Back to Journals » Infection and Drug Resistance » Volume 11
Impact of teicoplanin maintenance dose and MIC values on the clinical outcomes of patients treated for methicillin-resistant Staphylococcus aureus bacteremia
Authors Tsai CY, Lee CH , Chien CC, Chen IL
Received 16 April 2018
Accepted for publication 25 May 2018
Published 15 August 2018 Volume 2018:11 Pages 1205—1217
DOI https://doi.org/10.2147/IDR.S171236
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Ching-Yen Tsai,1 Chen-Hsiang Lee,1,2 Chun-Chih Chien,3 I-Ling Chen4
1Division of Infectious Diseases, Department of Medicine, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan; 2Chang Gung University College of Medicine, Kaohsiung, Taiwan; 3Department of Laboratory Medicine, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan; 4Department of Pharmacology, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan
Objectives: Teicoplanin, a glycopeptide, is regarded as among the drug choices for methicillin-resistant Staphylococcus aureus (MRSA) infections. Few studies have evaluated the relationship between teicoplanin minimal inhibitory concentrations (MICs) and outcomes among patients with serious MRSA infections.
Subjects and methods: We investigated the relationship between teicoplanin maintenance dose and clinical outcomes, on the completion of teicoplanin therapy, in bacteremia patients with MRSA infection, with different teicoplanin MICs. A total of 146 adult patients with MRSA bacteremia were enrolled at Kaohsiung Chang Gung Memorial Hospital between September 2012 and September 2015.
Results: A higher number of patients in the high-dose regimen group (6 mg/kg/12 h) had favorable outcomes than those in the standard-dose regimen group (6 mg/kg/24 h) (84.1% vs 41.2%; p<0.01), regardless of the teicoplanin MICs. In the multivariate analysis, a Pittsburgh bacteremia score ≥4 (OR, 0.07, 95% CI, 0.03–0.19) was a risk factor for an unfavorable final clinical response, whereas high-dose teicoplanin maintenance therapy for MRSA bacteremia was significantly associated with a favorable final response (OR, 25.3 [95% CI, 4.43–144.03] for isolates with a teicoplanin MIC ≥1.5 mg/L and OR, 5.6 [95% CI, 1.57–19.91] for isolates with a teicoplanin MIC <1.5 mg/L). Survival at 30 days was significantly better for patients receiving high-dose teicoplanin maintenance treatment, regardless of the teicoplanin MICs of the MRSA isolates. Patients were selected using propensity score matching, based on the independent predictors of a favorable final outcome. After appropriate propensity score matching, patients in the high-dose regimen group still had a statistically significant favorable outcome at the end of treatment (84.1% vs 40.9%; p<0.01).
Conclusion: The results suggested that high-dose teicoplanin maintenance treatment is associated with more favorable outcomes than standard-dose teicoplanin maintenance treatment, for patients with MRSA bacteremia, regardless of the teicoplanin MIC.
Keywords: glycopeptide, MRSA, serum concentration, therapeutic drug monitoring, outcomes
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is an important cause of several infectious syndromes in community and health care-associated settings.1 Of these syndromes, MRSA bacteremia seems to be associated with a longer hospital stay and higher mortality than bacteremia caused by other bacterial pathogens.2 Vancomycin has been the mainstay in the treatment of serious MRSA infections.3 Over the past decade, several studies have demonstrated higher rates of vancomycin treatment failure in patients with MRSA infection, who had vancomycin minimal inhibitory concentrations (MICs) ≥1.5 mg/L,4,5 despite these isolates being susceptible to vancomycin (MIC ≤2.0 mg/L, according to Clinical and Laboratory Standards Institute [CLSI] criteria).6 However, a meta-analysis of the published data concluded that there were no statistically significant differences in the risk of death between patients with MRSA infection exhibiting vancomycin MICs ≥1.5 mg/L and those infected with strains exhibiting vancomycin MICs <1.5 mg/L.7 Nevertheless, the MIC of vancomycin can still be considered when interpreting and determining if alternative antistaphylococcal agents are necessary for patients with MRSA bacteremia who have elevated but susceptible vancomycin MICs.4,5,7 The current guidelines for the management of patients with MRSA infections recommend that an initial vancomycin loading dose of 25–30 mg/kg be administered, followed by the administration of intermittent maintenance doses to achieve a target serum trough concentration of 15–20 mg/L.8 Clinicians may consider using alternative agents for MRSA infections if a patient’s condition worsens or the vancomycin MIC is ≥1.5 mg/L and the patient is critically ill.8
