Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 16
Implication of KDR Polymorphism rs2071559 on Therapeutic Outcomes and Safety of Postoperative Patients with Gastric Cancer Who Received S-1-Based Adjuvant Chemotherapy: A Real-World Exploratory Study
Authors Meng L, Cao J, Kang L, Xu G, Yuan DW, Li K, Zhu K
Received 26 July 2023
Accepted for publication 6 November 2023
Published 28 November 2023 Volume 2023:16 Pages 1027—1039
DOI https://doi.org/10.2147/PGPM.S432528
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Lei Meng,1,* Jun Cao,1,2,* Li Kang,3,* Gang Xu,1 Da-Wei Yuan,1 Kang Li,1 Kun Zhu1
1Department of Surgical Oncology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, 710061, People’s Republic of China; 2The Third Affiliated Hospital of Xi’an Medical University, Xi’an, 710068, People’s Republic of China; 3Department of Thoracic Surgery, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, 710061, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Kang Li; Kun Zhu, Department of Surgical Oncology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, 710061, People’s Republic of China, Tel +86 13087566643, Email [email protected]; [email protected]
Objective: Regimens of S-1-based adjuvant chemotherapy are of great significance in attenuating recurrence risk in postoperative patients with gastric cancer (GC). Kinase insert-domain receptor (KDR) gene plays an essential role in tumor growth and metastasis. This study aimed to investigate the implication of KDR genotyping on the therapeutic outcomes of patients with gastric cancer (GC) who received S-1-based adjuvant chemotherapy.
Methods: A total of 169 postoperative GC with pathological staging of II and III and no metastasis who received S-1-based adjuvant chemotherapy were included retrospectively. Peripheral blood specimens were collected and prepared for KDR genotyping and KDR mRNA expression. Correlation between KDR genotype status and prognosis was performed using Kaplan–Meier survival analysis, and multivariate analysis was ultimately adopted using Cox regression analysis.
Results: Median disease-free survival (DFS) of the 169 patients with GC was 5.1 years [95% confidence interval (CI): 4.25– 5.95] and median overall survival (OS) was 6.7 years (95% CI: 5.44– 7.96). Rs2071559 was located at the upstream region, and the prevalence among 169 patients with GC was as follows: AA genotype in 104 cases (61.5%), AG genotype in 57 cases (33.7%), and GG genotype in 8 cases (4.7%), yielding a minor allele frequency of 0.22, which was consistent with Hardy-Weinberg equilibrium (P=0.958). Median DFS of patients with AA and AG/GG genotypes was 6.0 years and 4.0 years, respectively (P=0.002). Additionally, patients with the AA genotype had longer OS than those with the AG/GG genotype [median OS: not reached (NR) vs 5.5 years, P=0.011]. Additionally, KDR mRNA expression was significantly higher in patients with the AG/GG genotype than that in those with the AA genotype (P< 0.001).
Conclusion: Rs2071559 in KDR gene might be a promising biomarker for evaluating the recurrence risk and OS of patients with GC who received S-1-based adjuvant chemotherapy. This conclusion should be confirmed in randomized clinical trials.
Keywords: gastric cancer, S-1, KDR, polymorphism, prognosis, biomarker
Introduction
Gastric cancer (GC) is one of the most common gastrointestinal tumors and the fifth most common type of tumor worldwide.1 It is reported that there are approximately 1,034,000 new cases and 783,000 new deaths of GC each year globally.2 Furthermore, there are approximately 479,000 new cases and 374,000 new deaths of GC in China currently.3 Given that early diagnosis in China is not prevalent, a considerable number of patients with GC are diagnosed with locally advanced or metastatic stage disease.4 Therapeutic outcomes of metastatic GC are still dismal with a 5-year overall survival (OS) rate of <10%.5 However, for patients with early stage GC, surgical resection is the most efficacious therapeutic option, and radical D2 surgery treatment reduced the risk of recurrence for Asian patients with GC significantly.6 Furthermore, fluoropyrimidine-based chemotherapy was the backbone of treatment for GC, including two oral derivatives: S-1 and capecitabine.7 Consequently, previous phase III clinical trial indicated that adjuvant chemotherapy of S-1 monotherapy significantly attenuated the risk of recurrence and elevated the overall survival (OS) of patients with stage II or III GC after radical D2 surgery treatment.8 Besides, different perioperative regimens were investigated in many clinical trials, including adjuvant treatment of S-1 with oxaliplatin (SOX) and S-1 with cisplatin.9 As a result, S-1-based adjuvant chemotherapy was widely used in patients with GC.
S-1 is made up of tegafur, gimeracil and oteracil, tegafur is a prodrug that must be metabolized to 5-FU by CYP2A6 in the liver to activate the antitumor activity.10 5-FU prevents DNA synthesis by inhibiting thymine nucleotide synthetase, playing the cytotoxic effect.11 Gimeracil is an inhibitor of dihydropyrimidine dehydrogenase, maintaining a high concentration of 5-FU. Oteracil inhibits fluorouracil phosphorylation in the gastrointestinal tract and attenuates gastrointestinal toxicity.8 As a compound preparation, S-1 demonstrates promising efficacy and controllable safety profile for GC.
