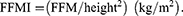Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Impaired Skeletal Muscle in Patients with Stable Chronic Obstructive Pulmonary Disease (COPD) Compared with Non-COPD Patients
Authors Wu ZY, Lu XM, Liu R, Han YX , Qian HY, Zhao Q, Niu M
Received 19 November 2022
Accepted for publication 3 July 2023
Published 19 July 2023 Volume 2023:18 Pages 1525—1532
DOI https://doi.org/10.2147/COPD.S396728
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Zhen-Yun Wu,1,* Xiang-Min Lu,2,* Rui Liu,1,* Yan-Xia Han,3 Hong-Ying Qian,1 Qian Zhao,1 Mei’e Niu3
1Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Soochow University, Suzhou, People’s Republic of China; 2School of Nursing, Suzhou Medical College of Soochow University, Suzhou, People’s Republic of China; 3Department of Nursing, The First Affiliated Hospital of Soochow University, Suzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mei’e Niu, Department of Nursing, the First Affiliated Hospital of Soochow University, No. 899, Pinghai Road, Suzhou, Jiangsu, 215006, People’s Republic of China, Email [email protected] Qian Zhao, Department of Pulmonary and Critical Care Medicine, the First Affiliated Hospital of Soochow University, No. 188, Shizi Street, Suzhou, Jiangsu, 215006, People’s Republic of China, Email [email protected]
Purpose: This study was designed to investigate the differences in skeletal-muscle atrophy between patients with stable chronic obstructive pulmonary disease (COPD) and healthy controls; associated factors were also considered. The study comprised selected residents of communities near the First Affiliated Hospital of Soochow University in Suzhou City, East China.
Patients and Methods: Included in this study were 123 COPD patients and 60 controls. All patients completed spirometry as well as examinations to determine their functional exercise capacity, body composition, and handgrip strength (HGS).
Results: COPD patients had less fat-free mass (FFM), a lower FFM index (FFMI), and a lower 6-min walking distance (6MWD) compared with controls (P = 0.007, P = 0.020, and P < 0.001, respectively) (FFMI: 17.59 ± 1.83 vs 18.34 ± 1.64). The HGS of these patients was also lower compared with that of controls (32.88 ± 7.84 vs 35.48 ± 7.42), and HGS tended toward statistical significance (P = 0.064, respectively). In multivariate analysis, age (β = − 0.107, P < 0.001), gender (β = 0.212, P < 0.001), body mass index (BMI) (β = 0.462, P < 0.001), FEV1% (β = 0.108, P = 0.009), and calf circumference (CC) (β = 0.457, P < 0.001) were significantly associated with FFMI.
Conclusion: Impaired skeletal muscle mass was more common in COPD patients than in controls. Multiple regression analysis showed that CC may be used to detect the degree of impairment, particularly by health-care providers working outside of the hospital.
Keywords: airflow obstruction, calf circumference, chronic obstructive pulmonary disease, fat-free mass index, skeletal muscle atrophy, skeletal muscle mass
Introduction
Chronic obstructive pulmonary disease (COPD), characterized by persistent airflow obstruction and respiratory symptoms, is a major cause of morbidity and mortality worldwide.1 Growing evidence suggests that COPD is associated with various extrapulmonary effects, including weight loss, cardiovascular disease, skeletal muscle atrophy (associated with inflammation2), and an oxidative-stress (OS) response,3 resulting in impaired functional capacity, worsening dyspnea, and a reduced health-related quality of life (QoL).