Teicoplanin is not approved for use in the USA, but is widely used in Europe and Taiwan. This glycopeptide has been reported to be comparable to vancomycin in efficacy and is associated with fewer adverse effects than vancomycin.9 While several studies have focused on the effects of vancomycin MICs on the clinical outcomes of MRSA bacteremia patients,4,5,7 few studies have evaluated the relationship between teicoplanin MICs and outcomes among patients with serious MRSA infections.10 A retrospective study conducted at a single center indicated that teicoplanin MICs ≥1.5 mg/L may predict unfavorable outcomes and higher mortality among patients receiving teicoplanin for MRSA bacteremia.11 However, another retrospective study conducted at 2 hospitals found no significant role for teicoplanin MICs in the prognosis of patients with teicoplanin-treated MRSA bacteremia.12
Theoretically, higher teicoplanin doses are indicated for infections caused by MRSA with high teicoplanin MICs. However, the pharmacokinetics of teicoplanin is complex,13 and the serum concentrations of the antibiotic are often not available in clinical practice. As a result, a great variety of dosage regimens, with different loading dosages and/or different maintenance dosages, have been reported.14 The findings of our previously conducted study highlighted the importance of a higher teicoplanin maintenance dose (6 mg/kg/12 h), especially for severe infections due to MRSA.15 Nevertheless, it is still unclear whether high-dose teicoplanin maintenance therapy confers a survival benefit to MRSA-infected bacteremia patients who have isolates with high teicoplanin MICs. The aim of this study was to compare the clinical outcomes of adult bacteremia patients infected by MRSA with different teicoplanin MICs, who received high-dose teicoplanin as maintenance therapy (6 mg/kg/12 h) with those of patients who received standard-dose therapy (6 mg/kg/24 h) after the loading dose.
Subjects and methods
Study design and patients
The study was conducted at the Kaohsiung Chang Gung Memorial Hospital, a tertiary-care medical center, with 2,700 beds, in southern Taiwan. In this current study (ethical approval and waiver of consent), the patient data were made anonymous to maintain confidentiality. The Institutional Review Board of the Chang Gung Memorial Hospital approved the study (No. 201601482B0) and waived the need for patient consent because of the retrospective nature of the study.
Between September 2012 and September 2015, blood culture records from all patients who had at least one positive result for S. aureus were collected. Glycopeptides (vancomycin and teicoplanin) were the only 2 first-line intravenous agents available for the treatment of MRSA bacteremia; quinupristin/dalfopristin was not available, and the use of linezolid, daptomycin, and tigecycline was restricted during the study period.16 Patients with a concurrent infection caused by microbes other than MRSA were excluded from this study. If a patient experienced >1 episode of MRSA bacteremia, only the first episode was included.
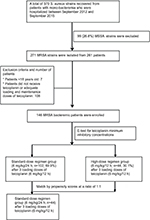
During the study period, a total of 261 MRSA mono-bacteremia patients were identified (Figure 1). Their medical charts were reviewed for the retrieval of demographic, clinical, and laboratory data for analyses. Adult patients (≥18 years of age) who fulfilled the following criteria were included: receipt of teicoplanin throughout the treatment course or receipt of <24 h of vancomycin followed by teicoplanin therapy for >3 days and adequate teicoplanin dosage. The dose of teicoplanin was decided upon based on the patient’s actual body weight. Adequate teicoplanin therapy was defined as a loading teicoplanin dose of 6 mg/kg, administered 3 times (at 12 h intervals), followed by a maintenance dose of 6 mg/kg/12 h (defined as the high-dose regimen) or 6 mg/kg/24 h (defined as the standard-dose regimen), with adjusted equivalent doses for patients with impaired renal function (package insert, Sanofi-Aventis S.A., Paris, France). The maintenance teicoplanin dosages for the included patients were prescribed at the discretion of their attending physicians. None of the patients received adjunctive antibiotics for the treatment of MRSA bacteremia.