Angiogenesis is an important theory in the development of numerous malignancies and has been confirmed as an important therapeutic target for solid tumors.12 Kinase insert-domain receptor (KDR) pathway is one of the most important pathways in tumor angiogenesis, tumor recurrence and metastasis.13 Located on chromosome 4q12, KDR consists of 30 exons, which may serve as one of the most important therapeutic targets of antiangiogenic drugs.14 Additionally, it should be noted that the function of KDR gene varied significantly among different populations and individual difference of KDR gene expression was noticed among different populations clinically.15 At present, rare studies investigated the clinical significance of the polymorphism in KDR among Chinese patients with GC who received surgical resection and adjuvant chemotherapy. Noteworthily, a recent study demonstrated that the 889C>T polymorphism in KDR gene was clinically significant among patients with extensive-stage small cell lung cancer who received apatinib monotherapy, and patients with CT/TT genotype of the polymorphism conferred a worse prognosis through the mediation of KDR gene expression.16 Furthermore, similar results were also reported among patients with advanced epithelial ovarian cancer who were treated with apatinib monotherapy, suggesting that patients with the TC/CC genotype of rs2071559 in KDR gene were associated with relatively worse progression-free survival (PFS) and OS.17 Interestingly, a previous study initiated by Zhu et al included 256 patients with GC and investigated the associations of VEGF/KDR proteins and KDR genetic variations with the prognosis of GC,18 which found that the KDR protein and rs1870377 polymorphism in KDR were prognostic factors for patients with GC regardless of the treatment regimens, highlighting the KDR polymorphism might be a potential biomarker to predict the prognosis of patients with GC clinically. Collectively, these findings indicated that KDR polymorphisms might be of encouraging implication in the development of GC. However, the clinical significance of KDR polymorphisms in GC patients who received surgical resection and S-1-based adjuvant chemotherapy remained unknown.
Consequently, this study aimed to identify the implication of KDR polymorphisms on the prognosis of postoperative GC who received S-1-based adjuvant chemotherapy in clinical practice.
Patients and Methods
Study Design
S-1-based adjuvant chemotherapy has been used in patients with GC for over ten years and numerous patients with GC are treated with S-1-based adjuvant chemotherapy in clinical practice. Therefore, patients with GC who received operative treatment at the Department of Surgical Oncology of the First Affiliated Hospital of Xi’an Jiaotong University between January 2012 and June 2021 were included in this study retrospectively. The main inclusion criteria were as follows: (1) histological diagnosis of gastric adenocarcinoma with pathological stage of II or III; (2) age ≥18 years; (3) Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2 score; (4) surgical resection and treatment with S-1-based adjuvant chemotherapy. Exclusion criteria were as follows: (1) previous administration of S-1-based systemic treatment in the neoadjuvant treatment; (2) concomitant cancer in the last three years or presence of serious disease that might compromise the living status; (3) patients had distant metastasis or tumor cells observed in patients’ ascites. The primary analysis in this study was the correlation analysis between KDR gene polymorphisms and prognosis. The research profile was shown in Figure 1. In total, 169 patients with GC were recruited. All patients were from China and their ethnicity was Han Chinese. This study was approved by the ethics committee of the First Affiliated Hospital of Xi’an Jiaotong University (approved number: XJTU1AF2021LSK-185) in 2021. Written informed consent was obtained from each subject according to the recommendations of the Declaration of Helsinki.
 |
Figure 1 Flow chart of this retrospective study regarding the implication of KDR polymorphism on the prognosis of patients with gastric cancer who received S-1-based adjuvant chemotherapy. |
Adjuvant Chemotherapy Regimens
All the patients enrolled in this study were treated with S-1-based adjuvant chemotherapy regimens within 3–6 weeks after surgery, which was consisted of S-1 monotherapy and S-1 plus oxaliplatin (SOX) regimen. Usage and dosage of S-1 monotherapy were as follows: S-1 capsule, 40 mg/m2, orally twice daily for day 1–28 and discontinued for 14 days every 42 days was deemed as one cycle.19 Additionally, usage and dosage of S-1 plus oxaliplatin (SOX) regimen were S-1 capsule, 80 mg/m2 orally twice daily for day 1–14 and discontinued for 7 days and oxaliplatin, 100–130 mg/m2, iv. infusion, day 1. Every 21 days was considered as one cycle. Adjuvant chemotherapy with S-1 monotherapy and the SOX regimen was planned to provide eight cycles to the patients. Patients were followed-up regularly to obtain the prognostic outcomes.
Blood Specimen Collection and KDR Polymorphism Genotyping
Peripheral blood samples were collected during the study. Genomic DNA was extracted from the cells. However, 21 patients’ biological peripheral blood specimens were not available, and 5 patients’ genomic DNA extraction was unsuccessful. As a result, 169 patients had available DNA specimens and were ultimately included in polymorphism analysis. KDR polymorphism analysis included the following three polymorphisms: rs2071559, rs2305948, and rs11941492.
