Back to Journals » Infection and Drug Resistance » Volume 15
Impacts of Corticosteroid Therapy at Acute Stage of Hospital-Onset Clostridioides difficile Infections
Authors Lee CC , Lee JC, Chiu CW, Tsai PJ , Ko WC , Hung YP
Received 9 June 2022
Accepted for publication 7 September 2022
Published 10 September 2022 Volume 2022:15 Pages 5387—5396
DOI https://doi.org/10.2147/IDR.S377967
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Ching-Chi Lee,1,2,* Jen-Chieh Lee,2,* Chun-Wei Chiu,3 Pei-Jane Tsai,4– 6 Wen-Chien Ko,2,7 Yuan-Pin Hung2,3,7,8
1Clinical Medicine Research Center, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, 704, Taiwan; 2Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, 704, Taiwan; 3Department of Internal Medicine, Tainan Hospital, Ministry of Health and Welfare, Tainan, 700, Taiwan; 4Department of Medical Laboratory Science and Biotechnology, College of Medicine, National Cheng Kung University, Tainan, 704, Taiwan; 5Department of Pathology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan; 6Centers of Infectious Disease and Signaling Research, National Cheng Kung University, Tainan, Taiwan; 7Department of Medicine, College of Medicine, National Cheng Kung University, Tainan, 704, Taiwan; 8Department of Microbiology & Immunology, College of Medicine, National Cheng Kung University, Tainan, Taiwan
*These authors contributed equally to this work
Correspondence: Yuan-Pin Hung, Department of Internal Medicine, Tainan Hospital, Ministry of Health and Welfare, Tainan, 700, Taiwan, Email [email protected] Wen-Chien Ko, Department of Medicine, College of Medicine, National Cheng Kung University, Tainan, 704, Taiwan, Email [email protected]
Introduction: The influence of corticosteroid therapy before or after the onset of Clostridioides difficile infections (CDIs) on the clinical outcomes of adults with hospital-onset CDIs was investigated.
Materials and Methods: A clinical study was conducted on the medical wards of a teaching hospital between January 2013 and April 2020. Adults (aged ≥ 20 years) with hospital-onset CDIs (ie, symptom onset at least 48 hours after hospitalization) were included. “Corticosteroid therapy during acute CDIs” was defined as the receipt of a corticosteroid at the prednisolone equivalent (PE) dose of ≥ 10 mg for at least 48 hours within one week after the CDI diagnosis. “Prior corticosteroid exposure” was defined as the receipt of a corticosteroid at the PE dose of ≥ 5 mg PE for at least 48 hours within one month before the CDI diagnosis.
Results: Of the 243 adults with hospital-onset CDIs, patients (44, 18.1%) who received corticosteroid therapy during acute CDIs were more likely to have prior corticosteroid exposure (86.4% vs 11.9%, P < 0.001) and CDI episodes in intensive care units (31.8% vs 10.8%, P =0.001). Of note, a crucial association between corticosteroid therapy during acute CDIs and CDI recurrence was evidenced (13.6% vs 1.5%, P =0.002). Prior corticosteroid exposure was not associated with favorable CDI outcomes in terms of successful treatment (78.3% vs 74.9%, P =0.89), in-hospital crude mortality (17.4% vs 24.0%, P =0.61), or CDI recurrence (4.3% vs 5.3%, P = 1.00). However, for 177 patients without prior corticosteroid exposure, corticosteroid therapy during acute CDIs was linked to a higher proportion of CDI recurrence (33.3% vs 5.3%, P =0.046).
Conclusion: Corticosteroid therapy during acute CDIs might impact the recurrence of CDIs, particularly in those with a lack of prior corticosteroid exposure.