Variables and definitions
Bacteremia was categorized as community-acquired, hospital-acquired, or health care-associated. Community-acquired bacteremia was defined as bacteremia onset within 48 h of admission without a previous hospital stay.17,18 Hospital-acquired bacteremia was defined as bacteremia onset at ≥48 h after admission. Health care-associated bacteremia was defined as follows: the bacteremia episode was associated with the presence of an invasive device; a recent history of surgery, hospitalization, or dialysis (performed within 3 months before the onset of infection); or residence in a long-term care facility within the preceding 12 months.11 The Chronic Kidney Disease Epidemiology Collaboration equation was used for the estimation of the glomerular filtration rate in this study.19 The clinical severity of illness, at the time of blood sampling for cultures, was stratified using the modified Pittsburgh bacteremia score; critical illness was defined as a Pittsburgh bacteremia score ≥4 points.20
Sources of bacteremia were identified according to clinical, microbiological, and imaging findings, as well as the judgment of the physicians. The isolation of MRSA from a patient in whom no apparent infection focus, other than the blood, had been identified was defined as bacteremia with no identified focus of infection; otherwise, it was referred to as bacteremia with an identified focus of infection. Pneumonia (PN) referred to the presence of clinical symptoms/signs of lower respiratory tract infection, accompanied by consistent radiographic findings.21 Bone and joint infection was diagnosed based on clinical manifestations with consistent histopathological and/or radiographical findings, regardless of the MRSA isolated from the infection site.21 Catheter-related bacteremia was diagnosed if the inserted catheter had been in place for ≥72 h, the culture yielded ≥15 colonies of MRSA by rolling the clipped 5 cm distant tip of the removed catheter on a culture medium, or if the culture of the purulent discharge from the catheter exit grew MRSA.22 Infective endocarditis (IE) was diagnosed in MRSA bacteremia patients if the histopathology was consistent with that observed in specimens obtained during surgery, or if valvular vegetation was sonographically revealed, regardless of the presence of an embolic phenomenon.23 Urinary tract infection was considered to be caused by MRSA when the microbe was the only identified pathogen with ≥105 colony-forming units/mL in the urine culture.21 Intra-abdominal infection was defined as an infection that extended into the peritoneal space, was associated with abscess formation or peritonitis, and if the peritoneal fluid, bile, or intra-abdominal abscess aspiration culture yielded MRSA.24,25 Soft tissue infection was considered to be caused by MRSA when the microbe grew from the specimen sampled from the affected site.21
Outcome measures
Early clinical response was assessed on day 7 after teicoplanin therapy initiation, while the evaluation upon the completion of teicoplanin therapy was defined as the final clinical response. Patients were evaluated on day 7 for the presence of 1) fatality resulting from MRSA bacteremia, 2) septic shock, 3) persistent bacteremia, 4) persistent fever, and 5) persistent leukocytosis; favorable and unfavorable early clinical responses were defined as the absence and presence of any of these findings, respectively.15 The final clinical responses were categorized as “cure,” “improvement,” or “failure.” “Cure” was defined as the resolution of clinical signs and symptoms and a negative culture report at the end of therapy. “Improvement” was defined as a partial resolution of clinical signs and symptoms based on clinical adjustment, with additional antibiotic therapy required. Patients with 1) clinical progression or relapse of sepsis that had previously improved clinically, 2) fatality, and/or 3) culture of the blood sample at the end of teicoplanin treatment testing positive for MRSA were defined as having treatment failure. “Cure” and “improvement” were classified as favorable outcomes, while “failure” was classified as an unfavorable outcome.15
Sepsis-related mortality was defined as fatality in an MRSA bacteremia patient with positive blood cultures or persistent signs of sepsis and no other definite causes of death. Thirty-day inhospital mortality was defined as all causes of mortality occurring within 30 days of hospitalization after the onset of MRSA bacteremia.15
Microbiological studies
