.2015.23.2.100
34. Leiferman KM, Gleich GJ, Peters MS, et al. Dermatologic manifestations of the hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2007;27(3):415–441. doi:10.1016/j.iac.2007.07.009
35. Pitini V, Arrigo C, Azzarello D, et al. Serum concentration of cardiac Troponin T in patients with hypereosinophilic syndrome treated with imatinib is predictive of adverse outcomes. Blood. 2003;102(9):3456–3457. doi:10.1182/blood-2003-07-2393
36. Eicher J-C, Bonnotte B, L’Huillier I, et al. Atteintes cardiaques au cours des hyperéosinophilies: une présentation clinique et échocardiographique polymorphe. Rev Med Interne. 2009;30(12):1011–1019. doi:10.1016/j.revmed.2009.03.355
37. Wright BL, Leiferman KM, Gleich GJ, et al. Eosinophil granule protein localization in eosinophilic endomyocardial disease. N Engl J Med. 2011;365(2):187–188. doi:10.1056/NEJMc1103005
38. Beedupalli J, Modi K. Early-Stage Loeffler’s endocarditis with isolated right ventricular involvement: management, long-term follow-up, and review of literature. Echocardiogr-J Card. 2016;33(9):1422–1427. doi:10.1111/echo.13264
39. Ho Hoffman M, Monroe DM. A cell-based model of hemostasis. Thromb Haemost. 2001;85(06):958–965. doi:10.1055/s-0037-1615947
40. Ruggeri ZM. Platelet interactions with vessel wall components during thrombogenesis. Blood Cells Mol Dis. 2006;36(2):145–147. doi:10.1016/j.bcmd.2005.12.012
41. Zhang Q, Si D, Zhang Z, et al. Loeffler endocarditis with intracardiac thrombus: case report and literature review. BMC Cardiovasc Disord. 2021;21(1):615. doi:10.1186/s12872-021-02443-2
42. Seol SH, Song P-S, Kim D-K, et al. Loeffler’s endocarditis presenting with acute abdominal aortic obstruction. Heart Lung Circ. 2013;22(11):966–967. doi:10.1016/j.hlc.2013.02.009
43. Gomes I, Mathur SK, Espenshade BM, et al. Eosinophil-fibroblast interactions induce fibroblast IL-6 secretion and extracellular matrix gene expression: implications in fibrogenesis. J Allergy Clin Immunol. 2005;116(4):796–804. doi:10.1016/j.jaci.2005.06.031
44. Noguchi H, Kephart GM, Colby TV, et al. Tissue eosinophilia and eosinophil degranulation in syndromes associated with fibrosis. Am J Pathol. 1992;140(2):521–528.
45. Brockington IF, Olsen EGJ. Loeffler's endocarditis and Davies’ endomyocardial fibrosis. Am Heart J. 1973;85(3):308–322. doi:10.1016/0002-8703(73)90365-7
46. Madhwal S, Goldberg J, Barcena J, et al. Unusual cause of acute mitral regurgitation: idiopathic hypereosinophilic syndrome. Ann Thorac Surg. 2012;93(3):974–977. doi:10.1016/j.athoracsur.2011.08.062
47. Lofiego C, Ferlito M, Rocchi G, et al. Ventricular remodeling in Loeffler endocarditis: implications for therapeutic decision making. Eur J Heart Fail. 2005;7(6):1023–1026. doi:10.1016/j.ejheart.2005.06.004
48. Salanitri GC. Endomyocardial fibrosis and intracardiac thrombus occurring in idiopathic hypereosinophilic syndrome. AJR Am J Roentgenol. 2005;184(5):1432–1433. doi:10.2214/ajr.184.5.01841432
49. Çetin S, Heper G, Gökhan Vural M, et al. Loeffler endocarditis: silent right ventricular myocardium! Wien Klin Wochenschr. 2016;128(13–14):513–515. doi:10.1007/s00508-016-0981-1
50. Ogbogu PU, Bochner BS, Butterfield JH, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009;124(6):1319–1325. doi:10.1016/j.jaci.2009.09.022
51. Alam A, Thampi S, Saba SG, et al. Loeffler endocarditis: a unique presentation of right-sided heart failure due to eosinophil-induced endomyocardial fibrosis. Clin Med Insights Case Rep. 2017;10:1179547617723643. doi:10.1177/1179547617723643
52. Van Kessel DJ, Jerzewski A, Kardux JJ, et al. Loeffler’s endocarditis. Eur Heart J Cardiovasc Imaging. 2015;16:343. doi:10.1093/ehjci/jeu208
53. Subhash HS, Asishkumar M, Jonathan M, et al. Unusual cardiac manifestation of hypereosinophilic syndrome. Postgrad Med J. 2002;78(922):490–491. doi:10.1136/pmj.78.922.490
54. Klion AD, Noel P, Akin C, et al. Elevated serum tryptase levels identify a subset of patients with a myeloproliferative variant of idiopathic hypereosinophilic syndrome associated with tissue fibrosis, poor prognosis, and imatinib responsiveness. Blood. 2003;101(12):4660–4666. doi:10.1182/blood-2003-01-0006