Genotype status of rs2071559 polymorphism was determined by PCR-RFLP. Forward primer: 5’- TCACTAGGGCTCTTCGTTGG-3’and the reverse primer: 5’- GAAGCGGATACTCAGCCAAG-3’. The PCR product size was 271bp, which was digested using the restriction enzyme BsmI. Genotyping of the polymorphism was distinguished by the size of the PCR bands as follows: AA genotype (one 271 bp stripe); GG genotype (one 108 bp stripe and one 163 bp stripe); AG genotype (one 271 bp stripe, one 108 bp stripe, and one 163 bp stripe).17 The representative images of the bands produced by PCR-RFLP regarding the genotype status of rs2071559 in the KDR gene were illustrated in Figure S1.
Collection of Peripheral Blood Mononuclear Cell (PBMC) Specimens and Analysis of KDR Gene mRNA Expression
PBMC specimens were initially collected from 91 randomly selected patients among 169 patients with GC. Finally, only 76 mRNA samples were available for the subsequent analysis. The methods for KDR mRNA expression were adopted from a previous study.20 The forward primer sequence used for KDR mRNA expression was 5’-ATGCAGAGCAAGGTGCTGC-3,’ and the reverse primer was 5’- TTAAACAGGAGGAGAGCTCAGTG-3’. Additionally, the forward primer sequence used for GAPDH (reference gene) mRNA expression was 5’-GAAGGTGAAGGTCGGAGTCAAC-3’ and the reverse primer was 5’-CAGAGTTAAAAGCAGCCCTGGT −3’.21
Statistical Analysis
All data presented in this study were collected and analyzed using SPSS version 25.0 (IBM, USA). We followed the methods of a previous study to analyze the statistical data.22 Disease-free survival (DFS) and OS were defined according to a previous study.23 The independent sample t-test method was used to analyze KDR mRNA expression according to the genotype status of rs2071559. Additionally, Cox analysis was adopted for DFS in the multivariate analysis. P<0.05 was statistically significant.
Results
Baseline and Demographic Characteristics of the 169 Patients with GC
Baseline characteristics of 169 patients with GC are presented in Table 1. The median age of 169 patients was 56 years. Male and female patients were observed in 120 and 49 cases, respectively. All patients had common early-stage GC and underwent surgical resection in clinical practice. Representative pathological information (HE staining and immunochemistry) of the two patients with GC were illustrated in Figures S2 and S3. S-1 monotherapy was administered to 57 patients, and the SOX regimen (S-1 plus oxaliplatin) was administered to 112 patients.
 |
Table 1 Baseline Characteristics of the 169 Patients with Gastric Cancer According to Genotype Status of KDR rs2071559 |
Regarding the KDR gene polymorphism analysis, as illustrated in Table 2, three polymorphisms were included in our study (rs2071559, rs2305948, and rs11941492), only rs2071559 was of clinical significance in preliminary analysis. The prevalence of the rs2071559 polymorphism among the 169 patients with GC was as follows: AA genotype, 104 cases (61.5%); AG genotype, 57 cases (33.7%); GG genotype, 8 cases (4.7%), resulted in a minor allele frequency of 0.22, which was consistent with the Hardy-Weinberg equilibrium (P=0.958).
 |
Table 2 The Preliminary Analysis Between Genotype Status of the Three Polymorphisms and Disease-Free Survival |
Prognostic Data of the 169 Patients with GC Who Received S-1 Based Adjuvant Chemotherapy
Patients included in this study were recruited between January 2012 and June 2021. The data cutoff date for this study was October 31, 2021, and the duration of this study was close to 10 years. The median follow-up duration from the patients included in this study to the last follow-up date was 6.3 years (follow-up range: 0.15–9.6 years). A total of 97 recurrence or death events were observed when the data were cut-off, which produced a DFS maturity of 57.4%. As illustrated in Figure 2, the median DFS of 169 patients with GC who received S-1-based adjuvant chemotherapy was 5.1 years [95% confidence interval (CI): 4.25–5.95]. Furthermore, the 3-year DFS rate was 74.14% (95% CI: 66.75–80.12%) and 5-year DFS rate was 50.16% (95% CI: 42.04–57.74%), respectively. As shown in Table S1, a total of 79 the 169 patients underwent subsequent treatment after relapsed. Of the 79 patients who received subsequent treatment, 31 patients were treated with chemotherapy (39.2%), 23 patients received PD-1/PD-L1-related regimens (29.1%), 12 patients received traditional Chinese medicine (15.2%), 8 patients were treated with anti-angiogenesis-related regimens (10.1%), and 5 patients were not available for subsequent treatments (6.3%). Additionally, the correlation between subsequent treatment and the rs2071559 genotype status in KDR suggested that patients with AA and AG/GG genotypes conferred similar and balanced subsequent regimens, and no statistically significant difference was observed (P>0.05). Ultimately, 79 deaths were observed when the data were cut-off, which yielded an OS maturity of 46.7%. As shown in Figure 2, the median OS of 169 GC who received S-1-based adjuvant chemotherapy was 6.7 years (95% CI: 5.44–7.96). Additionally, the 3-year OS rate was 87.39% (95% CI: 81.31–91.59%) and 5-year OS rate was 66.97% (95% CI: 59.07–73.69%).
