Back to Journals » Journal of Pain Research » Volume 14
Implantable, Programmable, and Wireless Device for Electrical Stimulation of the Dorsal Root Ganglion in Freely-Moving Rats: A Proof of Concept Study
Authors Vuka I, Marciuš T, Kovačić D, Šarolić A , Puljak L, Sapunar D
Received 3 August 2021
Accepted for publication 23 November 2021
Published 9 December 2021 Volume 2021:14 Pages 3759—3772
DOI https://doi.org/10.2147/JPR.S332438
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Supplementary video of "Implantable DRG stimulator" [ID 332438].
Views: 203
Ivana Vuka,1 Tihana Marciuš,1 Damir Kovačić,2 Antonio Šarolić,3 Livia Puljak,4,* Damir Sapunar1,*
1Laboratory for Pain Research, University of Split School of Medicine, Split, Croatia; 2Laboratory for Biophysics and Medical Neuroelectronics, University of Split Faculty of Science, Split, Croatia; 3Laboratory for Applied Electromagnetics (EMLab), FESB, University of Split, Split, Croatia; 4Centre for Evidence-Based Medicine and Health Care, Catholic University of Croatia, Zagreb, Croatia
*These authors contributed equally to this work
Correspondence: Damir Sapunar
Laboratory for Pain Research, University of Split School of Medicine, Šoltanska 2, Split, 21000, Croatia
Tel +385 21-557-809
Email [email protected]
Objective: This was a proof of concept study, based on systematic reviews of the efficacy and safety of the dorsal root ganglion (DRG) stimulation. The main objective was to develop an implantable, programmable, and wireless device for electrical stimulation of DRG and a methodology that can be used in translational research, especially to understand the mechanism of neuromodulation and to test new treatment modalities in animal models of pain.
Methods: We developed and tested a stimulator that uses a battery-powered microelectronic circuit, to generate constant current square biphasic or monophasic pulsed waveform of variable amplitudes and duration. It is controlled by software and an external controller that allows radio frequency communication with the stimulator. The stimulator was implanted in Sprague–Dawley (SD) rats. The lead was positioned at the L5 DRG level, while the stimulator was placed in the skin pocket at the ipsilateral side. Forty-five animals were used and divided into six groups: spinal nerve ligation (SNL), chronic compression injury of the DRG (CCD), SNL + active DRG stimulation, intact control group, group with the implanted sham stimulator, and sham lead. Behavioral testing was performed on the day preceding surgery and three times postoperatively (1st, 3rd, and 7th day).
Results: In animals with SNL, neurostimulation reduced pain-related behavior, tested with pinprick hyperalgesia, pinprick withdrawal test, and cold test, while the leads per se did not cause DRG compression. The rats well tolerated the stimulator. It did not hinder animal movement, and it enabled the animals to be housed under regular conditions.
Conclusion: A proof-of-concept experiment with our stimulator verified the usability of the device. The stimulator enables a wide range of research applications from adjusting stimulation parameters for different pain conditions, studying new stimulation methods with different frequencies and waveforms to obtain knowledge about analgesic mechanisms of DRG stimulation.
Keywords: neurostimulation, implantable stimulator, DRG, SNL, pain-related behavior
Introduction
Electrical stimulation of the dorsal root ganglion (DRG) is currently recognized as a safe and cost-effective therapeutic neuromodulation modality for chronic pain patients, especially for those who do not respond well to conventional treatments.1,2 Following experimentally induced nerve injury or inflammation, DRG plays an important role in pathological nociceptive signaling through increased neuronal excitability and generation of ectopic discharges.3,4 There is evidence indicating that the DRG can be a relevant target for analgesic treatment in the experimental animal models.5,6 More recent studies on the experimental model of painful diabetic peripheral neuropathy in rats confirmed a generally positive effect on animal pain-related behavior using conventional7,8 and burst DRG stimulation. Furthermore, a similar effect was obtained for experimental animal models of osteoarthritis 10 and rheumatoid arthritis.
A well-localized therapeutic approach at the level of the peripheral nervous system and, in particular the DRG, represents an alternative to spinal cord stimulation (SCS). This approach provides stability of the stimulation leads regardless of body position, improves the ability to achieve pain relief in locations that are hard to target with conventional SCS, reduces paresthesia and energy usage 14,15 and contributes to overall better clinical outcomes in selected clinical conditions.15,16
From the recent studies on patients, readers could get the impression that the electrical stimulation of the DRG has been proven to be highly effective in providing analgesia for chronic pain. These clinical studies show that the DRG could be a neuromodulation target for peripheral neuropathic pain following hernia surgery, complex regional pain syndrome,13,16,18 low back pain,19,20 phantom limb pain, disc-related back pain, and radicular pain. However, systematic reviews of the clinical and experimental studies give us a different view.6,23 A systematic review published in 2018 showed that few preclinical studies had investigated the effect of DRG stimulation. Experimental animal studies preceding clinical studies or performed in parallel with them can enhance the understanding of the effect of electrical stimulation on injured DRGs. However, a systematic review of the literature revealed only six publications that have analyzed DRG stimulation in experimental pain models 8,9,24–27 and two more that investigated the stimulation effect in healthy animals.28,29 In all these cases, the pathophysiological mechanisms behind DRG stimulation still remain largely unknown.