The microorganisms isolated from the blood cultures were detected using a BD BACTEC FX system (Becton, Dickinson Microbiology System, Sparks, MD, USA). Gram-positive cocci in clusters were detected by microscopic inspection after Gram staining. The subsequent identification of S. aureus was performed with catalase-positive, coagulase-positive, and ornithine-negative reactions, according to standard methods,26 and methicillin susceptibility testing was done using the disk diffusion method on Müeller–Hinton agar, in which cefoxitin (30 μg) produced an inhibition zone of ≤21 mm, according to the CLSI recommendation.6 The teicoplanin MIC was determined using Etest® teicoplanin strips (AB Biodisk, Solna, Sweden) with an inoculum that had a turbidity equivalent to that of a 0.5 McFarland standard, brain heart infusion agar, and incubation period of 24 h at 35°C–37°C. An internal control was testing S. aureus ATCC 29213. The MIC breakpoint for teicoplanin resistance was set at >2 mg/L, in accordance with the European Committee on Antimicrobial Susceptibility Testing.27
Statistical analysis
In the univariate analysis, a Student’s t-test or Mann–Whitney U test was used for comparisons between continuous variables, whereas a χ2 test or Fisher’s exact test was used for comparisons between dichotomous variables. Multivariate analysis was performed using logistic regression to identify the factors that independently affected the outcomes. Variables with a p-value ≤0.1 in the univariate analyses were included in the multivariate analysis. Kaplan–Meier estimation was used to plot the 30-day survival curve for each study group, with the start of the follow-up period defined as the date of teicoplanin maintenance therapy after the loading doses. A log-rank test was used to compare the 2 survival curves. Goodness of fit was assessed by the Hosmer and Lemeshow statistic. Receiver operating characteristic curve analysis was used to evaluate the predictive performance of the logistic regression model. To reduce the effects of the potential confounders that might result from the diverse characteristics among patients receiving high-dose teicoplanin and standard-dose teicoplanin therapies after a loading dose, we adjusted for differences in the baseline characteristics between the treatment groups by propensity score matching analysis. The propensity score was calculated using the independent predictors of favorable final outcome assessed using a multivariable logistic regression model. Each patient receiving high-dose teicoplanin therapy was matched on a 1:1 basis to a patient receiving standard-dose teicoplanin therapy who had a similar propensity score, as described previously.28 A maximum difference of 5% was allowed in the matching process. Statistical analysis was performed using SPSS version 21 (IBM Corporation, Armonk, NY, USA). All tests were 2-tailed. Variables with a p-value <0.05 were regarded as statistically significant.
Results
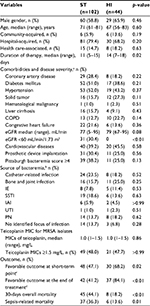
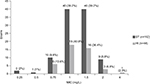
A total of 146 adult MRSA bacteremia patients were included and categorized into 2 groups based on the teicoplanin maintenance dosing: the standard-dose regimen group (ST; teicoplanin 6 mg/kg/24 h and adjusted equivalents for renal insufficiency, n=102) and the high-dose regimen group (HI; teicoplanin 6 mg/kg/12 h and adjusted equivalents for renal insufficiency, n=44) (Figure 1). The MIC distribution of teicoplanin against the 146 MRSA bloodstream infection strains is demonstrated in Figure S1. There were no statistical differences in the MIC distribution of teicoplanin for the MRSA isolates among these 2 groups. Similar demographic and clinical features (including MRSA teicoplanin MICs) were found in the 2 groups, except for a shorter duration of therapy (11 versus 14 days, p=0.02) and a higher proportion of impaired renal function (30.4% versus 0%, p<0.01) found in the ST. Patients in the HI had significantly favorable early clinical responses (p=0.02) and final clinical responses (p<0.01), lower 30-day overall mortality (p<0.01), and lower sepsis-related mortality (p=0.01) (Table 1).
Compared to the patients with a favorable clinical response (Table 2), those with unfavorable clinical responses had a higher proportion of coronary artery disease (34.3% versus 17.7%, p=0.02), Pittsburgh bacteremia score ≥4 (59.7% versus 12.7%, p<0.01), and IE (14.9% versus 3.8%, p=0.02). The patients in the ST who had MRSA isolates with teicoplanin MICs ≥1.5 mg/L also had more unfavorable clinical responses (49.3% versus 20.3%, p<0.01). Patients with favorable clinical responses had a higher proportion of community-acquired bacteremia (13.9% versus 1.5%, p=0.01), treatment with the high-dose regimen (46.8% versus 10.4%, p<0.01), treatment with the high-dose regimen for MRSA isolates with teicoplanin MICs <1.5 mg/L (22.8% versus 7.5%, p=0.01), and treatment with the high-dose regimen for MRSA isolates with teicoplanin MICs ≥1.5 mg/L (24.1% versus 3.0%, p<0.01).