 |
Figure 2 Disease free survival and overall survival of the 169 patients with gastric cancer who received S-1-based adjuvant chemotherapy. |
Clinical Significance of rs2071559 Polymorphism
The GG and AG genotypes were combined as one group in a dominant manner, and 65 patients had the AG/GG genotypes. Association analysis between genotype status of rs2071559 and DFS was performed firstly. As illustrated in Figure 3, the median DFS of patients with the AA and AG/GG genotypes of the rs2071559 polymorphism was 6.0 years (95% CI: 4.61–7.39) and 4.0 (95% CI: 3.31–4.69) years, respectively. This difference was statistically significant (χ2=9.14, P=0.002). Furthermore, as shown in Figure 4, the median OS of patients with the AA and AG/GG genotypes of the rs2071559 polymorphism was not reached (NR) (95% CI: NR-NR) and 5.5 (95% CI: 4.89–6.11) years, respectively. The 5-year OS rate of patients with the AA and AG/GG genotypes of the rs2071559 polymorphism was 76.00% (95% CI: 66.33–83.24%) and 52.22% (95% CI: 38.92–63.94%), respectively. This difference was also statistically significant (χ2=6.48, P=0.011).
 |
Figure 3 Disease free survival of the 169 patients with gastric cancer who received S-1-based adjuvant chemotherapy according to KDR rs2071559 genotype status. |
 |
Figure 4 Overall survival of the 169 patients with gastric cancer who received S-1-based adjuvant chemotherapy according to KDR rs2071559 genotype status. |
The median DFS and 95% CI were calculated according to the different baseline characteristic subgroups in the univariate analysis. Interestingly, as shown in Table 3, ECOG performance status score, pathological staging, and resection type were significantly associated with DFS in univariate analysis, which suggested that the median DFS of patients with ECOG 0 score was dramatically longer than that of patients with the 1–2 score (median DFS: 6.1 vs 4.4 years, P=0.008), the median DFS of patients with pathological staging of IIA-IIB was significant better than that of patients with IIIA-IIIC (median DFS: 6.3 vs 4.2 years, P=0.001). Additionally, patients who underwent total resection had worse DFS than those who underwent distal resection (median DFS: 4.4 vs 5.5 years, P=0.019). Furthermore, multivariate Cox regression analysis for DFS was performed, including baseline characteristics that were significant in the univariate analysis (P<0.05). Multivariate analysis results are shown in Table 3, and a statistically significant difference was still found between the KDR rs2071559 polymorphism and DFS, which demonstrated that the rs2071559 polymorphism was an independent factor for DFS (hazard ratio (HR) = 0.67, P = 0.009). Furthermore, as shown in Table 3, after adjusting for Cox regression analysis, the ECOG performance status score (HR = 0.69, P = 0.011), pathological staging (HR = 0.59, P = 0.007) and resection type (HR = 1.22, P = 0.023) were independent factors for DFS.
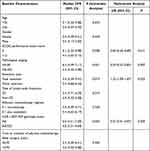 |
Table 3 Univariate and Multivariate Analyses of DFS Among the 169 Patients with Gastric Cancer |
Additionally, the safety profile of S-1-based adjuvant chemotherapy regimens and correlation analysis were shown in Table S2. No statistically significant difference was found between the safety profile and genotype status of rs2071559 (P>0.05), indicating that the rs2071559 genotype status in the KDR gene failed to influence the safety profile of S-1-based adjuvant chemotherapy.
Correlation Between rs2071559 Polymorphism and KDR mRNA Expression
Expression of KDR mRNA was implemented by extracting RNA from 76 PBMC specimens, and the relevance analysis between the genotype status of rs2071559 polymorphism and KDR mRNA expression was subsequently analyzed. The prevalence of the rs2071559 polymorphism in the 76 PBMC specimens was as follows: AA genotype, 47 cases (61.8%); AG genotype, 26 cases (34.2%); and GG genotype, three cases (3.9%), yielding an MAF of 0.21. Similarly, the AG and GG genotypes were combined for subsequent analysis. As illustrated in Figure 5, patients with the AG/GG genotype conferred a relatively higher KDR mRNA expression than patients with the AA genotype (AG/GG vs AA: 3.96 ± 0.675 vs 3.08 ± 0.755, t = 5.166, P < 0.001).