Keywords: steroid, prednisolone, Clostridioides difficile, diarrhea, recurrence, intensive care unit, immunosuppression
Introduction
Clostridioides difficile is well known to cause gastrointestinal infections, ranging from mild diarrhea to pseudomembranous colitis or toxic megacolon.1–3 Because innate immunity plays a crucial role in the occurrence and development of C. difficile infections (CDIs),1,4 impaired host immunity, such as the presence of hematological malignancy or polymorphisms of immune genes (such as IL-8 or toll-like receptors), is linked to the increased incidence and recurrence rates of CDIs.1,4,5
The association between immunosuppressant drugs and the occurrence or development of CDIs is ambiguous and seems to be one body two sides.6–12 In the past, recent immunosuppressant usage (within six months) has been recognized as an independent risk factor for asymptomatic carriage of C. difficile.7 Moreover, postoperative immunosuppressant usage has been regarded as a higher risk factor for the development of CDIs among patients who receive metabolic and bariatric surgeries.9 Nevertheless, it has been evident that numerous inflammatory responses in patients with CDIs can critically result in tissue damage and thus unfavorable outcomes,11 and treatment with oral immunosuppressants, such as a peroxisome proliferator-activated receptor (PPAR)-γ agonist, apparently ameliorated CDI colitis in a mouse model.10 Moreover, inhibiting the inflammatory process of inflammasome activation and IL-1β secretion could reduce the tissue damage caused by C. difficile toxins.12 In general the potential benefit of immunosuppressant therapy in CDIs has achieved little consensus because immunosuppressants can predispose patients to CDIs, but it may be evident in preventing tissue damage and diminishing its symptoms.
Corticosteroids are clinically adopted for their anti-inflammatory and immunosuppressive properties and have been shown to be associated with the asymptomatic carriage of C. difficile7 and the occurrence8 or recurrence6 of CDIs. However, the evidence detailing the efficacies of corticosteroid therapy in patients experiencing acute CDIs is limited. In this study, we aimed to investigate the prognostic effect of corticosteroid therapy in the acute stage of CDIs and/or previous corticosteroid exposure prior to CDI episodes among hospitalized adults.
Materials and Methods
Study Population and Sites
A retrospective clinical study was performed in the medical wards of the Tainan Hospital, Ministry of Health and Welfare, a regional hospital in southern Taiwan, between January 2013 and April 2020. The study was approved by the Institutional Review Board of National Cheng Kung University Hospital, Taiwan (approval number: B-ER-103-098). Because of a retrospective review of medical records, no specific ethical concerns, and minimal safety risk, the study was permitted to waive informed consent in compliance with the Declaration of Helsinki from the legally authorized representatives. The confidentiality of patient data was cautiously maintained.
Of patients diagnosed with CDIs during the study period, non-hospitalized patients, those aged < 18 years, and those with a lack of complete clinical information or certain survival at discharge (ie, transferred from other hospitals) were excluded. Adults (aged ≥ 20 years) with hospital-onset CDIs (ie, symptom onset at least 48 hours after hospitalization) were included.
Collection of Clinical Data
Clinical information, in terms of patient demographics (sex, age, and comorbidities), laboratory data and locations of CDI episodes (ward or intensive care unit [ICU]), severity and treatment response of CDIs, and patient outcomes, was captured from the electronic medical records using a predetermined record form. The type and dosing of medications prescribed within one month before the CDI diagnosis, including antibiotics, proton pump inhibitors, histamine 2 (H2) receptor antagonists, and corticosteroids, were also recorded. Information detailing the colonization or infection of vancomycin-resistant enterococci (VRE) during hospitalization was also recorded.
