Back to Journals » Nature and Science of Sleep » Volume 11
Impact of traumatic brain injury on sleep: an overview
Authors Aoun R , Rawal H , Attarian H , Sahni A
Received 29 May 2019
Accepted for publication 7 August 2019
Published 19 August 2019 Volume 2019:11 Pages 131—140
DOI https://doi.org/10.2147/NSS.S182158
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Steven A Shea
Raissa Aoun,1 Himanshu Rawal,2 Hrayr Attarian,3 Ashima Sahni4
1Department of Neurology, Lebanese American University Medical Center – Rizk Hospital, Beirut, Lebanon; 2Department of Medicine, Medstar Union Memorial Hospital, Baltimore, MD, USA; 3Department of Neurology, Northwestern Memorial Hospital, Chicago, IL, USA; 4Division of Pulmonary, Critical Care, Sleep and Allergy, Department of Medicine, University of Illinois at Chicago, IL, Chicago, USA
Correspondence: Ashima Sahni
Division of Pulmonary, Critical Care, Sleep and Allergy, Department of Medicine, University of Illinois at Chicago (MC 719), Chicago, IL 60612, USA
Tel +312 355 4806
Email [email protected]
Abstract: Traumatic brain injury (TBI) is a global health problem that affects millions of civilians, athletes, and military personnel yearly. Sleeping disorders are one of the underrecognized sequalae even though they affect 46% of individuals with TBI. After a mild TBI, 29% of patients have insomnia, 25% have sleep apnea, 28% have hypersomnia, and 4% have narcolepsy. The type of sleep disturbance may also vary according to the number of TBIs sustained. Diffuse axonal injury within the sleep regulation system, disruption of hormones involved in sleep, and insults to the hypothalamus, brain stem, and reticular activating system are some of the proposed theories for the pathophysiology of sleep disorders after TBI. Genetic and anatomical factors also come to play in the development and severity of these sleeping disorders. Untreated sleep disturbances following TBI can lead to serious consequences with respect to an individual’s cognitive functioning. Initial management focuses on conservative measures with progression to more aggressive options if necessary. Future research should attempt to establish the effectiveness of the treatments currently used, as well as identify manageable co-existing factors that could be exacerbating sleep disorders.
Keywords: traumatic brain injury, neurobiology, sleep disorders, TBI, sleep
Introduction
Traumatic brain injury (TBI) is defined as a disruption in brain function due to the impact of contact forces including rapid acceleration, deceleration, or collision, manifesting as altered state of consciousness, neurological deficits, and/or amnesia.1 It is classified as mild, moderate, or severe depending on the presence and duration of the symptoms.2 TBI is a significant public health problem affecting civilians, athletes, and military personnel as a result of motor vehicle accidents, falls, contact sports, assaults, and explosions.3,4 Over the years, the rates of TBI in the US population have increased with more emergency room visits, driving the health care costs, though the rate of hospitalization and deaths has been steady.5 In fact, it is estimated that 1.5 million people in the US suffer a brain injury every year. Approximately, 80% of these injuries are classified as mild in severity.1
Mild TBI (mTBI), the most common form of TBI in high-impact sports,6 can lead to a wide array of cognitive, emotional, and somatic symptoms.7 The most frequently reported symptoms include fatigue, headaches, memory impairment, concentration deficits, and sleep-related problems.6 The majority of patients achieve complete resolution of symptoms quickly after a mTBI, but 10–20% may develop persisting symptoms.7 In fact, neuropsychological testing revealed persistent cognitive impairments in boxers following mTBI extending beyond the subjectively symptomatic period.6
Sleep disorders are underrecognized consequences of TBI despite their high prevalence. In general, around 46% of individuals have sleep disorders following TBI including sleep apnea, insomnia, post-traumatic hypersomnia, and narcolepsy.3
It is important to recognize and manage sleep disorders in TBI patients because untreated sleep disturbances translate to longer hospital stays, higher cost of rehabilitation, and more disability.8
The aim of this review is to discuss the pathophysiology of sleep disturbances after TBI, the various sleep disorders encountered, their management, and future directions.