One reason for the scarcity of electrical stimulation data in experimental pain models stems from the lack of an appropriate stimulation apparatus for experimental animals. Although there are several technologically advanced devices used for stimulation following amputation, spinal cord injury, or bladder pain syndrome, 32 some of those devices are used in bigger experimental animals, some are not fully implantable, and none of them is intended for usage in chronic pain research.30,31 In addition to devices intended for the stimulation, there are state-of-the-art devices used for the recording of neuronal activity from the surface of DRG, 33 but again these are not applicable for the potential chronic pain treatment in animal pain models. The lack of usable implantable devices that can be used in animal experimental models is also evident in the research in which DRG stimulation is used to evoke motor responses following spinal cord injury.34,35
In this study, we provide a proof of concept for an implantable stimulator that is small, programmable, wireless, and suitable for DRG stimulation in small experimental animals. Additionally, we examined the behavioral effect of the stimulator implantation and placement of the leads with electrodes in healthy rats and rats with spinal nerve ligation (SNL).
Materials and Methods
Study Design
This was a proof of concept study.
Implantable Stimulator
Hardware Overview
The stimulator is a custom-made device developed to provide a programmable platform for DRG stimulation experiments in rats (Figure 1A and B). It uses a microelectronic circuit, powered by a standard non-rechargeable-battery (3V/280mAh, providing 56 hours of signal generation under current stimulation parameters), to generate constant-current square biphasic or quasi-monophasic pulsed waveform of variable amplitudes to stimulate the DRG via a bipolar custom-built lead. Importantly, the stimulator was designed for autonomous operation and wireless control. The implanted device is self-contained in terms of the power supplied by its own battery, while its stimulation protocol is controlled via wireless connection to the external controller. The external controller is connected to a PC via a USB port and controlled via a proprietary software application. Accordingly, the whole system provides the pre-determined chronic electrical stimulation to the free-moving animals without external connection wires.
The implantable part of the stimulator consists of an implantable neurostimulator (INS), an external controller that allows radio frequency (RF) wireless communication with the INS, stimulation electrodes, and the extension wires.
The implanted device consists of the battery-powered electronic circuitry connected to the bipolar lead via the extension wires. The circuitry consists of several major blocks (Supplementary Figure 1): (1) microcontroller unit (MCU), controlling the rest of the circuitry; (2) 3V battery supplying power to the circuitry; (3) wireless link to the external controller for data transfer; (4) magnetic switch for wireless initiation of protocols by magnet movements; (5) DC–DC converter providing the higher voltage required for the stimulation circuitry; (6) stimulation front-end circuitry (voltage-to-current and current-to-current converter), providing the stimulation signal to the lead via the extension wires.
The electrodes in the lead (CorTec GmbH, Freiburg, Germany; cat.no. 1031.2058.01) consist of a platinum-iridium core coated with iridium oxide to avoid fast material corrosion due to the high stimulation currents and small contact surface (Figure 1C). The impedance of the bipolar lead immersed in extracellular fluid and cerebrospinal fluid was previously examined and found to be in 600 Ω – 1 kΩ range (depending on the frequency), which is comparable to the SCS electrode impedance reported by Abejon et al. The lead impedance was also measured after implantation in live rats and found to be slightly larger but still in the kΩ range (2–8 kΩ, depending on the frequency). During initial ex vivo testing of the stimulator, we did not observe heating of the stimulator or any other problems that could interfere with the well-being of the experimental animals.
Coating and Usage
For stimulator coating, we used a two-component SILASTIC MDX4-4210 BioMedical Grade Elastomer (Dow Corning, Auburn, MI, USA). We prepared the elastomer by mixing one part of the curing agent with ten parts by mass of the base elastomer and left the mixture for 30 minutes for de-airing.
The stimulator was first wrapped in several layers of Parafilm M (Sigma-Aldrich, Inc., St. Louis, MO, USA); then, a layer of elastomer was applied on it by immersing a stimulator in elastomer mixture and putting additional amount by brush if needed. We cured the coating at room temperature for 24 h. The coating of the stimulator increased its weight from 6.6 g to 13.4 g, making it 3.4% to 4% of the total body mass (Figure 1B).
After implanting the stimulator in the rat, the stimulation parameters are defined using a computer and sent to the stimulator via the external controller connected to the PC via USB port. The stimulator is activated by passing a permanent magnet one centimeter over the skin near the implanted stimulator. A brief passage of the magnet connects the stimulator with the external controller and allows stimulation protocol transfer, while longer holding the magnet above the stimulator starts the stimulation protocol. Each stimulator also has red light-emitting diodes (LEDs) visible through the animal’s skin, providing a brief flash of light as a confirmation when the stimulator is switched on. The stimulator parameters are listed in Table 1. Each neurostimulator has a unique internal identifier that allows the researcher to identify the specific device through an external controller.
 |
Table 1 Stimulator Signal Parameters |
Software Overview
The PC application with a graphical user interface (GUI), shown in Figure 2, was developed in Java programming language using developmental environment NetBeans 8.0.2 IDE with installed JDK 8 update 11 (64 bit). GUI was created in the JavaFX Scene Builder 2.0 tool. For database access, the Jackcess-2.1.0 library was used. The application allows the user to define the stimulator operation parameters, store them in the database, and send them to the stimulator. The application does not access the RF communication device directly but through a communication service using UDP/IP packets. The main development tool for this communication service application was MS Visual Studio 2015, and the programming language was C++. The main library was Microsoft Foundation Class (MFC), and the Functional Test Case Serial Peripheral Interface (FTCSPI) library was used to access the USB module. The task of this communication service is bidirectional communication between the GUI user application and the implanted stimulator. The communication service application was installed on the MS Windows platform as a service and automatically runs each time the computer is turned on.