Definition
CDI was diagnosed as the presence of unexplained diarrhea and a positive result of glutamate dehydrogenase (GDH) and toxin A/B using an enzyme immunoassay (Abbott, Santa Clara, USA) in fecal samples or the detection of tcdB-carrying C. difficile isolates in stool culture.13 Diarrhea was defined as at least three unformed bowel movements per day for at least 48 hours. Fecal samples were sent for C. difficile cultures in plates of cycloserine-cefoxitin-fructose agar following the discretion of attending physicians and were incubated anaerobically for 24 to 48 hours. A multiplex polymerase chain reaction (PCR) was used to detect tcdA, tcdB, cdtA, cdtB, and tcdC deletions in C. difficile isolates, as described previously.14
CDI diagnosed after 48 hours of hospitalization was regarded as hospital-onset CDI. Corticosteroid therapy during acute CDIs was defined as the receipt of a corticosteroid at the prednisolone equivalent dose of ≥10 mg for at least 48 hours within one week after the CDI diagnosis.15 Prior corticosteroid exposure was defined as the receipt of a corticosteroid at the prednisolone equivalent dose of ≥5 mg for at least 48 hours within one month before the CDI diagnosis. The comorbidity of chronic kidney disease was defined as an estimated glomerular filtration rate of < 60 mL/min/1.73 m2 for at least three months.16 Other comorbidities were defined as described previously.17
Severity and Treatment Response of CDIs
The severity of CDIs was graded based on the Clinical Practice Guidelines of the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) issued in 2017.13 Briefly, patients with a leukocyte count ≥ 15,000 or serum creatinine > 1.5 mg/dL were considered to have a severe CDI, whereas those who lacked the above criteria were categorized to be nonsevere.18 Based on a modified definition,19 antimicrobial treatment for CDIs was successful if the following three criteria were fully met: the resolution of diarrhea within six days of therapy, a lack of the need to change therapeutic regimens, and survival at the end of therapy. The occurrence of relapsing diarrhea in combination with the detection of C. difficile toxin or tcdB-carrying C. difficile in unformed stools at least three weeks after the initial treatment success was regarded as the recurrence of CDIs.19 In addition to in-hospital mortality, CDI outcomes included treatment success and recurrence.
Statistical Analysis
Statistical analysis was performed using statistical software (IBM SPSS, version 22.0). Continuous and categorical variables were expressed as the means ± standard deviations and numbers (percentages), respectively. The χ2 test or Fisher’s exact test was used for categorical variables, and Student’s t test was used for continuous variables. To avoid the interaction of confounding factors, subgroup analysis after stratification was adopted. A two-tailed P value < 0.05 was considered statistically significant.
Results
Overall Population
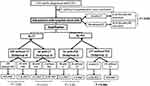
Of the 243 patients diagnosed as having CDIs, 238 (97.9%) patients were hospital-onset and included for the analysis (Figure 1). Their average age (± standard deviation) was 75.7 ± 12.7 years, and 47.4% of them were male (Table 1). A total of 44 (18.5%) patients had ever received corticosteroid therapy after the CDI diagnosis. Of these 44 patients, the types of corticosteroid therapy administered in acute CDIs included prednisolone (PE: 15–30 mg) in 21 patients, hydrocortisone (PE: 25–75 mg) in 19 patients, and methylprednisolone (prednisolone equivalent: 30–312.5 mg) in 4 patients for 3–7 days. Of the 238 patients, those who received corticosteroid therapy during acute CDIs were more likely to have prior corticosteroid exposure before the CDI diagnosis (86.4% vs 11.9%, P < 0.001) and episodes of CDIs in the ICU (31.8% vs 10.8%, P = 0.001) than those without corticosteroid therapy (Table 1). However, there were no significant differences between the two patient groups in the majority of patient demographics and clinical characteristics in terms of sex, age, underlying diseases, colonization of vancomycin-resistant enterococci (VRE), prior medication exposure before the CDI diagnosis, laboratory abnormalities during the CDI diagnosis, and severity of CDIs (Tables 1 and 2). Of note, corticosteroid therapy during acute CDIs was frequently associated with oral vancomycin therapy for CDIs (13.6% vs 1.5%, P = 0.002) and the subsequent recurrence of CDIs (11.4% vs 5.2%, P = 0.003) compared to those without corticosteroid therapy (Table 2). Only 172 patients with C. difficile strains available from stool culture; and of them infected by tcdC deletion C. difficile strains was not correlated with steroid use (Table 2).