Neurobiology of sleep
The suprachiasmatic nucleus in the hypothalamus is the master regulator of circadian rhythms. A cluster of neurons, known as the reticular activating system (RAS), expand into the hypothalamus from the tegmentum of the brainstem and play a crucial role in maintaining arousal. The network of neurons in the RAS process information from multiple projections in the brain through neurotransmitters and neuromodulations to control sleep and wakefulness.4
There are three distinct states of being: wakefulness, rapid eye movement sleep (REM) and non-rapid eye movement sleep (NREM). Monoaminergic and cholinergic system are predominantly wake-promoting systems, which also receive excitatory hypothalamic hypocretin/orexin input to promote wakefulness. GABAergic system is the important sleep-promoting system and is present in the brainstem, lateral hypothalamus, and preoptic area. REM sleep is primarily generated in the dorsolateral pons. Cholinergic activation promotes and maintains REM sleep.9
Sleep and wakefulness is a fine interplay between the homeostatic and circadian drive. Homeostatic drive is also called sleep drive, which increases continually during wakefulness and is reduced by sleeping. This drive is primarily regulated by adenosine through a partially understood mechanism that involves cortical neuronal nitric oxide (nNOS) neurons and neurokinin 1 receptors.10 On the other hand, the circadian rhythm is the alerting process driven by suprachiasmatic nuclei in the hypothalamus. It naturally dips in the late afternoon but is high during the day and low during the night. It is primarily regulated through the light-induced suppression and darkness-induced release of the pineal neurohormone melatonin.11 The integration of the two drives also takes place in the suprachiasmatic nucleus and may involve orexin A/hypocretin 1 input.12
Other neuropeptides involved in sleep–wake regulation include pituitary hormones, cytokines interleukin-1, interleukin-6, and tumor necrosis factor alpha13 as well as melanin-concentrating hormone (MCH). MCH coexists with GABA in hypothalamic neurons involved in REM control.14
Pathophysiology of sleep disturbance after TBI
Direct factors
Several theories have been proposed for the pathophysiology of sleep disorders after TBI. Diffuse axonal injury (DAI) occurring within the arousal and/or sleep regulation system has been implicated as a possible cause.15,17 DAI can occur across the spectrum of TBI severity and is considered the most important factor in determining morbidity in TBI patients.16 DAI leads to impairment in axonal membrane stability and intracellular function which results in signal disruption and accumulation of proteinaceous waste products.1 Moreover, the ruptured cell membranes release glutamate which provokes a toxic metabolic cascade of cell injury leading to acidosis and edema.18
Disruption of hormonal systems involved in sleep is another implicated cause of sleep disturbance after TBI. In research studies, TBI groups were found to have decreased levels of hypocretin, histamine, and melatonin in the CSF.15 Hypocretin and histamine are wake-promoting neurotransmitters; therefore, low CSF levels of these two are associated with hypersomnia.19,20 On the other hand, reduced melatonin is associated with decreased REM sleep in TBI.20 Studies showed that 95% of patients with acute TBI had low CSF hypocretin (less than 320 pg/mL),3 and autopsies of individuals who died from severe TBI showed a 41% reduction of histaminergic neurons in the tuberomammillary nucleus of the hypothalamus.19
Insults to key brain regions involved in sleep such as the hypothalamus, brain stem, and RAS also underpin sleep disturbances in TBI patients.3,20 Damage to the retino-hypothalamic tract, which coordinates the hypothalamic circadian pacemaker with the light–dark cycle, can lead to abnormally programmed circadian rhythms.20 Post-TBI animal models also show impairment in the expression of BMAL1 and Cry 1 genes indicating disruption in the circadian rhythm.21
There are also genetic factors that come to play in the development of sleeping disorders in mTBI patients. Per3 is one of the many polymorphic genes that are involved in the regulation of the circadian rhythm. Studies showed that carriers of Per3 have a shorter duration of sleep at 6 weeks post mTBI compared to baseline, and only Per3 noncarriers showed improvement in sleep quality with time.3