Muscle atrophy, a common phenomenon in COPD, is correlated with a decline in muscle mass. Muscle strength and endurance are the main physiologic prerequisites for skeletal muscle function; therefore, muscle atrophy is associated with the loss of this function. Schol et al4 showed that about 15% to 50% of patients with COPD have skeletal muscle atrophy, and a domestic survey indicates that up to 36% of COPD patients have this condition. This suggests that COPD is often accompanied by skeletal-muscle atrophy and that such patients require timely attention. Fat-free mass (FFM) is primarily composed of lean tissue and inorganic salts (ie, body weight minus fat), which can at least partly reflect the degree of an individual’s skeletal muscle atrophy. After adjusting for height and nutritional status in patients with COPD, several studies have found that the fat-free mass index (FFMI) is closely related to respiratory muscle function and exercise endurance.5
Anthropometric measurements in clinical settings can be useful tools for the assessment of nutrition. Currently, bioelectrical impedance analysis (BIA) and dual energy x-ray absorptiometry (DXA) are commonly used to measure body composition.6,7 However, their utilization has certain limitations: DXA may be costly and technically challenging, and these tools may be unavailable in the community.8 Because skeletal muscle atrophy is associated with a significantly higher risk of mortality in patients with COPD,9 there is a need for effective screening tools and early diagnosis in communities lacking resources such as BIA. Thus, the relationship between skeletal muscle mass and anthropometric indices (particularly CC) warrants further research.
A literature review10 found that skeletal-muscle atrophy was associated with dyspnea, exercise intolerance, and the likelihood of COPD. Over the long term, the risk of mortality in COPD patients at normal weight and with depleted FFM increases greatly. In other words, the prognoses of patients with skeletal muscle atrophy are not optimistic, and targeted intervention should be staged immediately to delay the atrophic process. However, previous domestic studies on skeletal muscle atrophy have mainly focused on its mechanism11 and the evaluation of patients’ nutrition;12 moreover, research subjects were mostly hospital inpatients or outpatients seeking medical care. In the developed eastern regions of China, many patients choose to receive healthcare in their homes. Additionally, owing to poorly equipped facilities and the lack of medications as well as, insufficient medical personnel, the primary health-care management of COPD in the community remains limited,13 and the prevalence of skeletal muscle atrophy among community residents with COPD is poorly understood. Therefore, this study aimed to explore differences in skeletal muscle atrophy between stable COPD patients and healthy controls and to assess the relationship between CC and skeletal muscle atrophy.
Materials and Methods
Study Design and Populations
Between September 2018 and September 2020, a total of 183 (123 with COPD and 60 controls) participants were recruited from communities near the First Affiliated Hospital of Soochow University in Suzhou City, East China; they were randomly recruited by posting flyers on bulletin boards and distributing flyers in communities as well as from data collected at the hospital. COPD was defined by post-bronchodilator fixed criteria (FEV1/FVC <0.7) per the Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (GOLD) guidelines.1 Stability was defined as the absence of exacerbation of COPD over the previous 30 days. Exclusion criteria were (1) cardiovascular disease, (2) neuromuscular impairment, (3) psychiatric disease, or (4) osteoarticular disorders. We recruited control subjects through advertisements. Inclusion criteria for controls were (1) post-bronchodilation FEV1/FVC≥0.7; (2) age matching with COPD patients; (3) willingness to participate in this study and to sign the informed consent. The exclusion criteria for controls were the same as for COPD. All procedures were approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (2017022), and all patients provided written informed consent. The study protocols were conducted in accordance with the principles outlined in the Declaration of Helsinki of the World Medical Association.
Data Collection
After informed consent had been obtained, a total of 123 patients and 60 controls were evaluated based on a self-designed questionnaire to collect their basic information (age, gender, body weight, height, body mass index (BMI), smoking status and living arrangements). Participants were consecutively enrolled in outpatient clinics and completed the relevant data collection examinations (spirometry, functional exercise capacity, body composition, and handgrip strength [HGS]).
Subjects were asked to keep their stomachs empty for 2 h before body composition measurement. After the subjects had removed mobile phones and voided their bladders to reduce unnecessary errors, they were instructed to measure their body composition using a Multifrequency Body Composition Analyzer BCA-2A (Tsinghua Tongfang Co., Ltd., Beijing, China).14 Furthermore, subjects were asked to stand barefoot on the metal platform, holding an electrode handle at a 15° angle from the body to remain upright with the arms abducted. During the test, the subjects were instructed to stay still for 2 min. We calculated FFMI by the following equation:
In accordance with the standard set by the General Administration of Sport of China, we tested the dominant hands of subjects using a dynamometer (YILIAN, BCA-2A, Shanghai, China). Three measurements with 1-min rest intervals were recorded, and the highest value was analyzed.