Subgroup Analyses
To achieve the stratification analyses, the eligible 238 patients were categorized into four subgroups (Figure 1). For 177 patients without prior corticosteroid exposure (subgroup 1), the receipt of corticosteroid therapy during acute CDIs was associated with a higher likelihood of blood leukocyte count ≥15,000 cells/mL (66.7% vs 23.4%, P = 0.03) and severe CDIs (66.7% vs 32.2%, P = 0.10), although the latter variable was not statistically significant (Table 3). However, focusing on 61 patients without prior corticosteroid exposure (subgroup 2), the receipt of corticosteroid therapy during acute CDIs was related to a higher likelihood of CDI recurrence (33.3% vs 5.3%, P = 0.046).
 |
Table 3 Clinical Characteristics and Outcomes of 177 Patients Without Prior Corticosteroid Exposure, Stratified by Corticosteroid Therapy During Acute Clostridioides difficile Infections (CDIs) |
Of 44 patients receiving corticosteroid therapy during acute CDIs (subgroup 3), patients who had prior corticosteroid exposure had lower proportions of metronidazole therapy for CDIs (44.7% vs 100%, P=0.02) and more frequent treatment success (81.6% vs 33.3%, P = 0.03) than those without prior corticosteroid exposure (Table 4). However, there were no differences in numerous clinical variables in terms of patient demographics (gender, age, and comorbidities), laboratory abnormalities, or the severity of CDIs (Table 4). Of 33 patients with treatment success, the leading antimicrobial administered was oral metronidazole (13 patients, 39.4%), followed by oral vancomycin (1, 3.0%), and the remaining 19 (57.6%) discontinued offending antibiotics and symptomatic treatment.
Of 194 patients who did not receive corticosteroid therapy during acute CDIs (subgroup 4), those with prior corticosteroid exposure were more likely to have the comorbidity of chronic obstructive pulmonary disease (30.4% vs 6.4%, P = 0.002) and less likely to have the comorbidity of congestive heart failure (0% vs 16.4%, P = 0.05) than those without prior corticosteroid exposure (Table 5). However, there were no differences in numerous outcomes assessed for CDIs, namely, treatment success, in-hospital crude mortality, and recurrence rates (Table 5).
Discussion
Although corticosteroid use has been regarded as a risk factor for C. difficile colonization7 or infection,8 its anti-inflammatory property might be beneficial in preventing the tissue damage caused by CDIs.11 In fact, amelioration of the inflammatory responses by immunosuppressant drugs might have protective effects during acute CDIs.10,12 However, such beneficial effects of immunosuppressant drugs have been shown in animal models of CDI and remain clinically undefined. Our principal finding is that the receipt of corticosteroid therapy during acute CDIs (ie, within seven days after the CDI diagnosis) was linked to a higher chance of CDI recurrence overall for adults and those without corticosteroid exposure prior to the CDI diagnosis. However, corticosteroid therapy during acute CDIs did not result in an unfavorable prognosis, as indicated by the crude in-hospital mortality rate herein. As mentioned, several host factors affect immunity status, such as age, underlying hematologic malignancy, and immune gene polymorphisms, and influence protective immunity during CDIs.1,4,5 In the clinical study in Iran, the receipt of anti-tumor necrosis factor (TNF)-containing regimens in combinations with other immunosuppressive medications may impact the susceptibility to CDI among patients with underlying inflammatory bowel disease.20 Immunosuppressant drugs might predispose relatively non-immunocompetent patients to develop, instead of protect from, CDI.20 We believe that the complexity of clinical settings among the cases of CDI might be one of the reasons why the prognostic impact of corticosteroid use was not evident in our study. Of importance, clinicians should pay attention to the adverse effects of corticosteroid therapy during acute CDIs on recurrence.
