Back to Journals » International Journal of General Medicine » Volume 15
Impact of Systemic Diseases on Olfactory Function in COVID-19 Infected Patients
Authors Awwad A, Abd Elhay OMM , Rabie MM , Awad EA , Kotb FM , Maghraby HM, Eldamarawy RH, Dawood YMA, Balat MIEI, Hasan AIM, Elsheshiny AH, El Sayed SSMM , Fouda AAB, Alkot AMF
Received 15 January 2022
Accepted for publication 28 April 2022
Published 17 June 2022 Volume 2022:15 Pages 5681—5691
DOI https://doi.org/10.2147/IJGM.S355974
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Ayat A Awwad,1 Osama MM Abd Elhay,2 Moustafa M Rabie,3 Eman A Awad,4 Fatma M Kotb,4 Hend M Maghraby,4 Rmadan H Eldamarawy,4 Yahia MA Dawood,1 Mostafa IEI Balat,1 Ahmed IM Hasan,5 Ahmed H Elsheshiny,6 Said SMM El Sayed,2 Albayoumi AB Fouda,2 Ahmad MF Alkot2
1Otorhinolaryngology department, Faculty of Medicine, Al-Azhar University, Cairo, Egypt; 2Medical Physiology Department, Faculty of Medicine, Al-Azhar University, Cairo, Egypt; 3Public Health and Community Medicine Department, Faculty of Medicine, Al-Azhar University, Cairo, Egypt; 4Internal medicine department, Faculty of Medicine, Al-Azhar University, Cairo, Egypt; 5Pediatric Department, Faculty of Medicine, Al-Azhar University, Cairo, Egypt; 6Neurology department, Faculty of Medicine, Al-Azhar University, Cairo, Egypt
Correspondence: Ayat A Awwad, Otorhinolaryngology department, Faculty of Medicine, Al-Azhar University, Al Zhraa University Hhospital, Alabasia, Cairo, 11517, Egypt, Email [email protected]; [email protected] Osama MM Abd Elhay, Medical Physiology Department, Faculty of Medicine, Al-Azhar University, Cairo, Egypt, Email [email protected]; [email protected]
Background: COVID-19 (SARS-CoV-2/2019-nCoV) is now a major public health threat to the world. Olfactory dysfunctions (ODs) are considered potential indicating symptoms and early case identification triaging for coronavirus disease 2019 (COVID-19). The most common reported comorbidities are diabetes mellitus, chronic lung disease, and cardiovascular disease. The objective of this study was to evaluate prevalence of different types of smell disorders in patients with laboratory-confirmed COVID-19 infection and impact of involved systemic diseases.
Methodology: A cross-sectional retrospective study has been done for patients with laboratory-confirmed COVID-19 infection (mild-to-moderate). The data collected from patient’s files and developed online electronic questionnaire (WhatsApp) based on the patients most common and recurrent reported data including: a) symptoms of olfactory dysfunction and associated covid19 symptoms fever and headache, cough, sore throat, pneumonia, nausea, vomiting and diarrhea, arthralgia and myalgia and taste dysfunction. b) Associated systemic diseases including: diabetes, hypertension, asthma, chronic renal disease, chorionic liver disease and hypothyroidism.
Results: Of 308 patients confirmed with Covid-19 infection, (72.4%) developed OD distributed as follows; complete anosmia (57.8%), troposmia (8.4%), hyposmia (2.9%), partial anosmia (2.6%) and euosmia (0.6%). Significantly increased prevalence of diabetes, hypertension asthma in the group with olfactory dysfunction (p < 0.001), chronic liver disease (p = 0.005), and hypothyroidism (p = 0.03).
Conclusion: The development of ODs after Covid-19 infection was associated with mild disease form and lower hospitalization. In addition, it showed significant relationship with preexisting systemic diseases. Anosmia is the common modality of ODs.
Keywords: COVID-19, anosmia, olfactory dysfunction
Introduction
World Health Organization (WHO) declared coronavirus disease 2019 (COVID-19) to be pandemic after it quickly spread all over the world.1 The involved cause is severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).2 Human-to-human transmission is extremely rapid.3 Coronavirus is contagious with an incubation period ranging from 2 to 14 days. Through this period, patients can transmit infection even if asymptomatic.4
Asia reported that the most prevalent symptoms as: fever, myalgia, arthralgia cough, dyspnea, headache, diarrhea, rhinorrhea, and sore throat.5 Also, respiratory complications as pneumonia, lung fibrosis, and even death have been reported.6 Later, atypical presentation of the disease is widely observed including olfactory and gustatory malfunction but without rhinorrhea or nasal obstruction which are usually associated to other respiratory viral infections.7
WHO considers smell disorders as key symptoms of COVID-19.8 The American Academy of Otolaryngology–Head and Neck Surgery Foundation, 20209 together with Ear, Nose, and Throat Society of the United Kingdom (ENTUK) recommended self-isolation for patients presenting with these clinical features. Many countries reported the association smell disorder and taste with COVID-1910–12, but evidence remains controversial. In addition, none of them was concerned the incidence of different types of dysfunction.