In multivariate analysis (Table 3), a Pittsburgh bacteremia score ≥4 was an independent risk factor for an unfavorable final response (OR, 0.07; 95% CI, 0.03–0.19, p<0.01). Patients in the HI were likelier to have a favorable final response (OR, 25.3 [95% CI, 4.43–144.03], p<0.01 for isolates with teicoplanin MICs ≥1.5 mg/L and OR, 5.6 [95% CI, 1.57–19.91], p=0.01 for isolates with teicoplanin MICs <1.5 mg/L) (Table 3).
A total of 26 (38.9%) patients including 23 in the standard-dose group and 3 in the high-dose group with an unfavorable clinical response to teicoplanin received alternative agents for MRSA bacteremia. Of the 15 (57.7%) patients who switched to daptomycin, with an overall mortality of 53.3%, 6 were found to have catheter-related bacteremia, 4 had IE, 2 had bone and joint infections, 2 had PN, and 1 had soft tissue infection. Of the 11 patients (42.3%) who switched to linezolid, with an overall mortality of 81.8%, 3 were found to have IE, 3 had bone and joint infection, 2 had PN, 2 had catheter-related bacteremia, and 1 had bacteremia with no identified focus of infection.
A total of 44 patients in the HI could be matched with 44 patients in the ST, based on the propensity score. Compared to the matched patients receiving the standard-dose teicoplanin regimen, those receiving the high-dose teicoplanin regimen had statistically significant favorable outcomes at the end of treatment (p<0.01), lower 30-day overall mortality (p=0.02), and lower sepsis-related mortality (p=0.02) (Table 4).
In the subgroup analysis of patients infected with MRSA isolates that had teicoplanin MICs ≥1.5 mg/L, the sepsis-related mortality of patients in the ST (42.9%, 21/49) was higher than that of patients in the HI (9.5%, 2/21). For those patients with bacteremic episodes caused by isolates with teicoplanin MICs <1.5 mg/L, the sepsis-related mortality was similar between the standard-dose regimen and HIs (30.1% [16/53] versus 17.3% [4/23], p=0.40) (Figure 2). On examining the sepsis-related mortality, according to the source of MRSA bacteremia, a higher mortality was observed in patients with bacteremic PN who were treated with the standard-dose regimen than in those treated with the high-dose regimen (85.7% [12/14] versus 0% [0/8]; p< 0.01; Figure 3).
Additionally, patients were grouped according to the teicoplanin maintenance dosage (standard-dose or HI) and further stratified by the teicoplanin MICs. Groups 1–4 represented the ST with MRSA isolates with teicoplanin MICs <1.5 mg/L, the ST with MRSA isolates with teicoplanin MICs ≥1.5 mg/L, the HI with MRSA isolates with teicoplanin MICs <1.5 mg/L, and the HI with MRSA isolates with teicoplanin MICs ≥1.5 mg/L, respectively. The log-rank tests for survival at 30 days favored the HIs, regardless of the teicoplanin MICs (Groups 3 and 4; Figure 4). Moreover, among patients who received the standard-dose regimen, those infected with isolates with teicoplanin MICs <1.5 mg/L had a higher survival rate than those infected with isolates with teicoplanin MICs ≥1.5 mg/L (Group 1 versus Group 2, p=0.03) (Figure 4).
Discussion
This study assessed the final clinical responses, sepsis-related mortality, and overall mortality of adult MRSA-infected bacteremia patients who received high-dose or standard-dose teicoplanin maintenance treatment after loading doses of teicoplanin. All the analyses suggested that the patients receiving high-dose teicoplanin maintenance treatment had favorable outcomes, regardless of the teicoplanin MICs. Standard-dose teicoplanin maintenance treatment may not be a suitable option for the treatment of MRSA infections, particularly if the teicoplanin MICs are ≥1.5 mg/L.
Although several studies have reported that vancomycin MICs ≥1.5 mg/L were associated with a higher rate of vancomycin treatment failure for MRSA infections,7,17 more studies are warranted to confirm whether the finding also applies to MRSA bacteremia with teicoplanin MICs ≥1.5 mg/L. Chang et al11 conducted a single-center study of 101 patients who received 6 mg/kg/d of maintenance teicoplanin therapy for MRSA bacteremia. In agreement with their finding, which showed that a teicoplanin MIC cutoff value of 1.5 mg/L could be used as a predictor for the outcomes of patients with teicoplanin-treated MRSA bacteremia, our study showed that patients in the ST (6 mg/kg/24 h) infected with MRSA with teicoplanin MICs ≥1.5 mg/L also had unfavorable final outcomes (49.3% versus 20.3%, p<0.01, Table 2). However, the variable, teicoplanin MIC ≥1.5 mg/L, was not an independent risk factor for the prediction of unfavorable final clinical outcomes in the multivariate analysis (Table 3).