The integrated circuits used in the implanted device also required programming. The microcontroller unit C8051F411 (Silicon Laboratories, Inc., Austin, TX, USA) software was written in the C programming language, using “Keil uVision5” as the development environment. The RF transceiver chip TI CC2500 (Texas Instruments, Inc., Dallas, TX, USA), used as the RF wireless link, was configured to generate current pulses based on parameters received via RF communication using “Texas Instruments SmartRF Studio 7” tool. The parameters are valid until a new set of parameters is sent to the unit. To ensure stable and reliable transfer of stimulation parameters, we deployed a check-back system by requesting the implanted device to send back the actual parameters calculated on the microcontroller because those parameters may vary slightly due to the microcontroller’s discretization and timer setting.
Experimental Animals
All experimental procedures and protocols followed the International Association for the Study of Pain (IASP) Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals and were approved by the Ethics Committee of the University of Split School of Medicine. A total of 47 male Sprague-Dawley rats were assigned to one of the following six groups: SNL group (n = 6), group with chronic compression injury of DRG (CCD) (n = 9), SNL group with active DRG stimulation (n = 11), intact control group (n = 6), and group with the implanted sham stimulator (n = 8) and sham lead (n = 7). The description of the performed interventions in the groups used in the study is presented in Table 2. Because of the size of the stimulator, we used older animals weighing 350–400 g.
 |
Table 2 Description of the Performed Interventions in the Groups Used in the Study |
Surgery Procedures for SNL and CCD
We used the well-established SNL 37 and CCD 38 models for the induction of pain-related behavior. All surgical procedures were performed under anesthesia induced with 5% isoflurane in oxygen (Forane®, Abbott laboratories Ltd., Queenborough, United Kingdom) and then maintained with 2% isoflurane.
For SNL, 37,39 after exposure to the right paravertebral region, the sixth lumbar transverse process was carefully removed to expose the L5 nerve, which was ligated with 6–0 silk suture and transected distally. Articular processes and paraspinal muscles were not removed since their destruction can lead to pain-related behavior.40,41
The insertion of the leads with stimulation electrodes can result in CCD;38,42–44 thus, we compared the effect of sham lead placement with the CCD model. CCD injury was performed according to the slightly modified procedure described previously. Briefly, under isoflurane anesthesia, intervertebral foramen of L5 was exposed, and a stainless steel L shaped rood (4 mm longer arm, 2 mm shorter arm, and 0.6 mm in diameter) was inserted 4 mm into the L5 intervertebral foramen at an angle of 30° to the midline. A slight twitch in the ipsilateral leg was typically observed during the insertion.
Surgical Procedure for Stimulator Implantation
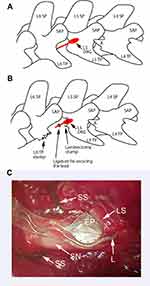
Following a skin incision in the sagittal line of the lumbosacral region, the skin pocket was created with blunt dissection cranially to the incision. The right paravertebral region was exposed. Connective tissue and remaining muscles were removed by iris scissors to expose the L5 laminae, L6 transverse, and articulate process between the L5 and L6 vertebrae. For the SNL and placement of leads with the electrodes, the transverse process of the L6 was removed. With the L6 transverse process removed, the L5 and L4 spinal nerves were clearly visible, enabling us to follow the L5 spinal nerve towards the L5 DRG. To expose the caudal part of the L5 DRG, a small part of the lamina on the right side, caudal to the L5 was removed using a small rongeur (Figure 3). To limit bleeding, which was the main problem during the laminectomy, we applied Surgicel Fibrillar (Absorbable Hemostat-oxidized regenerated cellulose; Ethicon, Inc., Somerville, NJ, USA) when bleeding occurred after the removal of the laminae rim. Surgicel was removed immediately after the bleeding stopped. After the caudal part of the ganglia was exposed, the lead was inserted into the foramen. This step was done with special care because the lead’s tip lacked sufficient firmness for easy insertion in the foramen. The lead was secured in place by tightly ligating it to the L5 spinal nerve (Figure 3). After the lead was secured with ligature, SNL was performed by transecting the spinal nerve distal to the ligation used to secure the lead. The leads were tunneled towards the cranial skin pocket on the rat’s back, where the stimulator was implanted. To minimize infection risk, we immersed the implantable stimulators and leads in 70% ethanol before implantation. Following the surgery, the animals did not receive any antibiotics or analgesic therapy.
Stimulation Protocol
Baseline behavioral testing was performed on the day preceding surgery, while postoperative behavioral testing was performed three times postoperatively (1st, 3rd, and 7th day). Stimulation was performed continuously, once per day for 5 days, from 3rd till 7th day postoperatively immediately before behavioral testing. After 60 minutes of acclimatization, the stimulator was activated and set for a session lasting 20 minutes. At the end of the stimulation session, we performed behavioral testing following additional 60 minutes of acclimatization. The timing of the behavioral test was selected based on the other short-term efficacy studies 7,26,27,45 and on the fact that the time course of hyperalgesic-type responses following SNL was most pronounced between 4th and 7th day. The duration of the sessions was identical,46,47 or comparable with previously published studies.7,26,27,45
For the group of animals implanted with an active stimulator, we set the DRG stimulation currents at 80% of the motor threshold (MT), ie, a rectangular pulse of 0.2 mA with a 0.2 ms pulse width and frequency of 20 Hz. The 80% of the MT was selected because it has been proven that it produces a better analgesic effect than 40% or 60% MT values.10,48 The MT was assessed in a pilot study preceding the experiment using a protocol with gradually increasing current amplitude at the following stimulator settings: frequency 20 Hz, pulse width 0.2 ms. When the MT was reached, muscle twitches were observed, and the current was recorded. Measured MT levels were 0.25 ± 0.02 mV (n = 6). To balance the charge and prevent a net ion flow between the electrodes, which can cause a toxic reaction in the tissue, we used biphasic pulses. The charge balance is passive with asymmetric pulses (Figure 2). The active phase is followed by a reverse polarity pulse with amplitude or duration calculated so that positive and negative phases have equal surfaces under the pulse.