In addition, some craniofacial phenotypes may be particularly vulnerable to the development or worsening of sleep disorders. In acceleration–deceleration injuries, variations in the structure of the tentorial notch may impact the level of brainstem distortion. Also, people with certain anatomical features may be more prone to injure the pineal gland, resulting in dysregulation of melatonin homeostasis. This impairs adequate circadian activity and leads to sleep disturbances.22 Sleep-disordered breathing such as obstructive sleep apnea can also be a consequence of injury to upper respiratory muscles along with TBI. Supine sleep, weight gain, and medication – induced reduced muscle tone and respiratory depression further contribute to sleep-disordered breathing in TBI patients.3
Indirect factors
It is important to acknowledge the contribution of secondary factors like fatigue and depression after TBI to the development of sleep disturbances.15 Generalized anxiety disorder within 3 months of TBI has been consistently correlated to onset of insomnia.23 Moreover, people with TBI tend to use more medications than the general population such as antidepressants, analgesics, and sedative-hypnotics which can add to the sleep deficits observed.3,20 Other secondary complications following TBI, such as medical comorbidities, may also play a role in sleeping difficulties.20
To conclude, taken together, the risk for sleep disturbances in a patient with TBI is the product of several internal and external influences, all acting on a genetically determined substrate.22 Untreated sleep disturbances following TBI can lead to potentiation of the injury at cellular level, leading to cognitive dysfunction: Sleep is essential for the optimal functioning of the glymphatic system, which is the waste clearance pathway of the central nervous system.24 Therefore, sleep disruption may lead to reduced clearance and subsequently increased accumulation of proteinaceous waste products like p-tau and beta-amyloid. Also, in TBI patients, resulting astroglial scars and inflammation may further the damage to the glymphatic system and increase the buildup of these neurotoxins. Persistent accumulation of waste products may potentiate neurodegenerative processes like Alzheimer’s disease and chronic traumatic encephalopathy.18
Various sleep disorders encountered in TBI
Studies have shown that mild TBI is more strongly associated with sleep disturbances than moderate to severe TBI.2 This may be because individuals with mild TBI are more likely to report sleep disturbances than people with severe TBI who perceive sleep disturbances as minor compared to other cognitive sequelae.2 However, research has also proposed that the location of the lesion is more important than the severity of TBI in predicting sleep outcomes, where damage to the arousal/sleep regulation centers causes the most sleep disturbances.20
Nearly 50% of patients report sleep disturbances after injury (Table1). Actigraphy and polysomnography, which are objective measures of sleep, verify the subjective complaints reported.19 A meta-analysis reported that after a TBI, 29% of patients have insomnia, 25% have sleep apnea, 28% have hypersomnia, and 4% have narcolepsy.19
Acutely after a TBI, individuals can experience early phase symptoms like headaches, fatigue, amnesia, and sleep disruption. These symptoms resolve over a few weeks in the majority of people. However, 10–15% develop prolonged symptoms that affect the quality of life.1 Studies have shown that individuals with TBI have longer sleep onset latencies, shorter total duration of sleep, and more nighttime awakenings than controls. Also, TBI patients were found to spend less time in REM sleep. They report poor sleep quality, more daytime dysfunction, and the use of more sleep medication. This was corroborated by objective sleep measures like the Epworth Sleepiness Scale and PSQI.20
The type of sleep disturbance might vary according to the number of TBIs. Following a first TBI, the most commonly reported complaint is onset insomnia. Following an additional TBI, maintenance insomnia intensifies.2 In military personnel, the number of lifetime TBIs was found to be a significant predictor of insomnia severity even when controlling for depression, PTSD, and severity of concussion symptoms.2 In fact, insomnia is one of the most commonly reported symptoms among patients with TBI, and it is the second most documented sleep disturbance in people with TBI, after snoring.22 Prevalence rates of insomnia, which are estimated to range from 6% to 10% of the general population, reach 25–29% in patients with TBI.2
In a prospective study, TBI patients were recruited from rehabilitation facilities and underwent sleep studies at least 3 months post-injury. 6% of the TBI patients met the diagnostic criteria for narcolepsy, which is significantly higher than the prevalence of the diseases in the general population (0.056%).3 The prevalence of sleep-disordered breathing in TBI patients was found to be 23% which is also significantly higher than the general population. In rehabilitation centers, parasomnias – most commonly REM sleep behavior disorder – were the presenting complaint in 25% of patients with brain trauma.3