In another study of 270 patients with teicoplanin-treated MRSA bacteremia conducted at 2 medical centers,12 teicoplanin MICs were not statistically significant predictors of clinical outcomes. Although all the patients in that study received teicoplanin as initial therapy for ≥3 days, at a maintenance dose of 6 mg/kg/d, adjusted for their renal function, only the severity of bacteremia, as reflected by the presence of septic shock and an elevated C-reactive protein level, was an independent risk factor for unfavorable outcomes at the end of teicoplanin therapy.12 In addition to the severity of bacteremia, the findings of our previously conducted study15 as well as those of the present study highlight the importance of high teicoplanin maintenance dosing in treating severe MRSA infections.
The poorer outcomes observed when the MRSA teicoplanin MICs were ≥1.5 mg/L could be attributed to ineffective treatment, secondary to suboptimal dosage, or other microbiological characteristics of the causative organism. Some experts have suggested that the serum teicoplanin trough level should be kept at ≥10 mg/L to ensure successful treatment, for MRSA bacteremia.29 Given that teicoplanin’s bactericidal activity is dependent upon the concentration–time curve to MIC ratio (area under the curve [AUC24/MIC]) and the peak concentration to MIC ratio, administering higher doses could have a positive impact on patient outcomes. According to previously conducted studies,14,30 the teicoplanin minimum concentration (Cmin) averaged 10 mg/L on day 6, in patients receiving teicoplanin maintenance doses of 6 mg/kg/24 h, after loading doses of 6 mg/kg/12 h; furthermore, the teicoplanin Cmin was 10 mg/L on day 2 in patients in whom teicoplanin maintenance doses of 6 mg/kg/12 h were continued to be administered after loading doses of 6 mg/kg/12 h.14,30 The abovementioned data may explain the presence of statistically significant favorable early responses, final clinical responses, and lower sepsis-related mortality in the HI in our study compared to those in the ST, as a Cmin ≥10 mg/L could only be anticipated in the ST on day 6, whereas a Cmin ≥10 mg/L could be achieved 4 days earlier in the HI (day 2). The higher rate of clinical failure in the ST is potentially a reflection of the inability to reach the AUC24/MIC target when the teicoplanin MIC exceeds 1 mg/L. It is to be noted that an increased maintenance dosage of teicoplanin did not increase the prevalence of adverse events in other studies.15,31 If the teicoplanin MICs are ≥1.5 mg/L, patients are still likely to safely tolerate higher teicoplanin doses than would be required to achieve the AUC24/MIC target to optimize activity against MRSA. Further pharmacodynamic studies focusing on teicoplanin for the treatment of such infections are warranted.
One previously conducted study showed that the MRSA isolates of ST5 had a significantly increased proportion of high teicoplanin MICs.12 ST5 is the predominant MRSA strain in some Asian countries, and the prevalence of ST5 has increased in Taiwan recently.32 In addition, an upward creep has been reported in the teicoplanin MICs for MRSA isolates.33 The proportions of teicoplanin MICs ≥1.5 mg/L among the MRSA strains isolated from our patients, who were hospitalized from September 2012 to September 2015, were 37.3%, 51.5%, and 54.9%, respectively (p for trend; <0.01). As a result, it is important to continue monitoring the molecular epidemiology of clinical MRSA isolates and the teicoplanin MICs among these MRSA isolates.
The finding that significantly favorable clinical outcomes were found in the subgroup of patients with MRSA bacteremic PN who received high-dose teicoplanin maintenance treatment (Table 3) is in agreement with other reports suggesting that a higher teicoplanin dose is indicated for the treatment of severe MRSA infections.15,29 The sepsis-related mortality of patients with IE did not significantly differ between the patients receiving high-dose teicoplanin maintenance therapy and those receiving standard-dose therapy. This could be attributed to the low number of IE cases in our cohort. Although more studies are needed to confirm these findings, an increasing trend of mortality with standard-dose teicoplanin maintenance treatment was observed among patients with bacteremia related to deep-seated infections.