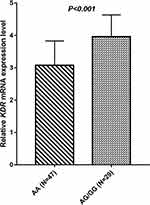 |
Figure 5 The relative expression level of KDR mRNA according to KDR rs2071559 genotype status. |
The KDR mRNA expression status was divided into KDR high expression (KDR-H) and KDR low expression (KDR-L) according to the median KDR mRNA relative expression threshold value to explore its association with DFS. Noteworthily, 39 and 37 patients had KDR-H and KDR-L, respectively. As shown in Figure 6, patients with KDR-H showed a trend of worse DFS than those with KDR-L (median DFS: 5.3 vs 6.8 years), although the difference was not statistically significant (χ2=3.023, P=0.082).
 |
Figure 6 Disease free survival of the 76 patients with gastric cancer who received S-1-based adjuvant chemotherapy according to KDR mRNA expression status. |
Discussion
Our study highlighted and provided real-world evidence regarding the prognostic outcomes of patients with postsurgical GC who received S-1-based adjuvant chemotherapy. Meanwhile, the prognostic correlation analysis identified that polymorphism rs2071559 in the KDR gene might be a promising biomarker for predicting the recurrence risk and prognosis of patients with GC who were administered S-1-based adjuvant chemotherapy.
GC is a highly heterogeneous digestive system malignancy and one of the most common malignant types worldwide with a poor prognosis.24 S-1-based or capecitabine-based treatments are the standard postoperative adjuvant chemotherapy regimens for patients with GC according to two Phase III clinical trials of ACTS-GC and CLASSIC, respectively.25 However, it was still necessary to investigate the potential biomarkers based on the individual genome that might predict the prognosis of adjuvant chemotherapy, thus providing the maximum survival benefit for GC patients who received adjuvant chemotherapy clinically.26 Interestingly, considerable pharmacogenomic research results that might predict the therapeutic outcomes of patients with GC have been reported over the past few years,27–29 suggesting that genomic polymorphisms in patients with GC might be involved in the therapeutic outcomes of conventional chemotherapy to some extent.30
The median DFS and OS of 169 patients enrolled in this study who received S-1-based adjuvant chemotherapy were 5.1 years and 6.7 years, respectively. It seemed that the prognosis was inferior to DFS and OS data in the ACTS-GC clinical trial (median DFS and OS in the study were both greater than 5.5 years).8 We speculated that the reasons might be as follows: First, this study was conducted retrospectively, which suggested that the management of the patients was not sufficient and normative compared with a well-designed phase III clinical trial, the similar findings were still observed in a previous retrospective study.31 Secondly, our study included more patients with an ECOG performance status of 2 than that in the ACTS-GC trial, and the results of the Cox analysis in our study demonstrated that patients with an ECOG of 1–2 score conferred a worse prognosis, which was consistent with the previous study as well.32 Thirdly, it should be noted that the proportion of stage II patients in this study was lower than the proportion of stage II patients in the ACTS-GC trial (37.9% vs 49.9%). Furthermore, a relatively superior prognosis of stage II patients was observed in the Cox multivariate analysis in our study. Collectively, all the above factors might contribute to the tendency that clinical outcomes in real-world retrospective studies might be relatively worse than those in phase III clinical trials.
Interestingly, many previous studies demonstrated that genetic variation in the drug metabolism gene might contribute to the therapeutic outcomes of chemotherapy to some extent.33 Polymorphism analysis in our study suggested that the AG/GG genotype of rs2071559 in KDR gene conferred a relatively worse prognosis among patients with GC who received postoperative adjuvant chemotherapy, which was in agreement with a previous study initiated by XR et al18 A total of 256 patients with GC who underwent gastrectomy were included in their study, and KDR genetic variation and protein expression were analyzed. The results indicated that KDR expression was associated with worse prognosis, and KDR rs1870377 was an independent prognostic marker for GC. Another previous study initiated by YJ et al explored the association between polymorphisms in the anti-angiogenesis signaling pathway and prognosis of patients.34 A total of 81 patients with advanced GC who were treated with systemic chemotherapy, and polymorphisms in the NRP-1 and KDR genes were genotyped. Correlation analysis highlighted that the TT genotype of rs1870377 in KDR is associated with superior OS and PFS. The design of this study was consistent with that of our study. Interestingly, another exploratory study initiated by ZY et al explored the implication of KDR polymorphism on the clinical outcomes of patients with advanced epithelial ovarian cancer (EOC) who received apatinib monotherapy.17 A total of 118 patients with advanced EOC who received apatinib monotherapy were recruited, and KDR gene polymorphism was genotyped, which indicated that TC/CC genotype of rs2071559 in KDR gene was associated with worse PFS and OS. The findings of this study were consistent with those of the present study. Collectively, these studies suggested that the AG/GG genotype of rs2071559 might contribute to KDR gene expression, resulting in the potentiation of angiogenesis among patients with GC.