Using a nonelastic tape, we measured the calf circumference (CC) of each subject’s dominant leg to about 0.1 cm while the subject sat with the knee of that leg at a 90° angle and the soles flat on the floor. The tape was moved along the thickest part of the calf without compressing the subcutaneous tissue. We calculated the average of two measurements for our analysis.
Functional-exercise capacity was evaluated by the 6-min walking distance (6MWD) test in accordance with ATS guidelines. The subjects were instructed to complete the test within 6 min at maximal gait. If significant discomfort such as chest pain or unbearable dyspnea occurred, the test was terminated.
Sample Size
Assuming a two-sided significance level of 5% and 90% power, a sample size of 55 subjects for each group was needed for a paired data comparison with an approximate 10% dropout rate. Then, to calculate the sample size for multiple linear regression, we estimated that multiple correlation squared of 0.50, 90% power, and α = 5%; this led us to a sample size of 110 COPD patients. Finally, 123 COPD and 60 non-COPD controls were enrolled.
Statistical Analyses
Results were expressed as mean ± standard deviation (SD) or percentage. We analyzed the data using SPSS statistical software version 20 (IBM Corp., Armonk, NY). Data were assessed using Kolmogorov–Smirnov tests for normality. We examined normally distributed data using the independent-sample t-test or one-way analysis of variance (ANOVA). Categorical data were analyzed using a chi-squared test or Fisher’s exact test. To analyze possible relationships among the different parameters, we calculated Pearson’s or Spearman correlation coefficients. A linear stepwise-regression model was applied to the data to reveal which variables contributed to the variation in FFMI in patients with stable COPD.
Results
As shown in Table 1, COPD patients did not differ from controls in age, gender, BMI, smoking habits, or living arrangements, but COPD patients presented with lower FFM, FFMI, and 6MWD (P = 0.007, P = 0.020 and P < 0.001, respectively); patients’ HGS was lower than that of controls. However, HGS tended toward statistical significance (P = 0.064).
 |
Table 1 Comparison of Demographics and Baseline Characteristics Between the Two Groups |
We assessed FFMI according to each objective variable by sorting COPD patients into different groups (Table 2). There were no significant differences between groups in educational level, marital status, smoking status, or course of COPD. However, patients with lower FFMI tended to be older, female, retired, underweight, and with more dyspnea.
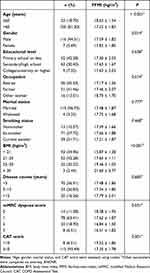 |
Table 2 Fat-Free Mass Index Across Different Demographic Characteristics |
As shown in Table 3, FFMI correlated positively with FEV1%, FEV1/FVC, BMI, HGS, and CC and negatively with the modified Medical Research Council (mMRC) dyspnea scale and COPD Assessment Test (CAT) scores. However, 6MWD did not correlate with FFMI in this study.
 |
Table 3 Correlations Between Fat-Free Mass Index and the Variables Studied in COPD Patients (n = 123) |
The results of the forward stepwise linear-regression analysis are shown in Table 4. In the multivariate analyses, approximately 80% of the variation in FFMI was accounted for when age, gender, BMI, FEV1%, and CC were combined into a multiple regression model.
 |
Table 4 Multiple Regression Analysis of Determinants of Fat-Free Mass Index in COPD Patientsa |
Discussion
The results of this study suggest that impaired skeletal muscle was more common in COPD patients than controls. We enrolled 123 patients with COPD to investigate the factors associated with the parameters of FFMI, and multiple regression analysis showed that BMI, gender, age, lung function and CC were significantly correlated with FFMI.