There are several limitations to our study. First, this was a retrospective, chart review study at a single institution, which enrolled a limited number of patients with MRSA bacteremia, and therefore, there were missing data in the charts, which may compromise our study’s generalizability, analytic results, and causal inference. The maintenance dose was prescribed at the discretion of the attending physician, and this could be heavily biased. To minimize the confounding factors, subgroup analysis, multivariate analysis, and propensity score matching analysis were performed. All these analytical results led to the same finding that high-dose teicoplanin maintenance therapy was associated with favorable outcomes in patients with MRSA bacteremia, regardless of the teicoplanin MIC values. However, prospective, randomized, controlled comparative efficacy trials are needed to validate our findings. Second, molecular analyses were not performed to identify the specific MRSA strains and clones that may have been prevalent in the hospital during the study period. MRSA strains with specific resistance genes may have an influence on MICs.18,34 Third, there is a large variability in the pharmacokinetics of teicoplanin,13,31 and this could also be important in our study. Analyses of the serum teicoplanin levels of the affected patients were not performed, as no commercialized methods were available to determine the serum teicoplanin levels during the study period. Although all the patients with impaired renal function (estimated glomerular filtration rate <60 mL/min/1.73 m2) received standard-dose teicoplanin maintenance therapy, impaired renal function was not identified as an independent risk factor for unfavorable outcomes in the multivariable analysis.
Conclusion
Demonstrating a relationship between teicoplanin doses and clinical outcomes would be extremely useful to inform clinical practice. Our study showed that a high Pittsburgh bacteremia score was an independent risk factor for unfavorable final clinical outcomes. Patients receiving high-dose teicoplanin maintenance treatment had favorable outcomes for MRSA bacteremia, regardless of the teicoplanin MIC values. A suboptimal clinical outcome may be anticipated when standard-dose teicoplanin is administered as maintenance therapy for bacteremia caused by MRSA with MICs ≥1.5 mg/L. To determine whether teicoplanin MICs and/or high-dose teicoplanin maintenance therapy play a significant role in the outcomes of patients with teicoplanin-treated MRSA bacteremia, further studies are needed.
Acknowledgments
This work was partially funded by Chang Gung Memorial Hospital, Taiwan [CMRPG 8F1801]. We thank Dr Chien-Ching Hung at the Department of Internal Medicine, National Taiwan University Hospital, for his critical review of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666–674. | ||
Cosgrove SE, Qi Y, Kaye KS, Harbarth S, Karchmer AW, Carmeli Y. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol. 2005;26(2):166–174. | ||
Karchmer AW. Staphylococcus aureus and vancomycin: the sequel. Ann Intern Med. 1991;115(9):739–741. | ||
Moise PA, Sakoulas G, Forrest A, Schentag JJ. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2007;51(7):2582–2586. | ||
Soriano A, Marco F, Martínez JA, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46(2):193–200. | ||
Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: 25th Informational Supplement, CLSI Document M100-S25. Wayne, PA: CLSI; 2015. | ||
Kalil AC, Van Schooneveld TC, Fey PD, Rupp ME. Association between vancomycin minimum inhibitory concentration and mortality among patients with Staphylococcus aureus bloodstream infections: a systematic review and meta-analysis. JAMA. 2014;312(15):1552–1564. | ||
Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):285–292. | ||
Svetitsky S, Leibovici L, Paul M. Comparative efficacy and safety of vancomycin versus teicoplanin: systematic review and meta-analysis. Antimicrob Agents Chemother. 2009;53(10):4069–4079. | ||
Wilson AP, Cepeda JA, Hayman S, Whitehouse T, Singer M, Bellingan G. In vitro susceptibility of Gram-positive pathogens to linezolid and teicoplanin and effect on outcome in critically ill patients. J Antimicrob Chemother. 2006;58(2):470–473. | ||
Chang HJ, Hsu PC, Yang CC, et al. Influence of teicoplanin MICs on treatment outcomes among patients with teicoplanin-treated methicillin-resistant Staphylococcus aureus bacteremia: a hospital-based retrospective study. J Antimicrob Chemother. 2012;67(3):736–741. | ||