Additionally, potential mechanisms regarding the clinical significance of rs2071559 among 169 GC patients needed to be elucidated, and 76 randomly selected PBMC specimens were collected for mRNA expression analysis. We found that KDR mRNA expression was significantly higher in patients with the AG/GG genotype. Interestingly, two previous studies also found that rs2071559 also changed KDR mRNA expression, and AG/GG genotypes were associated with higher KDR mRNA expression.17,20 We speculated that the explanation regarding how this polymorphism changed gene expression might be as follows. First, located at upstream of the KDR gene, rs2071559, might be highly in linkage disequilibrium with another polymorphism located at the 3’UTR of the KDR gene. In this context, the different genotypes of the polymorphism could influence the binding site of the promoter, thus changing the gene expression of KDR to some extent, which was consistent with a previous study showing that 889C>T was highly in linkage disequilibrium with another polymorphism, rs7667298, which was located at the 5’UTR of the KDR gene.35 However, this hypothesis should be confirmed in a mechanistic study. This might explain how this polymorphism changed gene expression. Additionally, prognostic analysis between KDR mRNA expression status and DFS suggested that patients with high KDR expression tended to have worse DFS than those with low KDR expression, although the difference was not statistically significant (P=0.082) owing to the limited sample size (N=76). This result was consistent with that of a previous study initiated by Geng et al who investigated the implication of KDR genetic variation on the efficacy and safety of patients with advanced NSCLC who received first-line bevacizumab plus chemotherapy regimens.36 To the best of our knowledge, KDR was the most important receptor with the strongest binding ability to VEGF and played an essential role in angiogenesis.37 Previous study highlighted that KDR protein expression conferred a vital role in the process of tumor proliferation and metastasis, demonstrating that higher levels of KDR expression in tumor cells were associated with a greater chance for tumor cells to regenerate blood vessels and relapse or metastasize.38 Furthermore, previous study found that patients with higher expression of KDR conferred worse PFS and OS in patients with GC.18 As a result, these results were consistent with our study to some extent. In addition, Schacher et al investigated the changes in KDR expression due to shear stress for each gene polymorphism in HUVEC. Unfortunately, no significant association was observed between polymorphism genotype status and basal expression of KDR in HUVECs,39 which contradicted the findings of our study. We speculated that this might be due to discrepancies in the included specimens. KDR played an important role in the process of epithelial-mesenchymal transition.40 Previous study highlighted that its mRNA expression in cancer tissue was significantly higher than that in normal tissue of endometrial carcinoma,41 suggesting that the mRNA expression of KDR in different tissues in vivo showed great differences. The specimen used in our study for KDR mRNA expression analysis was a PBMC specimen, different from that used in the study by Schacher et al, which might have resulted in the difference in KDR mRNA expression. Additionally, another clinical study initiated by Yoshinori Hirashima et al investigated the impact of VEGFR 1, 2 and 3 expression on the outcome of patients with gastric cancer included a total of 86 patients who underwent gastrectomy and received chemotherapy for recurrent or residual tumor.42 And they found that KDR (VEGFR2) expression in stromal vessels was correlated with shorter survival and lower response to S-1 based regimens. This study suggested that KDR might be related to response of S-1, which was in line with the result in our study to some extent. Therefore, it was clear currently that KDR was associated with the prognosis of patients with GC, but whether it was related to response of S-1 still needed to be further confirmed by larger sample studies, which was part of the reason why we conducted this study and explored the association between KDR and S-1. Collectively, the prognosis of GC who received S-1-based adjuvant chemotherapy might be affected by the KDR rs2071559 polymorphism by mediating the mRNA expression of KDR. This conclusion should be confirmed in prospective clinical trials.
Our study existed some limitations. Firstly, as a real-world study, the sample size was relatively small, and we failed to perform KDR protein expression analysis to identify the underlying mechanisms. Secondly, our study was designed as a retrospective analysis, and bias might not be avoided. We believed that an inherent bias in the retrospective study might have existed in our study: 1. Selection bias: This bias occurred when the selection of participants was not random or representative of the target population. This might have led to over- or under-representation of certain groups, potentially compromising the study results; 2. Information bias: Retrospective studies relied on existing data and medical records, which might have contained errors or incomplete information. This bias might have deteriorated the quality and reliability of the data analyzed; 3. Publication bias: Retrospective studies with positive or significant findings were more likely to be published than those with negative or nonsignificant results. This bias might have led to an overestimation of the treatment effects or associations. Thirdly, given that the number of patients in our study was limited, we failed to perform propensity score-matching analysis. Fourthly, for patients with GC who underwent surgical resection, only DFS and OS were available for clinical outcomes, which was different from patients with metastatic GC, who might also use objective response rate (ORR) and disease control rate (DCR) to describe the clinical outcomes.
In a word, clinical significance of rs2071559 in KDR was identified in univariate and multivariate analysis, which demonstrated that rs2071559 in KDR gene might be a promising biomarker for evaluating the recurrence risk and prognosis of patients with GC who received S-1-based adjuvant chemotherapy. In our opinion, patients with AG/GG genotypes in KDR gene might not benefit from S-1-based adjuvant chemotherapy, and more therapeutic regimens should be adopted if the AG/GG genotype in rs2071559 was detected clinically.
Acknowledgments
This study was funded by the Natural Science Basic Research Program of the Shanxi Province (Program No.2021JM-263).