First—as assessed by FFM, FFMI, and 6MWD—there was a significant difference in skeletal muscle atrophy between COPD patients and healthy controls; this was in accordance with the findings of van de Bool et al15 Owing to chronic airway inflammation, COPD is often accompanied by long-term low levels of systemic inflammation, which induces changes in the skeletal-muscle fibers (mainly from fiber I to fiber II). However, the increase in the proportion of type II fiber, which is dominated by anaerobic metabolism and more prone to cause fatigue, contributes to a decrease in muscle strength and endurance. In addition, related mediators stimulated by chronic hypoxia in COPD patients can reduce the oxygen content in muscle cells and oxygen transport capacity, leading to changes in muscle structure. Meanwhile, as respiratory symptoms increase, patients typically become more sedentary to avoid dyspnea, and this accelerates muscle wasting.16 Indeed, multiple factors involved in muscle atrophy interact with other factors,17 resulting in a decline in skeletal muscle mass and function compared with controls.
One recent study reported that muscle mass decreases with aging;18 this is in line with our findings, which indicate that muscle mass tends to increase in younger individuals. Aging is associated with decreased daily activity and, in myocytes, with reductions in oxidase and mitochondrial density.19 Harmful substances that accelerate apoptosis and muscle atrophy are released by this process. In addition, gender plays a role in muscle atrophy;20 this could be because aging women tend to have more fat and lower skeletal-muscle mass, which makes muscle atrophy in female patients more obvious.
Our results suggest that FFMI plays an important role in the progression of COPD. The airflow limitation is common in COPD patients and has significant implications when the prognosis of COPD is evaluated. A previous study,21 in accordance with our findings, has shown that FFMI has a positive association with FEV1%. As a frequently reported symptom, dyspnea is thought to be associated with multiple outcomes. To prevent dyspnea caused by exercise, COPD patients enter a feedback loop of skeletal muscle deconditioning, breathlessness, and inactivity.22 Therefore, it is essential for patients with stable COPD to improve the management of their disease and delay its procession.23 We also found a significant correlation between the two groups divided by CAT score = 10, which indicated that skeletal-muscle mass was normal in patients who were in the early asymptomatic stage of COPD or who had fewer symptoms. This is why current pulmonary-rehabilitation programs are primarily targeted at patients with moderate to severe COPD. Furthermore, another study24 has confirmed that prophylactic intervention for patients with early asymptomatic or less symptomatic COPD can ameliorate the decline in lung function. In the long run, the level of skeletal-muscle mass is also effectively controlled, which can yield clinical benefits.
In this study, patients with lower BMI were likely to experience a decrease in muscle mass. Although previous studies25 have also found that underweight is a risk factor in the progression of COPD, the definition of underweight in COPD patients is still unclear. A relevant study26 showed that the risk of mortality significantly increased when patients had a BMI below 20 or 21 kg/m2; therefore, we adopted BMI below 21 kg/m2 as our cutoff. Additionally, 20% to 30% of COPD patients with normal BMI experience a decline in skeletal muscle mass.27 This suggests that BMI can be used only as an indicator of body weight change and is inappropriate for evaluating skeletal muscle mass, especially in COPD patients with a normal BMI.
Anthropometry is a convenient and noninvasive method to assess the size and composition of the human body, thus being suitable for nutritional assessment in clinical settings. As a common tool, the CC is also used to evaluate nutrition in clinical settings. Previous studies have shown that skeletal muscle mass in the lower extremities accounts for about 30% of total body muscle mass and has a lower fat content than in other parts.28,29 As the widest region of the leg and consistent with our findings, the CC is closely correlated with the skeletal muscle mass.30 As shown in Table 3 and Table 4, CC is a statistically significant factor of skeletal muscle mass (β = 0.457, P < 0.001). Thus, to some extent, CC can reflect skeletal muscle atrophy in patients with COPD. The muscles of the leg constitute an important weight-bearing group that is critical to many daily activities, such as walking; minor changes to these can directly or indirectly affect the patient’s level of self-care and quality of life.31 Patients suffering from serious illnesses subconsciously choose less challenging lifestyles. Therefore, their leg muscles appear atrophied, and their loss of volume can manifest as a reduction in CC. This makes CC a simple marker for health-care workers, especially outside the hospital, for detecting skeletal muscle impairment in patients with COPD. Future studies should calculate an optimal cutoff value for the quicker and easier use of the CC in clinical practice.