Increased sleep need after mTBI, also known as post-traumatic hypersomnia, affects around 20% of individuals after brain injury. It manifests as fatigue, longer sleep durations, or excessive daytime sleepiness. Several notions have been proposed as causative factors. The first hypothesis is that persistent pain is an important cause for both the development and maintenance of excessive sleep need.25 Decreased quality of life due to pain was significantly associated with sleep need exceeding eight hours per day as well as increased number of naps.25 This is because patients with pain were found to have increased ß power frequency – brain waves associated with active thinking and concentration – in the prefrontal and frontal cortices during NREM and REM sleep compared to mTBI patients with no pain, which could cause a relative state of “arousal” even when asleep. This impedes the restorative function of sleep leading to increased sleep need to compensate. Another hypothesis implicated the hypocretin system in the development of increased sleep need after brain injury. As mentioned previously, low levels of hypocretin have been found in the CSF of most TBI patients, which can explain increased sleepiness.25
Untreated sleep disorders impact sleep architecture. Moderate to severe TBI is characterized by increased slow wave sleep (SWS) and reduced stage 2 sleep, reflecting cortical reorganization and restructuring after injury. These alterations in sleep architecture have not been observed in mild TBI.1,19 Both obstructive sleep apnea and insomnia patients have reduced REM and slow wave sleep, but these tend to normalize with effective treatment.1,19
Impact on quality of life
Sleep disorders significantly impact quality of life and contribute to disability. Inadequate sleep can negatively affect rehabilitation and optimal recovery in inpatients with TBI, leading to poorer outcomes and cognitive dysfunction.26 Moreover, insomnia has been associated with increased disability and delayed recovery from brain injury in workers independent of age, gender, and other psychiatric co-morbidities. This was determined using the Sheehan disability scale (SDS), which measures items pertaining to impairment in work, social life, and family responsibilities.4 This emphasizes the importance of identifying and treating sleep disorders to improve quality of life and work-related outcomes.4
Diagnosis
It is important to start by ruling out modifiable causes of sleep disturbances like pain, bowel/bladder issues, and mood disturbances.8
Adequate management starts with accurate diagnosis. Subjective sleep symptoms in TBI patients can be unreliable; therefore, objective testing with polysomnography and MSLT are essential to diagnose sleep disorders like sleep apnea, narcolepsy, and post-traumatic hypersomnia.3 Actigraphy and sleep logs at home over several nights are also good ways of assessing sleep after TBI.3,15 The sleep lab can create a new and confounding environment for the individual, which makes actigraphy a useful alternative since it assesses sleep at home over several nights.3,15
Management
Conservative management
Initial management should focus on conservative measures with progression to more aggressive options if necessary.
Sleep hygiene
Sleep hygiene is the first factor to address, including avoiding stimulating activities before bed, fixed schedules, and avoiding late afternoon caffeine and alcohol. If the patient is not asleep within 30 mins of being in bed, he or she should engage in any stimulating activity in another room and return to bed only when drowsy.1,8 Regular aerobic exercise has also been shown to improve sleep quality in TBI patients.8
Cognitive behavioral therapy (CBT) is also a technique that helps alter a patient’s negative associations with sleep. Examples include relaxation training and CBT.8 CBT for insomnia (CBTi) is considered the first-line approach for management of insomnia. It works by targeting and altering dysfunctional beliefs and attitudes about sleep.3,27 It is associated with increased total sleep time and decreased sleep–onset latency as evidenced by objective sleep measurements like actigraphy and polysomnography,28 and it has been found to be superior to pharmacological therapy.3,8
Phototherapy or light exposure
A study also looked into the effect of ocular light exposure as a stimulant in the TBI population, and it showed reduced fatigue and daytime sleepiness with blue light therapy compared with the use of modafinil for 6 weeks.8 Some patients with TBI are very sensitive to light; therefore, avoidance of full spectrum bright light is advised.