55. Bomken S, Haigh S, Bown N, et al. Cutaneous B-lymphoblastic lymphoma with IL3/IgH translocation presenting with hypereosinophilia and acute endocarditis. Pediatr Blood Cancer. 2015;62(6):1055–1057. doi:10.1002/pbc.25318
56. Park J, Hemu M, Kalra D, et al. Loeffler’s endocarditis: a diagnosis made with cardiac magnetic resonance (CMR) imaging. J Am Coll Cardiol. 2019;73(9):2267. doi:10.1016/S0735-1097(19)32873-6
57. Gastl M, Behm P, Jacoby C, et al. Multiparametric Cardiac Magnetic Resonance Imaging (CMR) for the diagnosis of Loeffler’s endocarditis: a case report. BMC Cardiovasc Disord. 2017;17(1):74. doi:10.1186/s12872-017-0492-7
58. Ommen SR, Seward JB, Tajik AJ, et al. Clinical and echocardiographic features of hypereosinophilic syndromes. Am J Cardiol. 2000;86(1):110–113. doi:10.1016/S0002-9149(00)00841-9
59. Shah R, Ananthasubramaniam K. Evaluation of cardiac involvement in hypereosinophilic syndrome: complementary roles of transthoracic, transesophageal, and contrast echocardiography. Echocardiography. 2006;23(8):689–691. doi:10.1111/j.1540-8175.2006.00288.x
60. Kalra DK, Park J, Hemu M, et al. Loeffler endocarditis: a diagnosis made with cardiovascular magnetic resonance. J Cardiovasc Imaging. 2019;27(1):70–72. doi:10.4250/jcvi.2019.27.e5
61. Raza S. A curious case of Loeffler endocarditis. JACC. 2018;71(11):11. doi:10.1016/S0735-1097(18)32803-1
62. Radovanovic M, Jevtic D, Calvin AD, et al. ”Heart in DRESS”: cardiac manifestations, treatment and outcome of patients with drug reaction with eosinophilia and systemic symptoms syndrome: a systematic review. J Clin Med. 2022;11(3):704. doi:10.3390/jcm11030704
63. Kleinfeldt T, Ince H, Nienaber CA, et al. Hypereosinophilic syndrome: a rare case of Loeffler’s endocarditis documented in cardiac MRI. Int J Cardiol. 2011;149(1):e30–32. doi:10.1016/j.ijcard.2009.03.059
64. Bonanad C, Monmeneu JV, López-Lereu MP, et al. Loeffler endocarditis of the left ventricle: cardiac magnetic resonance findings. Heart Lung Circ. 2013;22(12):1056–1057. doi:10.1016/j.hlc.2013.03.087
65. Polito MV, Hagendorff A, Citro R, et al. Loeffler’s endocarditis: an integrated multimodality approach. J Am Soc Echocardiog. 2020;33(12):1427–1441. doi:10.1016/j.echo.2020.09.002
66. Scully PR, Bastarrika G, Moon JC, et al. Myocardial extracellular volume quantification by cardiovascular magnetic resonance and computed tomography. Curr Cardiol Rep. 2018;20(3):15. doi:10.1007/s11886-018-0961-3
67. Katre RS, Sunnapwar A, Restrepo CS, et al. Cardiopulmonary and gastrointestinal manifestations of eosinophil-associated diseases and idiopathic hypereosinophilic syndromes: multimodality imaging approach. Radiographics. 2016;36(2):433–451. doi:10.1148/rg.2016150145
68. Metze M, Davierwala PM, Andreas H, et al. Progression of left ventricular thrombus in Loeffler’s endocarditis without eosinophilia-case report and review of the literature. Clin Res Cardiol. 2019;108(10):1163–1170. doi:10.1007/s00392-019-01483-1
69. Klion AD, Bochner B, Gleich G, et al. The hypereosinophilic syndromes working, g. approaches to the treatment of hypereosinophilic syndromes: a workshop summary report. J Allergy Clin Immunol. 2006;117(6):1292–1302. doi:10.1016/j.jaci.2006.02.042
70. Simon HU, Cools J. Novel approaches to therapy of hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2007;27(3):519–527. doi:10.1016/j.iac.2007.07.003
71. Zhuang Q, Zheng Z-Y, Mao W, et al. Right ventricular apical obstruction in a patient with hypereosinophilia: Loeffler endocarditis. Heart Lung. 2015;44(2):165–169. doi:10.1016/j.hrtlng.2014.11.003
72. Gotlib J. World Health Organization-defined eosinophilic disorders: 2014 update on diagnosis, risk stratification, and management. Am J Hematol. 2011;89(3):325–337. doi:10.1002/ajh.23664
73. Tefferi A, Patnaik MM, Pardanani A, et al. Eosinophilia: secondary, clonal and idiopathic. Br J Haematol. 2006;133(5):468–492. doi:10.1111/j.1365-2141.2006.06038.x
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.