Behavioral Tests
Four behavioral tests were used, with mechanical and thermal stimuli. The tests were performed during the morning hours immediately after the stimulation session in the following sequence: needle pin prick test, von Frey fibers, acetone, and hot plate test. The animals were tested in a cohort of two. The acclimatization of the animals to the laboratory environment prior to the stimulation session was 60 minutes. The duration of the behavioural testing session per cohort was approximately one hour including 15-minute acclimatization to the rack and the hot plate. Blinded testing was possible only between rats with an implanted active and sham stimulator. Stimuli were applied to the plantar skin of hind paws of unrestrained rats, including the following procedures in each case.
Needle Pinprick Test
The point of a 22-gauge spinal anesthesia needle was applied to the center of the paw with enough force to indent the skin but not to injure it. The incidence of pinprick withdrawal (brisk, simple withdrawal with immediate return of the paw to the cage floor) and hyperalgesia (more complex response with sustained paw lifting, licking, chewing, and grooming) responses during ten applications to hind paws (separated by at least 10 seconds) were recorded and averaged.
Acetone Test for Cold Allodynia/Hyperalgesia
Sensitivity to cold was assessed using the application of acetone, which was expelled from a syringe attached to the tubing to form a drop that was applied to the mid-plantar surface of the hind paw without contact of the tubing with the skin. The response was scored as either none or positive if the paw was withdrawn. Three applications were spaced at least 30 seconds apart and averaged.
Hot Plate Test
To assess sensitivity to heat, the hot plate analgesia meter (IITC Life Science, Woodland Hills, CA, USA) was used. The rats were placed in the device chamber with an aluminum plate maintained at 25°C. The temperature-provoking withdrawal response of the hind paw was measured for each rat. The cut-off temperature was 50°C. Withdrawal temperature was measured three times, separated by 3 min, and averaged.
Von Frey Fibers
Punctate mechanical stimulation was applied to the paws by von Frey fibers (North Coast Medical Inc., Gilroy, CA, USA). As described previously, calibrated fibers, starting with the 2.8 g filament (3,84 filament), were applied in increasing order from the weakest to the strongest to determine the threshold stiffness required for 50% paw withdrawal. The withdrawal response was scored either as none or positive if the paw was removed. If there was no response to the stiffest filament, the value of 25 g was assigned as threshold.
Computerized Tomography (CT)
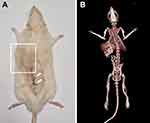
To verify the position of the stimulator within the rat body, we used a CT scan on one additional rat (not included in the behavioral analysis). The rat was anesthetized and scanned in a coronal plane at 0.6 mm slice thickness on the Somatom Definition AS 128-Lines Multislice CT (Siemens, Germany). CT imaging parameters were set to a tube voltage of 120 kV and 18–39 mA. The analysis of the scans and 3D reconstructions was performed with SynGo software (Siemens, Germany) using Osseous –metal reconstruction modality Figure 4B.
Statistical Analysis
Behavioral test scores were analyzed with one-way repeated measures analyses of variance (ANOVA). The repeated measures were performed with the factors Time (baseline and three postoperative tests), Group (4 groups: SNL, CCD, SNL + stimulation, and merged control and sham groups), and Time by Group interaction, with behavioral outcomes as dependent variables. The presented analyses are Time × Group interactions, and when they were significant, post hoc assessment of within comparison was performed using the Bonferroni post hoc test (Statistica 7.0; StatSoft, Tulsa, OK, USA). Graphs show means ± SEM. Any difference with p < 0.05 was considered to be statistically significant.
Results
Tolerance of the Stimulator by Animals
Once implanted, the stimulator was well tolerated by the rats. It did not hinder animal movement, and it enabled the animals to be housed in regular animal facilities since no external contacts were necessary for the usage of the stimulator. One rat from the CCD group kept the injured paw in a guarding position. The guarding posture was observed on postoperative day 1 but ceased at postoperative day 2. None of the rats exhibited signs of autotomy or nail changes. The skin above the implanted stimulator did not exhibit any changes, and the sutures were healing well (Figure 4A).
The position of the stimulator within the body of the experimental animal was confirmed by CT imaging (Figure 4B) and 3D reconstructions (Supplementary video). The CT scans allowed successful visualization of both the implanted stimulator and the lead. The position of the stimulator was just below the skin and without compression of neighboring organs. The position of the leads can be easily identified, and the position of the pad with electrodes can only be vaguely located in the area of the intervertebral foramen (Figure 4B). However, the autopsy findings confirmed the desirable position of the pad with electrodes in all animals.
Pain-Related Behavior
To test if the implantation of the stimulator and placement of the leads can produce any effect on pain-related behaviors, we used groups with sham leads and sham stimulator. Since both groups did not reveal any behavioral changes during the postoperative period in the tested modalities compared to the control group, the sham groups were merged with the control group.
The expected pro-algesic effect of SNL and CCD was confirmed with behavioral tests. Figures 5A and 4B show that SNL and CCD models resulted in the expected increase in pain-related behavior tested by the pinprick method. The SNL model affected all tested sensory modalities except the heat responses (Figure 5D). In the CCD model, increased pain-related behavior was observed only in pinprick hyperalgesia, pinprick withdrawal responses, and cold response (Figure 5A-C). Post hoc analysis showed a significant increase in pinprick withdrawal (Figure 5A) and hyperalgesia (Figure 5B) responses on the 1st, 3rd, and 7th day following induction of the SNL or CCD model compared to corresponding control values. In the SNL model, the same pattern was observed only for cold and on 3rd and 7th day for von Frey testing. The CCD model did not affect von Frey threshold or heat, but it did affect the cold response on the 3rd day (Figure 5C).