The present study had certain limitations. First, the sample size, especially for women, was small. Therefore, further studies with larger sample sizes are needed to confirm our data. Second, few women were enrolled in this study despite the absence of gender-related criteria; therefore, we were unable to analyze the FFMI based on gender. This may be related to the gender distribution of the COPD population in the region. In general, future work should consider the effect of gender in studies of patients with COPD. Third, although we found the community-based evaluation of CC to be useful, the specific cutoff values remain to be explored.
Conclusion
This study demonstrated the role of impaired skeletal muscle mass—including FFM, FFMI, and functional-exercise capacity assessed by 6MWD—in patients with COPD compared with paired controls. Multiple linear regression analysis showed that BMI, age, gender, FEV1%, and CC were significantly correlated with FFMI. In the future, we should concentrate on the role of community-based evaluation of calf circumference to detect skeletal muscle impairment in patients with COPD; this parameter might be especially relevant for health-care workers outside the hospital.
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript. Qian Zhao and Mei-e Niu are equal corresponding authors.
Funding
This research was supported by National Natural Science Foundation of China (No.72204182), the Suzhou Livelihood Science and Technology Project Fund [No. SYS2020110] and the Nursing Society Talents “Seedling” Project Fund (No.SHQM202301).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Agusti A, Beasley R, Celli B, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease [EB/OL]; 2022. Available from: https://goldcopd.org/2023-gold-report-2/.
2. Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46(5):705–716. doi:10.1016/0092-8674(86)90346-6
3. Abdulai RM, Jensen TJ, Patel NR, et al. Deterioration of limb muscle function during acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197(4):433–449. doi:10.1164/rccm.201703-0615CI
4. Schols AM, Soeters PB, Dingemans AM, et al. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis. 1993;147(5):1151–1156. doi:10.1164/ajrccm/147.5.1151
5. Schols AM, Wouters EF, Soeters PB, et al. Body composition by bioelectrical-impedance analysis compared with deuterium dilution and skinfold anthropometry in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 1991;53(2):421–424. doi:10.1093/ajcn/53.2.421
6. Chen LK, Woo J, Assantachai P, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300–307.e2. doi:10.1016/j.jamda.2019.12.012
7. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi:10.1093/ageing/afy169
8. Yamada Y, Nishizawa M, Uchiyama T, et al. Developing and validating an age-independent equation using multi-frequency bioelectrical impedance analysis for estimation of appendicular skeletal muscle mass and establishing a cutoff for sarcopenia. Int J Environ Res Public Health. 2017;14(7):809. doi:10.3390/ijerph14070809
9. Xu J, Wan CS, Ktoris K, et al. Sarcopenia is associated with mortality in adults: a systematic review and meta-analysis. Gerontology. 2022;68(4):361–376. doi:10.1159/000517099
10. Vestbo J, Prescott E, Almdal T, et al. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med. 2006;173(1):79–83. doi:10.1164/rccm.200506-969OC
11. L Y, W-G X. Skeletal muscle dysfunction and oxidative stress in patients with chronic obstructive pulmonary disease. Int J Respir Pulm Med. 2010;30:294–297.
12. L K-Y, M L-J, Q Y. Review on the mechanism of skeletal muscle loss for COPD patients with malnutrition. Chin J Respir Crit Care Med. 2013;12:313–315.
13. M MC, K LY, Z JX, et al. Current situation and prospect of chronic obstructive pulmonary disease community management in China. Chin Gen Pract. 2020;23(3):251–256.