Other non-pharmacotherapies
Osteopathic manipulative treatments (craniosacral therapy, visceral and neural manipulation) as well acupuncture also showed benefits in sleep quality for TBI patients.8
Pharmacotherapy
Pharmacotherapy should be initially attempted as a short-term treatment using the lowest effective dose and intermittently to avoid tolerance and dependence.
Pharmacotherapy is divided into three categories based on the predominant sleep symptomatology.
Insomnia
A commonly used drug to treat insomnia after head injury is trazodone, an antidepressant with both sedative and anxiolytic properties.3 Zolpidem, zaleplon, zopiclone, and eszopiclone are also non-benzodiazepine hypnotics that are frequently used.3 Common interventions used for insomnia are summarized in Table 2. If insomnia is due to PTSD, studies on veterans with mild TBI have shown that prazosin, a selective α-1 receptor antagonist, improves symptoms.8
 |
Table 1 Summary of sleep disorders in TBI patients and their management |
 |
Table 2 Insomnia management in TBI patients |
Post-traumatic hypersomnia/post-traumatic narcolepsy
Stimulant therapy would be warranted for post-traumatic hypersomnia. Positive results have been seen with modafinil. Increased aggression was noted in patients after initiation of methylphenidate, but beta blockers have shown some potential in mitigating drug-exacerbated aggression.29,30
Circadian rhythm disorders
Sleep agents like melatonin (0.5–3 mg/night), valerian root (300–600 mg per day), and L-tryptophan have been used, but studies have shown limited effectiveness in the TBI population.3,27 In fact, the management of circadian rhythm disorders in TBI is mostly the same as the general population except that prolonged hospitalization, decreased mobility, sedative effects of medications, and tube feeding with no proper light exposure could be challenging.31 Secondly, the reduction in the intrinsic melatonin secretion in patients with TBI may warrant higher doses of melatonin for management of circadian rhythm disorders.32
Revised strategies for managing various circadian rhythm disorders include a combination of light and melatonin therapy.33 In patients with advanced sleep–wake phase disorder, light therapy 2 hrs in the evening is recommended. Figure 1 demonstrates both the pretreatment and post-light therapy sleep–wake cycles (Figure 1). For delayed sleep–wake phase disorder, melatonin between 0.5–3 mg is recommended 5 hrs before habitual bedtime, as well as light therapy (at least 5000 lux) 30 mins to 2 hrs upon awakening. Figure 2 demonstrates both the pretreatment and post light and melatonin therapy sleep–wake cycles (Figure 2). It is difficult to treat patients with irregular sleep–wake phase disorder, and a trial of melatonin between 0.5 mg and 3 mg is suggested at bedtime, as well as light therapy during daytime along with structured daytime activities. Figure 3 demonstrates both the pretreatment and post-light therapy sleep–wake cycles (Figure 3). For non-4-h sleep–wake phase disorder, management differs between sighted and blind patients. In blind patients, melatonin 0.5 mg or tasimelteon is suggested 1 h before habitual bedtime. In sighted individuals, bright light is recommended upon awakening along with regular sleep–wake schedule with or without melatonin.34
Management of other comorbid sleep disorders
Obstructive sleep apnea
TBI patients with sleep apnea are treated effectively with continuous positive airway pressure, though cognitive impairment and/or co-morbid post-traumatic stress disorder can be a major hurdle.
Periodic limb movements
The patients with periodic movements are well treated with pramipexole at a dose of 0.375 mg/day.