Our included patients receiving corticosteroid therapy during acute CDIs were more likely to have prior corticosteroid exposure and episodes of CDIs in the ICU setting. Although the detailed reasons that corticosteroids were administered for those with CDIs were not analyzed, we believe that corticosteroid therapy is a common strategy for ICU patients, in accordance with numerous clinical demands.21 Herein, corticosteroid therapy was maintained or added after the CDI diagnosis in the majority (38 patients, 62.3%) of 61 patients (subgroup 2) who received corticosteroid therapy before the CDI diagnosis. Furthermore, even among patients not receiving corticosteroid therapy during acute CDIs (subgroup 4), patients having the comorbidity of chronic obstructive pulmonary disease characteristically reasonably had a higher proportion of prior corticosteroid exposure, and prior corticosteroid exposure heralded no prognostic significance in terms of treatment success, in-hospital mortality, or recurrence of CDI in the present study.
However, of patients without prior corticosteroid exposure (subgroup 1), corticosteroid therapy during acute CDIs was significantly associated with the recurrence of CDI in the univariate analysis. Likewise, the use of steroids or other immunosuppressants within six months after surgery has been associated with an increased risk of recurrent CDIs.6 In the literature, patients in the ICU commonly receive corticosteroids,22 and they heralded a 5–15-fold increased rate of CDI recurrence compared with non-ICU patients.23 In our study, the reason for CDI recurrence was not analyzed and might be due to the critical illness or the side effects of corticosteroids.
There were some limitations in this study. First, corticosteroid therapy was arbitrarily defined as a prednisolone equivalent of at least 10 mg for more than 48 hours to analyze the anti-inflammatory influence of corticosteroids. Our definition was adopted according to the previously established definition in the study detailing hemato-oncologic diseases, not CDIs.15 Second, corticosteroid therapy was arbitrarily defined as corticosteroid prescription within one week after the CDI diagnosis herein. In the real world, the usage of corticosteroids might be continuous before the CDI diagnosis and/or beyond one week after the CDI diagnosis. Third, trivial effects of corticosteroid therapy during acute CDIs on treatment efficacy and patient survival might be related to the limited case number and statistical power. Finally, there was no correlation between CDIs due to C. difficile strains with tcdC deletion and steroid use in our study. However, the ribotypes of our clinical C. difficile strains were not determined, and thus the impact of C. difficile ribotypes on clinical outcomes of patients with CDI receiving steroid needs further evaluation.
In conclusion, adults experience hospital-onset CDIs. The impact of corticosteroid therapy during the acute stage on the recurrence of CDIs was evidenced in all patients and those with a lack of corticosteroid exposure prior to the CDI diagnosis. Although our findings underscore the adverse effects of corticosteroid therapy on patients experiencing CDIs, prospective studies designed to enroll larger populations are needed to ensure reliable conclusions.
Author Contributions
All authors made a significant contribution to the work reported, whether it was in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The present study was supported by research grants from the Ministry of Science and Technology, Taiwan (MOST 108-2321-B-006-004, 108-2320-B-006-043-MY3, 109-2314-B-006-089-MY3, 110-2320-B-006-024, and 110-2314-B-675-001), Ministry of Health and Welfare, Taiwan (MOHW 110-TDU-B-211-124003), and National Cheng Kung University Hospital, Tainan, Taiwan (NCKUH-11004029).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hung YP, Tsai CS, Tsai BY, et al. Clostridioides difficile infection in patients with hematological malignancy: a multicenter study in Taiwan. J Microbiol Immunol Infect. 2021;54(6):1101–1110. doi:10.1016/j.jmii.2021.02.002
2. Chiu CW, Tsai PJ, Lee CC, et al. Inhibition of spores to prevent the recurrence of Clostridioides difficile infection - A possibility or an improbability? J Microbiol Immunol Infect. 2021;54(6):1011–1017. doi:10.1016/j.jmii.2021.06.002
3. Lee CC, Lee JC, Chiu CW, et al. Clinical significance of toxigenic Clostridioides difficile growth in stool cultures during the era of nonculture methods for the diagnosis of C. difficile infection. Microbiol Spectr. 2021;9(2):e0079921.