Stimulation with our device reduced pain-related behavior in SNL rats on the 3rd and 7th postoperative day, tested with pinprick hyperalgesia and cold test. In the pinprick withdrawal test, the reduction in pain-related behavior was evident only on 3rd day. The stimulation did not affect heat response (Figure 5D) and von Frey withdrawal threshold in the SNL model (Figure 5E).
Autopsy
The serosanguinous fluid accumulation (seroma) within a surgically created pocket was noticed during an autopsy in three rats (one with sham and two with active stimulator). Other signs of inflammation or behavior disturbances were not observed in rats with implanted stimulators.
The extraction of the stimulator during the autopsy was very easy. However, the extraction of the leads was difficult because the connective tissue bonded to them. The problematic extraction resulted in only half of the leads salvaged, while the remaining leads were destroyed. The position of the pad with electrodes was checked, and in all animals, the tip of the lead was still located in the intervertebral foramen. The electrodes in the lead did not exhibit any visible corrosion after usage.
In the first two SNL rats with implanted stimulators, we observed leakage of fluid under the SILASTIC elastomer coating. The leakage interfered with the electronics and made stimulators unusable after the first stimulation cycle. The source of leakage, examined under a stereomicroscope, was the connection between the lead and stimulator and path along with the lead coating. Those rats were excluded from further analysis. The leakage problem is immediately addressed by adding an additional layer of coating in the area where leads connect to the stimulator. The additional layer of coating did not increase the size or thickness of the stimulator.
Discussion
This was a proof-of-concept study, testing the software and hardware characteristics of a small, implantable, programmable, and wireless DRG stimulator for small experimental animals. The presented implantable neurostimulator addresses technical limitations of the previously described systems used for DRG stimulation or closely related systems, like those used for spinal cord stimulation.26,50,51
Advancements in the material sciences have led to use of more advanced devices and technologies with a variety of applications, such as closed-loop optogenetic device for peripheral neuromodulation in rat models 32 or soft neurotechnology bioelectric interfaces used in dura mater and epidural cervical stimulation in non-human primates.52,53 Soft subdural implants were also used in rat models, combining electrical and chemical stimulations of the spinal cord in animal models of paralysis. Advancements are also seen in the introduction of soft materials when designing electrode systems for the treatment of neurological disorders. Although these technical advancements contributed to the better outcomes in selected clinical condition in which the DRG stimulation is used,15,16 experimental animal research on DRG stimulation did not benefit adequately from those advancements.
Devices for DRG stimulation that were used earlier were not completely wireless since they had external wires, limiting the experimental animals’ movement and housing, or had limitations regarding type and stimulation parameters. Furthermore, those devices can increase the chance of infection.26,50,51,55,56 The external connections were prone to damage and breaks in the leads near the connection hub, which turns out to be the main problem with the external connection.7,26 However, previously designed devices for stimulation of the DRG were well tolerated by the experimental animals. In Pan et al, study rats reminded healthy during the implantation and stimulations, with no signs of autotomy or change in posture or behavior. A similar level of tolerance in the sham group with implanted leads was observed in rats with experimentally induced diabetic polyneuropathy.7,57 Although different in size and construction, those leads and electrodes were also well tolerated by the animals and allowed reliable delivery of the stimulation sessions.7,26,57
Battery-powered implantable stimulators are internally implanted inside the experimental animals’ body, yielding multiple advantages, eg, minimizing the risk of infection associated with percutaneous connectors or being capable of housing the animals under their regular conditions. The programmability of the implanted stimulator enables the generation of customized and complex protocols that can be changed during experiments. This level of flexibility is crucial since no single stimulation protocol is optimal for all situations.
The first fully implantable stimulator for rodents was developed by Huang et al and was used for auditory nerve stimulation in deaf rodents. The device was used to test monopolar and bipolar stimulation parameters, especially to achieve charge balance and avoid the residual direct current. Another fully implantable stimulator was designed by Millard et al. Their stimulator was powered by a pulsed magnetic field generated by wire coiled around the outside portion of the animal cage. Despite very small in size and adjustable for various uses, their stimulator requires special excitation coil assembly, and can thus affect pain-related behavior.
Qian et al developed a similar device implanted subcutaneously for SCS or fixed outside the skull for deep brain stimulation. The stimulator was also used for DRG stimulation in the hind paw for the prevention and treatment of disused bone loss after 14 days 46 and after 6 weeks. The characteristics of their stimulator are similar to the one described here. The main difference is the range of parameters. Our stimulator has a broader range of stimulation amplitudes, pulse width, and frequency, allowing a greater possibility of testing different treatment parameters depending on pain condition. Furthermore, our stimulator has a bipolar lead designed specifically for DRG stimulation and is tested for use in healthy animals and for treatment of neuropathic pain after SNL.
Pan et al used an implantable neurostimulator to treat chronic neuropathic pain in the tibial nerve injury model of neuropathic pain in rats. Their device was not implantable since the leads were placed in plastic tubing and tunneled to the head, where they were secured to the skull with dental cement and screws and leads. For stimulation, the connection hub needed to be connected to the external pulse generator, hindering animals’ free movement during the stimulation. The stimulating electrodes in the lead were built from two platinum-iridium wires to provide bipolar contact for DRG stimulation. This design caused breaks in a portion of the wires. Similar to our findings, they also found that insertion of the leads caused no evidence of pain-related behavior, while DRG stimulation resulted in decreased pain-related behavior, which is in agreement with our findings. The observed reversal of the pain-related behavior induced by the SNL model in our study occurred immediately after the DRG stimulation, which corresponds to the clinical experience 18 and experimental results. The behavioral results were in line with our previously published results in which we confirmed that simple withdrawal in the von Frey test is not a less effective measure of peripheral nerve injury pain in rats than hyperalgesic-type response. In addition to the different study designs, that is probably the reason why we did not observe a similar response to stimulation in von Frey test.