Wang JT, Wu HS, Weng CM, Hsu LY, Wang FD. Prognosis of patients with methicillin-resistant Staphylococcus aureus bloodstream infection treated with teicoplanin: a retrospective cohort study investigating effect of teicoplanin minimum inhibitory concentrations. BMC Infect Dis. 2013;13:182. | ||
Wilson AP. Clinical pharmacokinetics of teicoplanin. Clin Pharmacokinet. 2000;39(3):167–183. | ||
Brink AJ, Richards GA, Cummins RR, Lambson J; Gauteng Understanding Teicoplanin Serum levels (GUTS) Study Group. Recommendations to achieve rapid therapeutic teicoplanin plasma concentrations in adult hospitalized patients treated for sepsis. Int J Antimicrob Agents. 2008;32(5):455–458. | ||
Lee CH, Tsai CY, Li CC, Chien CC, Liu JW. Teicoplanin therapy for MRSA bacteraemia: a retrospective study emphasizing the importance of maintenance dosing in improving clinical outcomes. J Antimicrob Chemother. 2015;70(1):257–263. | ||
Chen IL, Lee CH, Su LH, Tang YF, Chang SJ, Liu JW. Antibiotic consumption and healthcare-associated infections caused by multidrug-resistant gram negative bacilli at a large medical center in Taiwan from 2002 to 2009: implicating the importance of antibiotic stewardship. PLoS One. 2013;8(5):e65621. | ||
Lodise TP, Graves J, Evans A, et al. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteraemia treated with vancomycin. Antimicrob Agents Chemother. 2008;52(9):3315–20. | ||
Khatib R, Jose J, Musta A, et al. Relevance of vancomycin-intermediate susceptibility and heteroresistance in methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother. 2011;66(7):1594–1599. | ||
Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. | ||
Paterson DL, Ko WC, Von Gottberg A, et al. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum β-lactamase production in nosocomial infections. Ann Intern Med. 2004;140(1):26–32. | ||
Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16(3):128–140. | ||
Brun-Buisson C, Abrouk F, Legrand P, Huet Y, Larabi S, Rapin M. Diagnosis of central venous catheter-related sepsis. Critical level of quantitative tip cultures. Arch Intern Med. 1987;147(5):873–877. | ||
El-Ahdab F, Benjamin DK Jr, Wang A, et al. Risk of endocarditis among patients with prosthetic valves and Staphylococcus aureus bacteraemia. Am J Med. 2005;118(3):225–229. | ||
Christou NV, Turgeon P, Wassef R, Rotstein O, Bohnen J, Potvin M. Management of intra-abdominal infections. The case for intra-operative cultures and comprehensive broad-spectrum antibiotic coverage. The Canadian Intra-abdominal Infection Study Group. Arch Surg. 1996;131(11):1193–1201. | ||
Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133–164. | ||
Bannerman TL, Peacock SJ. Staphylococci, micrococci, and other catalase-positive organisms. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Manual of Clinical Microbiology. Washington, DC: ASM Press; 2015:354–382. | ||
European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 6.0. EUCAST. Basel, Switzerland: European Committee on Antimicrobial Susceptibility Testing. Available from: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_6.0_Breakpoint_table.pdf. Valid from January 1, 2016. Accessed July 2, 2018. | ||
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. | ||
Harding I, MacGowan AP, White LO, Darley ES, Reed V. Teicoplanin therapy for Staphylococcus aureus septicaemia: relationship between pre-dose serum concentrations and outcome. J Antimicrob Chemother. 2000;45(6):835–841. | ||
LeFrock J, Ristuccia A. Teicoplanin in the treatment of bone and joint infections: an open study. J Infect Chemother. 1999;5(1):32–39. | ||
Matthews PC, Chue AL, Wyllie D, et al. Increased teicoplanin doses are associated with improved serum levels but not drug toxicity. J Infect. 2014;68(1):43–49. | ||
Chen CJ, Hsueh PR, Su LH, Chiu CH, Lin TY, Huang YC. Change in the molecular epidemiology of methicillin-resistant Staphylococcus aureus bloodstream infection in Taiwan. Diagn Mirobiol Infect Dis. 2009;65(2):199–201. | ||
Robert J, Bismuth R, Jarlier V. Decreased susceptibility to glycopeptides in methicillin-resistant Staphylococcus aureus: a 20 year study in a large French teaching hospital, 1983–2002. J Antimicrob Chemother. 2006;57(3):506–510. | ||
Wang JL, Wang JT, Sheng WH, Chen YC, Chang SC. Nosocomial methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia in Taiwan: mortality analyses and the impact of vancomycin, MIC =2 mg/L, by the broth microdilution method. BMC Infect. Dis. 2010;10:159. |
Supplementary material
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.