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
1. Li Y, Zhou D, Liu Q, et al. Gene polymorphisms of m6A erasers FTO and ALKBH1 associated with susceptibility to gastric cancer. Pharmgenomics Pers Med. 2022;15:547–559. doi:10.2147/pgpm.s360912
2. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
3. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
4. Chen J, Chen J, Lv X, Yang Q, Yao S. Epidermal growth factor in exhaled breath condensate as diagnostic method for non-small cell lung cancer. Technol Cancer Res Treat. 2019;18:153303381987227. doi:10.1177/1533033819872271
5. Wang H, Guo W, Hu Y, et al. Superiority of the 8th edition of the TNM staging system for predicting overall survival in gastric cancer: comparative analysis of the 7th and 8th editions in a monoinstitutional cohort. Mol Clin Oncol. 2018;9(4):423–431. doi:10.3892/mco.2018.1683
6. Han SU, Hur H, Lee HJ, et al. Surgeon quality control and standardization of D2 lymphadenectomy for gastric cancer: a prospective multicenter observational study (KLASS-02-QC). Ann Surg. 2021;273(2):315–324. doi:10.1097/sla.0000000000003883
7. Park SH, Lim DH, Sohn TS, et al. A randomized phase III trial comparing adjuvant single-agent S1, S-1 with oxaliplatin, and postoperative chemoradiation with S-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: the ARTIST 2 trial(☆). Ann Oncol. 2021;32(3):368–374. doi:10.1016/j.annonc.2020.11.017
8. Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810–1820. doi:10.1056/NEJMoa072252
9. Terashima M, Iwasaki Y, Mizusawa J, et al. Randomized phase III trial of gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer, the short-term safety and surgical results: Japan clinical oncology group study (JCOG0501). Gastric Cancer. 2019;22(5):1044–1052. doi:10.1007/s10120-019-00941-z
10. Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9(3):215–221. doi:10.1016/s1470-2045(08)70035-4
11. Yang L, Zou S, Shu C, et al. CYP2A6 polymorphisms associate with outcomes of S-1 plus oxaliplatin chemotherapy in Chinese gastric cancer patients. Geno Prot Bioinfor. 2017;15(4):255–262. doi:10.1016/j.gpb.2016.11.004
12. Folkman J, Parris EE, Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. doi:10.1056/nejm197111182852108
13. Zhao L, Chen H, Lu L, et al. New insights into the role of co-receptor neuropilins in tumour angiogenesis and lymphangiogenesis and targeted therapy strategies. J Drug Target. 2021;29(2):155–167. doi:10.1080/1061186x.2020.1815210
14. Liu K, Ren T, Huang Y, et al. Apatinib promotes autophagy and apoptosis through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death Dis. 2017;8(8):e3015. doi:10.1038/cddis.2017.422
15. Li Z, Wang M, Gu J, et al. Missense variants in hypoxia-induced VEGFA/VEGFR2 signaling predict the outcome of large artery atherosclerotic stroke. Cell Mol Neurobiol. 2021;41(6):1217–1225. doi:10.1007/s10571-020-00890-7
16. Geng N, Ding CM, Liu ZK, Song S, Hu WX. Influence of VEGFR2 gene polymorphism on the clinical outcomes of apatinib for patients with chemotherapy-refractory extensive-stage SCLC: a real-world retrospective study. Int J Clin Oncol. 2021;26(4):670–683. doi:10.1007/s10147-020-01849-w
17. Yan Z, Gu YY, Hu XD, et al. Clinical outcomes and safety of apatinib monotherapy in the treatment of patients with advanced epithelial ovarian carcinoma who progressed after standard regimens and the analysis of the VEGFR2 polymorphism. Oncol Lett. 2020;20(3):3035–3045. doi:10.3892/ol.2020.11857
18. Zhu X, Wang Y, Xue W, et al. The VEGFR-2 protein and the VEGFR-2 rs1870377 A>T genetic polymorphism are prognostic factors for gastric cancer. Cancer Biol Ther. 2019;20(4):497–504. doi:10.1080/15384047.2018.1537575
19. Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29(33):4387–4393. doi:10.1200/jco.2011.36.5908
20. Bai M, Li ZG, Ba Y. Influence of KDR genetic variation on the efficacy and safety of patients with chemotherapy refractory metastatic CRC who received apatinib treatment. Int J Gen Med. 2021;14:1041–1055. doi:10.2147/ijgm.s300968
21. Barber RD, Harmer DW, Coleman RA, Clark BJ. GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol Genomics. 2005;21(3):389–395. doi:10.1152/physiolgenomics.00025.2005
22. Zhi DB, Wang ZY, Xie T, Tu WW. Influence of GSTP-1 polymorphism on the prognosis of patients with high-grade glioma who received temozolomide plus radiotherapy adjuvant treatment. Int J Gen Med. 2021;14:10173–10183. doi:10.2147/ijgm.s328810