Regardless of the relatively numerous publications on DRG stimulation, the mechanisms of how DRG stimulation alleviates chronic pain are still not fully understood. In vitro studies on healthy DRGs reported that DRG simulations resulted in reduced excitability of the DRG neurons. It is proposed that DRG stimulation amplifies the filtering ability of the DRG T-junction and limits the propagation of trains of action potentials.59,60 Recent in vivo studies evaluated the effect of tonic and burst DRG stimulation as well as the possible mechanism of DRG stimulation in rats after tibial nerve injury.61,62 The authors confirmed that both burst and tonic stimulation can be used for the treatment of neuropathic pain; furthermore, they confirmed findings from in vitro studies about a possible mechanism where DRG stimulation blocks propagation of afferent action potentials and enhances T-junction filtering of action potentials in the sensory neuron.61,62
Our stimulator can be easily used in future animal studies using various neuropathic pain models designed to investigate and clarify the DRG stimulation mechanisms. Furthermore, studies on animal models can serve as a great starting point for optimizing stimulation parameters, in terms of the number of sessions, session duration, variable constant current square biphasic or monophasic pulsed waveform of variable amplitudes to achieve better intervention outcomes for different pain conditions. In addition, the stimulator can also be used for testing new treatment modalities, such as high-frequency stimulation, 57,63 but also in SCS and peripheral nerve stimulation preclinical studies.
Although the value of our stimulator can be questioned because of the already established clinical practice of DRG stimulation, research on the physiological mechanisms of neuromodulation can hardly be done in the clinical setting. The value of the device can be additionally justified when a lack of evidence in this field presented in recent systematic reviews is considered.6,23
Conclusion
We have developed and validated an implantable, programmable, and wireless DRG stimulator for rats, and our initial animal experiment verified the usability of the stimulator system in healthy animals as well as in animals with neuropathic pain model. We present a stimulator design that is economical, easy to use and enables a broad spectrum of research applications, from adjusting parameters of stimulation for different pain conditions, trying new stimulation modes such as high-frequency stimulation to get answers about analgesic mechanisms of DRG stimulation.
Acknowledgments
We are very grateful to the engineers Zdravko Koceić and Damir Gašperov for the construction of the stimulator and for the software development. This study was supported by the Croatian Science Foundation (HRZZ) grants for Young Scientist Career Development (HRZZ-I-2238-2016 and HRZZ DOK-2014-06-5195) and an HRZZ grant for Treating Neuropathic Pain with Dorsal Root Ganglion Stimulation, awarded to Damir Sapunar (HRZZ-IP-2013-11-4126). The production and market distribution of the presented stimulator is regulated by the agreement of the transfer of technology between the School of Medicine, University of Split, and the IRI Center d.o.o. (Kaštel Gomilica, Croatia).
Author Contributions
Authorship statement: All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest in this work to disclose.
References
1. Huygen F, Kallewaard JW, Nijhuis H, et al. Effectiveness and safety of Dorsal Root Ganglion stimulation for the treatment of chronic pain: a pooled analysis. Neuromodulation. 2020;23(2):213–221.
2. Mekhail N, Deer TR, Poree L, et al. Cost-effectiveness of dorsal root ganglion stimulation or spinal cord stimulation for complex regional pain syndrome. Neuromodulation. 2020;24:4.
3. Sapunar D, Ljubkovic M, Lirk P, McCallum JB, Hogan QH. Distinct membrane effects of spinal nerve ligation on injured and adjacent dorsal root ganglion neurons in rats. Anesthesiology. 2005;103(2):360–376.
4. Xie WR, Deng H, Li H, Bowen TL, Strong JA, Zhang JM. Robust increase of cutaneous sensitivity, cytokine production and sympathetic sprouting in rats with localized inflammatory irritation of the spinal ganglia. Neuroscience. 2006;142(3):809–822.
5. Sapunar D, Kostic S, Banozic A, Puljak L. Dorsal root ganglion - a potential new therapeutic target for neuropathic pain. J Pain Res. 2012;5:31–38.
6. Vuka I, Vucic K, Repic T, Ferhatovic Hamzic L, Sapunar D, Puljak L. Electrical stimulation of dorsal root ganglion in the context of pain: a systematic review of in vitro and in vivo animal model studies. Neuromodulation. 2018;21(3):213–224.
7. Koetsier E, Franken G, Debets J, et al. Effectiveness of dorsal root ganglion stimulation and dorsal column spinal cord stimulation in a model of experimental painful diabetic polyneuropathy. CNS Neurosci Ther. 2019;25(3):367–374.
8. Franken G, Douven P, Debets J, Joosten EAJ. Conventional dorsal root ganglion stimulation in an experimental model of painful diabetic peripheral neuropathy: a quantitative immunocytochemical analysis of intracellular gamma-aminobutyric acid in dorsal root ganglion neurons. Neuromodulation. 2021. doi:10.1111/ner.13398
9. Franken G, Debets J, Joosten EAJ. Nonlinear relation between burst dorsal root ganglion stimulation amplitude and behavioral outcome in an experimental model of painful diabetic peripheral neuropathy. Neuromodulation. 2020;23(2):158–166.
10. Yu G, Segel I, Zhang Z, Hogan QH, Pan B. Dorsal root ganglion stimulation alleviates pain-related behaviors in rats with nerve injury and osteoarthritis. Anesthesiology. 2020;133(2):408–425.