14. Xue C, Liu Y, Wang J, et al. Consumption of medium- and long-chain triacylglycerols decreases body fat and blood triglyceride in Chinese hypertriglyceridemic subjects. Eur J Clin Nutr. 2009;63(7):879–886. doi:10.1038/ejcn.2008.76
15. Van De Bool C, Gosker HR, Van Den Borst B, et al. Muscle quality is more impaired in sarcopenic patients with chronic obstructive pulmonary disease. J Am Med Dir Assoc. 2016;17(5):415–420. doi:10.1016/j.jamda.2015.12.094
16. Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428–1446. doi:10.1183/09031936.00150314
17. Sepúlveda-Loyola W, De Castro LA, Matsumoto AK, et al. NOVEL antioxidant and oxidant biomarkers related to sarcopenia in COPD. Heart Lung. 2021;50(1):184–191. doi:10.1016/j.hrtlng.2020.06.001
18. Buford TW, Cooke MB, Manini TM, et al. Effects of age and sedentary lifestyle on skeletal muscle NF-kappaB signaling in men. J Gerontol a Biol Sci Med Sci. 2010;65(5):532–537. doi:10.1093/gerona/glp196
19. Lexell J, Downham D. What is the effect of ageing on type 2 muscle fibres? J Neurol Sci. 1992;107(2):250–251. doi:10.1016/0022-510X(92)90297-X
20. Caram LM, Ferrari R, Bertani AL, et al. Smoking and early COPD as independent predictors of body composition, exercise capacity, and health status. PLoS One. 2016;11(10):e0164290. doi:10.1371/journal.pone.0164290
21. Mason SE, Moreta-Martinez R, Labaki WW, et al. Respiratory exacerbations are associated with muscle loss in current and former smokers. Thorax. 2021;76(6):554–560. doi:10.1136/thoraxjnl-2020-215999
22. Donaldson AV, Maddocks M, Martolini D, et al. Muscle function in COPD: a complex interplay. Int J Chron Obstruct Pulmon Dis. 2012;7:523–535. doi:10.2147/COPD.S28247
23. Schneider LP, Sartori LG, Machado FVC, et al. Physical activity and inactivity among different body composition phenotypes in individuals with moderate to very severe chronic obstructive pulmonary disease. Braz J Phys Ther. 2021;25(3):296–302. doi:10.1016/j.bjpt.2020.07.005
24. Zhou Y, Zhong NS, Li X, et al. Tiotropium in early-stage chronic obstructive pulmonary disease. N Engl J Med. 2017;377(10):923–935. doi:10.1056/NEJMoa1700228
25. Wilson DO, Rogers RM, Wright EC, et al. Body weight in chronic obstructive pulmonary disease. The national institutes of health intermittent positive-pressure breathing trial. Am Rev Respir Dis. 1989;139(6):1435–1438.
26. Cao C, Wang R, Wang J, et al. Body mass index and mortality in chronic obstructive pulmonary disease: a meta-analysis. PLoS One. 2012;7(8):e43892. doi:10.1371/journal.pone.0043892
27. Beijers R, Van De Bool C, Van Den Borst B, et al. Normal weight but low muscle mass and abdominally obese: implications for the cardiometabolic risk profile in chronic obstructive pulmonary disease. J Am Med Dir Assoc. 2017;18(6):533–538. doi:10.1016/j.jamda.2016.12.081
28. Real GG, Frühauf IR, Sedrez JHK, et al. Calf circumference: a marker of muscle mass as a predictor of hospital readmission. JPEN J Parenter Enteral Nutr. 2018;42(8):1272–1279. doi:10.1002/jpen.1170
29. Pagotto V, Santos KFD, Malaquias SG, et al. Calf circumference: clinical validation for evaluation of muscle mass in the elderly. Rev Bras Enferm. 2018;71(2):322–328. doi:10.1590/0034-7167-2017-0121
30. Tresignie J, Scafoglieri A, Pieter Clarys J, et al. Reliability of standard circumferences in domain-related constitutional applications. Am J Hum Biol. 2013;25(5):637–642. doi:10.1002/ajhb.22423
31. Adami A, Corvino RB, Calmelat RA, et al. Muscle oxidative capacity is reduced in both upper and lower limbs in COPD. Med Sci Sports Exerc. 2020;52(10):2061–2068. doi:10.1249/MSS.0000000000002364
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.