Bruxism (sleep-related teeth grinding)
The standard of care is a mouth guard that protects the teeth from the wear and tear of grinding. A single study, however, also showed success in the treatment of bruxism with botulinum toxin in a TBI patient.3
Future direction
Given the importance of early identification and management of sleep disorders in TBI patients, future research should continue to focus on diagnosis and treatment questions. Biomarkers could be established to characterize the presence or absence of brain injury, like protein or neuroimaging markers.1 Emphasis on pain management and further research into its role in the pathophysiology of sleep disturbances is warranted.25 Also, fatigue co-exists with sleep disturbances in most cases and may exacerbate it. Therefore, targeting both fatigue and sleep disturbance in treatment is worth investigating.35 Finally, more research is needed to establish the effectiveness of various treatment and management approaches in TBI patients.1
Conclusion
Sleep disorders are common in people who suffer TBIs due to disruption in the arousal/sleep regulation system and hormonal systems. Timely diagnosis and management of these sleep disorders is very important for adequate neuro-recovery. Failure to do so can lead to impaired cognition and quality of life. Management should start with conservative measures such as sleep hygiene and CBT followed by pharmacological agents if the former approaches do not succeed.
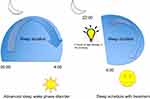 |
Figure 1 Management of advanced sleep–wake phase disorder. |
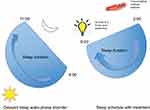 |
Figure 2 Management of delayed sleep–wake phase disorder. |
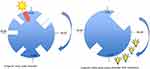 |
Figure 3 Management of irregular sleep–wake phase disorder. |
Disclosure
The authors report no conflicts of interest in this work.
References
1. Katz DI, Cohen SI, Alexander MP. Mild traumatic brain injury. Handb Clin Neurol. 2015;127:131–156.
2. Bryan CJ. Repetitive traumatic brain injury (or concussion) increases severity of sleep disturbance among deployed military personnel. Sleep. 2013;36(6):941–946. doi:10.5665/sleep.2730
3. Castriotta RJ, Murthy JN. Sleep disorders in patients with traumatic brain injury. CNS Drugs. 2011;25(3):175–185. doi:10.2165/11584870-000000000-00000
4. Mollayeva T, Pratt B, Mollayeva S, Shapiro CM, Cassidy JD, Colantonio A. The relationship between insomnia and disability in workers with mild traumatic brain injury/concussion. Sleep Med. 2016;20:157–166. doi:10.1016/j.sleep.2015.09.008
5. Frieden TR, Houry D, Baldwin G. Traumatic brain injury in the United States: epidemiology and rehabilitation. CDC NIH Rep to Congr. 2015;1–74.
6. Ling H, Hardy J, Zetterberg H. Neurological consequences of traumatic brain injuries in sports. Mol Cell Neurosci. 2015;66:114–122. doi:10.1016/j.mcn.2015.03.012
7. Bergersen K, JØ H, Tryti EA, Taylor SI, Olsen A. A systematic literature review of psychotherapeutic treatment of prolonged symptoms after mild traumatic brain injury. Brain Inj. 2017;31(3):279–289. doi:10.1080/02699052.2016.1255779
8. Thomas A, Greenwald BD. Nonpharmacological management of sleep disturbances after traumatic brain injury. NeuroRehabilitation. 2018;43(3):355–360. doi:10.3233/NRE-182535
9. Steriade M, Datta S, Pare D, Oakson G, Dossi RC. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci. 1990;10(8):2541–2559.