11. Pan B, Zhang Z, Chao D, Hogan QH. Dorsal root ganglion field stimulation prevents inflammation and joint damage in a rat model of rheumatoid arthritis. review. Neuromodulation. 2018;21(3):247–253.
12. Kramer J, Liem L, Russo M, Smet I, Van Buyten JP, Huygen F. Lack of body positional effects on paresthesias when stimulating the dorsal root ganglion (DRG) in the treatment of chronic pain. Clinical Trial. Neuromodulation. 2015;18(1):50–57.
13. Van Buyten JP, Smet I, Liem L, Russo M, Huygen F. Stimulation of dorsal root ganglia for the management of complex regional pain syndrome: a prospective case series. Pain Pract. 2015;15(3):208–216.
14. Abejon D, Feler CA. Is impedance a parameter to be taken into account in spinal cord stimulation? Pain Physician. 2007;10(4):533–540.
15. Esposito MF, Malayil R, Hanes M, Deer T. Unique characteristics of the dorsal root ganglion as a target for neuromodulation. Pain Med. 2019;20(Suppl 1):S23–S30.
16. Deer TR, Levy RM, Kramer J, et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain. 2017;158(4):669–681.
17. Liem L, Mekhail N. Management of postherniorrhaphy chronic neuropathic groin pain: a role for dorsal root ganglion stimulation. review. Pain Pract. 2016;16(7):915–923.
18. van Bussel CM, Stronks DL, Huygen FJ. Successful treatment of intractable complex regional pain syndrome type I of the knee with dorsal root ganglion stimulation: a case report. Neuromodulation. 2015;18(1):
19. Huygen F, Liem L, Cusack W, Kramer J. Stimulation of the L2-L3 dorsal root ganglia induces effective pain relief in the low back. Pain Pract. 2018;18(2):205–213.
20. Deer TR, Grigsby E, Weiner RL, Wilcosky B, Kramer JM. A prospective study of dorsal root ganglion stimulation for the relief of chronic pain. Neuromodulation:. 2013;16(1):
21. Eldabe S, Burger K, Moser H, et al. dorsal root ganglion (DRG) stimulation in the treatment of Phantom Limb Pain (PLP). Neuromodulation. 2015;18(7):
22. Liem L, Russo M, Huygen FJ, et al. One-year outcomes of spinal cord stimulation of the dorsal root ganglion in the treatment of chronic neuropathic pain. Neuromodulation. 2015;18(1):41–48.
23. Vuka I, Marcius T, Dosenovic S, et al. Neuromodulation with electrical field stimulation of dorsal root ganglion in various pain syndromes: a systematic review with focus on participant selection. Review. J Pain Res. 2019;12:803–830.
24. Eisenach JC, Zhang Y, Duflo F. Alpha2-adrenoceptors inhibit the intracellular Ca2+ response to electrical stimulation in normal and injured sensory neurons, with increased inhibition of calcitonin gene-related peptide expressing neurons after injury. Neuroscience. 2005;131(1):189–197.
25. Ma W, Zhang Y, Bantel C, Eisenach JC. Medium and large injured dorsal root ganglion cells increase TRPV-1, accompanied by increased alpha2C-adrenoceptor co-expression and functional inhibition by clonidine. Pain. 2005;113(3):386–394.
26. Pan B, Yu H, Fischer GJ, Kramer JM, Hogan QH. Dorsal root ganglionic field stimulation relieves spontaneous and induced neuropathic pain in rats. J Pain. 2016;17(12):1349–1358.
27. Pawela CP, Kramer JM, Hogan QH. Dorsal root ganglion stimulation attenuates the BOLD signal response to noxious sensory input in specific brain regions: insights into a possible mechanism for analgesia. NeuroImage. 2017;147:10–18.
28. Duflo F, Zhang Y, Eisenach JC. Electrical field stimulation to study inhibitory mechanisms in individual sensory neurons in culture. Anesthesiology. 2004;100(3):740–743.
29. Koopmeiners AS, Mueller S, Kramer J, Hogan QH. Effect of electrical field stimulation on dorsal root ganglion neuronal function. Neuromodulation. 2013;16(4):
30. King KW, Cusack WF, Nanivadekar AC, et al. DRG microstimulation evokes postural responses in awake, standing felines. J Neural Eng. 2019;17(1):016014.
31. Hogan MK, Barber SM, Rao Z, et al. A wireless spinal stimulation system for ventral activation of the rat cervical spinal cord. Sci Rep. 2021;11(1):14900.
32. Mickle AD, Won SM, Noh KN, et al. A wireless closed-loop system for optogenetic peripheral neuromodulation. Nature. 2019;565(7739):361–365.
33. Kashkoush AI, Gaunt RA, Fisher LE, Bruns TM, Weber DJ. Recording single- and multi-unit neuronal action potentials from the surface of the dorsal root ganglion. Sci Rep. 2019;9(1):2786.
34. Soloukey S, Drenthen J, Osterthun R, et al. How to identify responders and nonresponders to dorsal root ganglion-stimulation aimed at eliciting motor responses in chronic spinal cord injury: post hoc clinical and neurophysiological tests in a case series of five patients. Neuromodulation. 2021;24(4):719–728.
35. Soloukey S, de Rooij JD, Osterthun R, et al. The dorsal root ganglion as a novel neuromodulatory target to evoke strong and reproducible motor responses in chronic motor complete spinal cord injury: a case series of five patients. case reports. Neuromodulation. 2021;24(4):779–793.
36. Sarolic A, Skalic I, Deftu A, Sapunar D. Impedance Measurement of Bipolar Stimulation Electrodes Immersed in Medium. IEEE 2018 EMF-Med 1st World Conference on Biomedical Applications of Electromagnetic Fields (EMF-Med), IEEE Explore, 2018. Available from: https://ieeexplore.ieee.org/document/8526008. Accessed December 4, 2021.
37. Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50(3):355–363.
38. Hu SJ, Xing JL. An experimental model for chronic compression of dorsal root ganglion produced by intervertebral foramen stenosis in the rat. Pain. 1998;77(1):15–23.
39. Hogan Q, Sapunar D, Modric-Jednacak K, McCallum JB. Detection of neuropathic pain in a rat model of peripheral nerve injury. Anesthesiology. 2004;101(2):476–487.
40. Kosta V, Kojundzic SL, Sapunar LC, Sapunar D. The extent of laminectomy affects pain-related behavior in a rat model of neuropathic pain. Eur J Pain. 2009;13(3):243–248.
41. Busic Z, Kostic S, Kosta V, Carija R, Puljak L, Sapunar D. Postlaminectomy stabilization of the spine in a rat model of neuropathic pain reduces pain-related behavior. Spine (Phila Pa 1976). 2012;37(22):1874.
42. Song XJ, Hu SJ, Greenquist KW, Zhang JM, LaMotte RH. Mechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root ganglia. J Neurophysiol. 1999;82(6):3347–3358.
43. Zhang JM, Song XJ, LaMotte RH. Enhanced excitability of sensory neurons in rats with cutaneous hyperalgesia produced by chronic compression of the dorsal root ganglion. J Neurophysiol. 1999;82(6):3359–3366.
44. Yao H, Donnelly DF, Ma C, LaMotte RH. Upregulation of the hyperpolarization-activated cation current after chronic compression of the dorsal root ganglion. J Neurosci. 2003;23(6):2069–2074.
45. Franken G, Debets J, Joosten EAJ. Dorsal root ganglion stimulation in experimental painful diabetic peripheral neuropathy: burst vs. conventional stimulation paradigm. Neuromodulation. 2019;22(8):943–950.
46. Lau YC, Qian X, Po KT, Li LM, Guo X. Electrical stimulation at the dorsal root ganglion preserves trabecular bone mass and microarchitecture of the tibia in hindlimb-unloaded rats. Osteoporosis Int. 2015;26(2):481–488.
47. Yuen-chi Lau R, Qian X, Po KT, Li LM, Guo X. Response of rat Tibia to prolonged unloading under the influence of electrical stimulation at the dorsal root Ganglion. Neuromodulation. 2017;20(3):284–289.
48. Chao D, Zhang Z, Mecca CM, Hogan QH, Pan B. Analgesic dorsal root ganglionic field stimulation blocks conduction of afferent impulse trains selectively in nociceptive sensory afferents. Pain. 2020;161(12):2872–2886.
49. Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59(3):369–376.
50. Millard RE, Shepherd RK. A fully implantable stimulator for use in small laboratory animals. J Neurosci Methods. 2007;166(2):168–177.
51. Qian X, Hao HW, Ma BZ, et al. Programmable and implantable neurostimulator with novel stimulus waveforms for rat models. Electron Lett. 2012;48(17):1035–1036.
52. Schiavone G, Fallegger F, Kang X, et al. Soft, implantable bioelectronic interfaces for translational research. Adv Mater. 2020;32(17):e1906512.
53. Greiner N, Barra B, Schiavone G, et al. Recruitment of upper-limb motoneurons with epidural electrical stimulation of the cervical spinal cord. Nat Commun. 2021;12(1):435.
54. Capogrosso M, Gandar J, Greiner N, et al. Advantages of soft subdural implants for the delivery of electrochemical neuromodulation therapies to the spinal cord. J Neural Eng. 2018;15(2):026024.
55. Linderoth B, Gazelius B, Franck J, Brodin E. Dorsal column stimulation induces release of serotonin and substance P in the cat dorsal horn. Neurosurgery. 1992;31(2):
56. Tilley DM, Vallejo R, Kelley CA, Benyamin R, Cedeno DL. A continuous spinal cord stimulation model attenuates pain-related behavior in vivo following induction of a peripheral nerve injury. Neuromodulation:. 2015;18(3):
57. Koetsier E, Franken G, Debets J, et al. Dorsal root ganglion stimulation in experimental painful diabetic polyneuropathy: delayed wash-out of pain relief after low-Frequency (1Hz) stimulation. Neuromodulation. 2020;23(2):177–184.
58. Huang CQ, Shepherd RK, Carter PM, Seligman PM, Tabor B. Electrical stimulation of the auditory nerve: direct current measurement in vivo. IEEE Trans Biomed Eng. 1999;46(4):461–470.
59. Koopmeiners AS, Mueller S, Kramer J, Hogan QH. Effect of electrical field stimulation on dorsal root ganglion neuronal function. Neuromodulation. 2013;16(4):304–311.
60. Krames ES. The dorsal root ganglion in chronic pain and as a target for neuromodulation: a review. Review. Neuromodulation. 2015;18(1):24–32.
61. Yu G, Segel I, Tran H, et al. Analgesic effects of tonic and burst dorsal root ganglion stimulation in rats with painful tibial nerve injury. Neuromodulation. 2021. doi:10.1111/ner.13472
62. Chao D, Mecca CM, Yu G, et al. Dorsal root ganglion stimulation of injured sensory neurons in rats rapidly eliminates their spontaneous activity and relieves spontaneous pain. Pain. 2021. doi:10.1097/j.pain.0000000000002284
63. Pluijms WA, van Kleef M, Honig WM, Janssen SP, Joosten EA. The effect of spinal cord stimulation frequency in experimental painful diabetic polyneuropathy. Eur J Pain. 2013;17(9):1338–1346.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.