23. de Steur WO, van Amelsfoort RM, Hartgrink HH, et al. Adjuvant chemotherapy is superior to chemoradiation after D2 surgery for gastric cancer in the per-protocol analysis of the randomized CRITICS trial. Ann Oncol. 2021;32(3):360–367. doi:10.1016/j.annonc.2020.11.004
24. Matsuoka T, Yashiro M. Biomarkers of gastric cancer: current topics and future perspective. World J Gastroenterol. 2018;24(26):2818–2832. doi:10.3748/wjg.v24.i26.2818
25. Kanaji S, Suzuki S, Matsuda Y, et al. Recent updates in perioperative chemotherapy and recurrence pattern of gastric cancer. Ann Gastroenterol Surg. 2018;2(6):400–405. doi:10.1002/ags3.12199
26. Matsuzaki J, Tsugawa H, Suzuki H. Precision medicine approaches to prevent gastric cancer. Gut Liver. 2021;15(1):3–12. doi:10.5009/gnl19257
27. Juarez I, Gutierrez A, Vaquero-Yuste C, et al. TGFB1 polymorphisms and TGF-β1 plasma levels identify gastric adenocarcinoma patients with lower survival rate and disseminated disease. J Cell Mol Med. 2021;25(2):774–783. doi:10.1111/jcmm.16131
28. Kim BS, Lee I, Yook JH, Song K, Kim BS. Association between the MUC1 rs4072037 polymorphism and risk of gastric cancer and clinical outcomes. J Gastric Cancer. 2020;20(2):127–138. doi:10.5230/jgc.2020.20.e11
29. Zhao X, Dai D, Li X, et al. A polymorphism within the mismatch repair gene predicts prognosis and adjuvant chemotherapy benefit in gastric cancer. Gastric Cancer. 2019;22(6):1121–1129. doi:10.1007/s10120-019-00962-8
30. Joo Kang S, Shin CM, Sung J, Kim N. Association between ALDH2 polymorphism and gastric cancer risk in terms of alcohol consumption: a meta-analysis. Alcohol Clin Exp Res. 2021;45(1):6–14. doi:10.1111/acer.14508
31. Su J, Dai B, Yuan W, et al. The influence of PD-L1 genetic variation on the prognosis of R0 resection colorectal cancer patients received capecitabine-based adjuvant chemotherapy: a long-term follow-up, real-world retrospective study. Cancer Chemother Pharmacol. 2020;85(5):969–978. doi:10.1007/s00280-020-04069-1
32. Yang J, Xu H, Guo X, et al. Pretreatment inflammatory indexes as prognostic predictors for survival in colorectal cancer patients receiving neoadjuvant chemoradiotherapy. Sci Rep. 2018;8(1):3044. doi:10.1038/s41598-018-21093-7
33. de Man FM, Goey AKL, van Schaik RHN, Mathijssen RHJ, Bins S. Individualization of irinotecan treatment: a review of pharmacokinetics, pharmacodynamics, and pharmacogenetics. Clin Pharmacokinet. 2018;57(10):1229–1254. doi:10.1007/s40262-018-0644-7
34. Zhuo YJ, Shi Y, Wu T. NRP-1 and KDR polymorphisms are associated with survival time in patients with advanced gastric cancer. Oncol Lett. 2019;18(5):4629–4638. doi:10.3892/ol.2019.10842
35. Zhang J, Yang J, Chen Y, et al. Genetic variants of VEGF (rs201963 and rs3025039) and KDR (rs7667298, rs2305948, and rs1870377) are associated with glioma risk in a han Chinese population: a case-control study. Mol Neurobiol. 2016;53(4):2610–2618. doi:10.1007/s12035-015-9240-0
36. Geng N, Su J, Liu Z, et al. The influence of KDR genetic variation on the efficacy and safety of patients with advanced NSCLC receiving first-line bevacizumab plus chemotherapy regimen. Technol Cancer Res Treat. 2021;20:153303382110194. doi:10.1177/15330338211019433
37. Zhong M, Li N, Qiu X, et al. TIPE regulates VEGFR2 expression and promotes angiogenesis in colorectal cancer. Int J Biol Sci. 2020;16(2):272–283. doi:10.7150/ijbs.37906
38. Xie Y, Mansouri M, Rizk A, Berger P. Regulation of VEGFR2 trafficking and signaling by Rab GTPase-activating proteins. Sci Rep. 2019;9(1):13342. doi:10.1038/s41598-019-49646-4
39. Schacher NM, Raaz-Schrauder D, Pasutto F, et al. Impact of single nucleotide polymorphisms in the VEGFR2 gene on endothelial cell activation under non‑uniform shear stress. Int J Mol Med. 2019;44(4):1366–1376. doi:10.3892/ijmm.2019.4301
40. Du Y, Chen Q, Huang L, et al. VEGFR2 and VEGF-C suppresses the epithelial-mesenchymal transition via YAP in retinal pigment epithelial cells. Curr Mol Med. 2018;18(5):273–286. doi:10.2174/1566524018666181004115304
41. Wang J, Taylor A, Showeil R, et al. Expression profiling and significance of VEGF-A, VEGFR2, VEGFR3 and related proteins in endometrial carcinoma. Cytokine. 2014;68(2):94–100. doi:10.1016/j.cyto.2014.04.005
42. Hirashima Y, Yamada Y, Matsubara J, et al. Impact of vascular endothelial growth factor receptor 1, 2, and 3 expression on the outcome of patients with gastric cancer. Cancer Sci. 2009;100(2):310–315. doi:10.1111/j.1349-7006.2008.01020.x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