10. Dittrich L, Morairty SR, Warrier DR, Kilduff TS. Homeostatic sleep pressure is the primary factor of activation of cortical nNOS/NK1 neurons. Neuropsychopharmacology. 2014;40(3):632–639. doi:10.1038/npp.2014.212
11. Lewy AJ. Melatonin and human chronobiology. Cold Spring Harb Symp Quant Biol. 2007;72(1):623–636. doi:10.1101/sqb.2007.72.055
12. Schwartz MD, Kilduff TS. The neurobiology of sleep and wakefulness. Psychiatr Clin North Am. 2015;38(4):615–644. doi:10.1016/j.psc.2015.07.002
13. Krueger JM, Obál F, Fang J, Kubota T, Taishi P. The role of cytokines in physiological sleep regulation. Ann N Y Acad Sci. 2006;933(1):211–221. doi:10.1111/j.1749-6632.2001.tb05826.x
14. Apergis-Schoute J, Iordanidou P, Faure C, et al. Optogenetic evidence for inhibitory signaling from orexin to MCH neurons via local microcircuits. J Neurosci. 2015;35(14):5435–5441. doi:10.1523/JNEUROSCI.5269-14.2015
15. Sinclair KL, Ponsford J, Rajaratnam SMW. Actigraphic assessment of sleep disturbances following traumatic brain injury. Behav Sleep Med. 2013;12(1):13–27. doi:10.1080/15402002.2012.726203
16. Vieira RDCA, Paiva WS, Oliveira DVD, Teixeira MJ, Andrade AFD, Sousa RMCD. Diffuse axonal injury: epidemiology, outcome and associated risk factors. Front Neurol. 2016;7:178. doi:10.3389/fneur.2016.00178
17. Baumann CR, Werth E, Stocker R, Ludwig S, Bassetti CL. Sleep-wake disturbances 6 months after traumatic brain injury: a prospective study. Brain. 2007;130(7):1873–1883. doi:10.1093/brain/awm109
18. Sullan MJ, Asken BM, Jaffee MS, Dekosky ST, Bauer RM. Glymphatic system disruption as a mediator of brain trauma and chronic traumatic encephalopathy. Neurosci Biobehav Rev. 2017;84:316–324. doi:10.1016/j.neubiorev.2017.08.016
19. Mantua J, Grillakis A, Mahfouz SH, et al. A systematic review and meta-analysis of sleep architecture and chronic traumatic brain injury. Sleep Med Rev. 2018;41:61–77. doi:10.1016/j.smrv.2018.01.004
20. Grima N, Ponsford J, Rajaratnam SM, Mansfield D, Pase MP. Sleep disturbances in traumatic brain injury: a meta-analysis. J Clin Sleep Med. 2016;12(3):419–428. doi:10.5664/jcsm.5598
21. Boone DR, Sell SL, Micci M-A, et al. Traumatic brain injury-induced dysregulation of the circadian clock. PLoS One. 2012;7(10):e46204. doi:10.1371/journal.pone.0046204
22. Mollayeva T, Mollayeva S, Colantonio A. The risk of sleep disorder among persons with mild traumatic brain injury. Curr Neurol Neurosci Rep. 2016;16(6):55. doi:10.1007/s11910-016-0657-2
23. Collen J, Orr N, Lettieri CJ, Carter K, Holley AB. Sleep disturbances among soldiers with combat-related traumatic brain injury. Chest. 2012;142(3):622–630. doi:10.1378/chest.11-1603
24. Bacyinski A, Xu M, Wang W, Hu J. The paravascular pathway for brain waste clearance: current understanding, significance and controversy. Front Neuroanat. 2017;11:101. doi:10.3389/fnana.2017.00101
25. Suzuki Y, Khoury S, El-Khatib H, et al. Individuals with pain need more sleep in the early stage of mild traumatic brain injury. Sleep Med. 2017;33:36–42. doi:10.1016/j.sleep.2016.06.033
26. Gardani M, Morfiri E, Thomson A, Oneill B, Mcmillan TM. Evaluation of sleep disorders in patients with severe traumatic brain injury during rehabilitation. Arch Phys Med Rehabil. 2015;96(9):1691–1697. doi:10.1016/j.apmr.2015.05.006
27. Sullivan KA, Edmed SL, Allan AC, Karlsson LJE, Smith SS. Characterizing self-reported sleep disturbance after mild traumatic brain injury. J Neurotrauma. 2015;32(7):474–486. doi:10.1089/neu.2013.3284
28. Capaldi VF, Kim JR, Grillakis AA, Taylor MR, York CM. Insomnia in the military: application and effectiveness of cognitive and pharmacologic therapies. Curr Psych Rep. 2015;17(10):85. doi:10.1007/s11920-015-0622-9
29. Plantier D, Luauté J. Drugs for behavior disorders after traumatic brain injury: systematic review and expert consensus leading to french recommendations for good practice. Ann Phys Rehabil Med. 2016;59(1):42–57. doi:10.1016/j.rehab.2015.10.003
30. Wheaton P, Mathias JL, Vink R. Impact of pharmacological treatments on cognitive and behavioral outcome in the postacute stages of adult traumatic brain injury. J Clin Psychopharmacol. 2011;31(6):745–757. doi:10.1097/JCP.0b013e318235f4ac
31. Wolfe LF, Sahni AS, Attarian H. Sleep disorders in traumatic brain injury. NeuroRehabilitation. 2018;43(3):257–266. doi:10.3233/NRE-182583
32. Grima NA, Ponsford JL, Hilaire MAS, Mansfield D, Rajaratnam SM. Circadian melatonin rhythm following traumatic brain injury. Neurorehabil Neural Repair. 2016;30(10):972–977. doi:10.1177/1545968316650279
33. Kendis H, Zee PC. Sleepless patient: circadian rhythm sleep–wake disorders. In: Malhotra RK, editor. Sleepy or Sleepless: Clinical approach to sleep patient. Basel: Springer International Publishing; 2015:133–149.