4. Lai YH, Tsai BY, Hsu CY, et al. The role of Toll-Like receptor-2 in Clostridioides difficile infection: evidence from a mouse model and clinical patients. Front Immunol. 2021;12:691039.
5. DuPont HL, Garey K, Caeiro JP, Jiang ZD. New advances in Clostridium difficile infection: changing epidemiology, diagnosis, treatment and control. Curr Opin Infect Dis. 2008;21(5):500–507.
6. Messick CA, Hammel JP, Hull T. Risk factors that predict recurrent Clostridium difficile infections in surgical patients. Am Surg. 2017;83(6):653–659.
7. Eyre DW, Griffiths D, Vaughan A, et al. Asymptomatic Clostridium difficile colonisation and onward transmission. PLoS One. 2013;8(11):e78445.
8. Brown RJ, Raabe M, McCullough LD, Zhu L, Chokshi RV. Clostridium difficile infection does not impact outcomes in stroke patients. Neurologist. 2021;27(3):125–129.
9. Morales-Marroquin E, Xie L, Uppuluri M, Almandoz JP, Cruz-Munoz N, Messiah SE. Immunosuppression and Clostridioides (Clostridium) difficile infection risk in metabolic and bariatric surgery patients. J Am Coll Surg. 2021;233(2):223–231.
10. Viladomiu M, Hontecillas R, Pedragosa M, et al. Modeling the role of peroxisome proliferator-activated receptor γ and MicroRNA-146 in mucosal immune responses to Clostridium difficile. PLoS One. 2012;7(10):e47525. doi:10.1371/journal.pone.0047525
11. Donlan A, Petri WA
12. Ng J, Hirota SA, Gross O, et al. Clostridium difficile toxin-induced inflammation and intestinal injury are mediated by the inflammasome. Gastroenterology. 2010;139(2):
13. Loo VG, Bourgault AM, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365(18):1693–1703.
14. Hung YP, Cia CT, Tsai BY, et al. The first case of severe Clostridium difficile ribotype 027 infection in Taiwan. J Infect. 2015;70(1):98–101.
15. Skribek M, Rounis K, Afshar S, et al. Effect of corticosteroids on the outcome of patients with advanced non-small cell lung cancer treated with immune-checkpoint inhibitors. Eur J Cancer. 2021;145:245–254.
16. Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67(6):2089–2100.
17. Islam MM, Valderas JM, Yen L, Dawda P, Jowsey T, McRae IS. Multimorbidity and comorbidity of chronic diseases among the senior Australians: prevalence and patterns. PLoS One. 2014;9(1):e83783.
18. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):987–994.
19. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302–307.
20. Gholam-Mostafaei FS, Yadegar A, Aghdaei HA, Azimirad M, Daryani NE, Zali MR. Anti-TNF containing regimens may be associated with increased risk of Clostridioides difficile infection in patients with underlying inflammatory bowel disease. Curr Res Transl Med. 2020;68(3):125–130.
21. Somagutta MKR, Pormento MKL, Khan MA, et al. The Efficacy of vitamin C, thiamine, and corticosteroid therapy in adult sepsis patients: a systematic review and meta-analysis. Acute Crit Care. 2021;36(3):185–200.
22. Na SJ, Ha TS, Koh Y, et al. Characteristics and clinical outcomes of critically ill cancer patients admitted to Korean intensive care units. Acute Crit Care. 2018;33(3):121–129.
23. Jasiak NM, Alaniz C, Rao K, Veltman K, Nagel JL. Recurrent Clostridium difficile infection in intensive care unit patients. Am J Infect Control. 2016;44(1):36–40.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.