34. Abbott SM, Reid KJ, Zee PC. Circadian rhythm sleep–wake disorders. Psych Clin North Am. 2015;38(4):805–823. doi:10.1016/j.psc.2015.07.012
35. Nguyen S, Mckay A, Wong D, et al. Cognitive behavior therapy to treat sleep disturbance and fatigue after traumatic brain injury: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2017;98(8):1508–1517. doi:10.1016/j.apmr.2017.02.031
36. Elliott JE, Opel RA, Weymann KB, et al. Sleep disturbances in traumatic brain injury: associations with sensory sensitivity. J Clin Sleep Med. 2018;14(7):1177–1186. doi:10.5664/jcsm.7220
37. Kaufman Y, Tzischinsky O, Epstein R, Etzioni A, Lavie P, Pillar G. Long-term sleep disturbances in adolescents after minor head injury. Pediatr Neurol. 2001;24(2):129–134.
38. Valko PO, Gavrilov YV, Yamamoto M, et al. Damage to histaminergic tuberomammillary neurons and other hypothalamic neurons with traumatic brain injury. Ann Neurol. 2014;77(1):177–182. doi:10.1002/ana.24298
39. Duclos C, Dumont M, Blais H, et al. Rest–activity cycle disturbances in the acute phase of moderate to severe traumatic brain injury. Neurorehabil Neural Rep. 2013;28(5):472–482. doi:10.1177/1545968313517756
40. Wilde MC, Castriotta RJ, Lai JM, Atanasov S, Masel BE, Kuna ST. Cognitive impairment in patients with traumatic brain injury and obstructive sleep apnea. Arch Phys Med Rehabil. 2007;88(10):1284–1288. doi:10.1016/j.apmr.2007.07.012
41. Brown RM, Tang X, Dreer LE, et al. Change in body mass index within the first-year post-injury: a VA Traumatic Brain Injury (TBI) model systems study. Brain Inj. 2018;32(8):986–993. doi:10.1080/02699052.2018.1468575
42. Castriotta RJ, Lai JM. Sleep disorders associated with traumatic brain injury. Arch Phys Med Rehabil. 2001;82(10):1403–1406. doi:10.1053/apmr.2001.26081
43. Lequerica A, Jasey N, Tremont JNP, Chiaravalloti ND. Pilot study on the effect of ramelteon on sleep disturbance after traumatic brain injury: preliminary evidence from a clinical trial. Arch Phys Med Rehabil. 2015;96(10):1802–1809. doi:10.1016/j.apmr.2015.05.011
44. Shan RSLP, Ashworth NL. Comparison of lorazepam and zopiclone for insomnia in patients with stroke and brain injury. Am J Phys Med Rehabil. 2004;83(6):421–427.
45. Ouellet M-C, Morin CM. Efficacy of cognitive-behavioral therapy for insomnia associated with traumatic brain injury: a single-case experimental design. Arch Phys Med Rehabil. 2007;88(12):1581–1592. doi:10.1016/j.apmr.2007.09.006
46. Zollman FS, Larson EB, Wasek-Throm LK, Cyborski CM, Bode RK. Acupuncture for treatment of insomnia in patients with traumatic brain injury. J Head Trauma Rehabil. 2012;27(2):135–142. doi:10.1097/HTR.0b013e3182051397
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