COVID-19 virus appears to be more severe in severe older people and people with systemic conditions (such as diabetes, hypertension and asthma).13
The previous studies confirmed on olfactory dysfunction alone, neither its types nor impact of chronic diseases on OD so the aim of this study is to evaluate prevalence of different types of smell disorders in patients with laboratory-confirmed COVID-19 infection and impact of involved systemic diseases on ODs.
Methodology
The study was approved from Ethical Committee of the Faculty of medicine of Al-Azhar University (IRP) which complies with the Declaration of Helsinki. A cross-sectional retrospective study to patients with laboratory-confirmed COVID-19 infection (mild-to-moderate) who admitted in Al - Azhar University hospitals, fever hospitals, in addition to some of our patient’s clinics in Cairo, Egypt between the period from June 2020 to December 2020. The patients were divided into asymptomatic and symptomatic. The severity of the symptomatic diseases was classified into mild, moderate and severe.14
Informed consent was obtained from all patients (or a parent or legal guardian of patients under 18 years of age). The data collected from patient’s files and developed online electronic questionnaire (WhatsApp). Electronic questionnaire was designed by Professional otorhinolaryngologist, so that each participant could complete the survey. Questionnaire was based the patients most common and recurrent reported data including:
- 4 items for assessment of ODs including: presence of smell dysfunction (yes or no), types of smell dysfunction (anosmia, hyposmia, partial anosmia, euosmia and troposmia).
- 8 items for symptoms associated with covid-19 including: fever, headache, cough, sore throat, pneumonia, nausea, vomiting, diarrhea, arthralgia, myalgia and taste dysfunction (yes/no)
- 6 items for associated systemic diseases including: diabetes, hypertension, asthma, chronic renal disease, chorionic liver disease and hypothyroidism (yes or no)
Inclusion criteria were: (> 12 years old of both genders); laboratory-confirmed COVID-19 infection (reverse transcription polymerase chain reaction, RT-PCR); native speaker patients, and patients clinically able to fulfill the questionnaire.
Patients with history of smell disorders, and not confirmed COVID-19, were unable to fulfill the questionnaire in addition to patients admitted to intensive care were also excluded from the study.
The data were collected, tabulated, and analyzed by Statistical Package for Social Sciences (version 21; SPSS Inc., Chicago, IL, USA). Two types of statistics were done:
- Descriptive statistics [eg percentage (%), mean (x) and standard deviation (SD)],
- Analytic statistics: which include the following:
- Chi-square test (χ2): was used to indicate presence or absence of a statistically significant difference between two qualitative variables.
- P-value of <0.05 was considered statistically significant.
Results
Demographic characteristics, comorbidities, and symptoms at the onset were reported in all patients confirmed with COVID-19 as shown in Table 1.
 |
Table 1 Characteristics of Study Participants (N = 308) |
The prevalence of ODs were 72% (223) with anosmia being the most common presented type (57.8%) while euosmia was the least presented type being only in (0.6%) as shown in Table 2 and Figure 1.
 |
Table 2 Prevalence of Different Types of Olfactory Dysfunction Among COVID-19 Infected Patients (N = 308) |
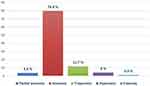 |
Figure 1 Prevalence of olfactory dysfunction types among cases who suffers from this dysfunction. |
The frequency of ODs were significantly high with increasing in age (P value =0.000). But there was no significant difference between genders (P value =0.167) as reported in Table 3. Significant increases in different types of ODs with increasing in age (P value =0.000) while, there was no significant difference regarding gender (P value = 0.564) as shown in Table 4. Anosmia was the commonest presenting type of smell dysfunction in both genders (Figure 2).
 |
Table 3 Frequency Distribution of Olfactory Dysfunction Occurrence According to Age and Sex |
 |
Table 4 Frequency Distribution of Olfactory Dysfunction Types in Relation to Age and Sex |
 |
Figure 2 Percent distribution of olfactory dysfunction types according to sex. |
Regarding to other symptoms, the frequency of ODs were significantly associated to fever and headache, arthralgia, myalgia, taste dysfunction (P value =0.000), cough (P value =0.001), sore throat (P value =0.037), diarrhea, nausea and vomiting (P value =0.002). But it was not significantly associated with Pneumonia (P value =0.077) as shown in Table 5. ODs were the only presenting symptoms in 59.7% of patients Figure 3.
 |
Table 5 Frequency Distribution of Olfactory Dysfunction Occurrence According to the Other Symptoms Experienced |
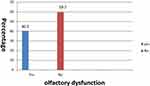 |
Figure 3 Percent distribution of olfactory dysfunction as the only presenting symptom. |
The frequency of ODs were significantly associated with diabetes, hypertension, asthma (P value=0.000), chronic liver disease, hypothyroidism (P value =0.003) and chronic renal disease (P value =0.005) as reported in Table 6. The different types of smell dysfunction showed significant association with asthma, chronic renal disease (P value =0.000), diabetes (P value =0.003), and hypertension (P value =0.002) while, there was no significant association with chronic liver disease and hypothyroidism (P value =0.158 and 0.524 respectively). Anosmia was the most common type of OD in association with diabetes, hypertension, asthma and chronic liver disease while, troposmia was the most common type of OD associated with chronic renal disease. The only case presented with euosmia was reported in chronic liver disease Table 7.
 |
Table 6 Frequency Distribution of Olfactory Dysfunction Occurrence According to the Different Comorbidities |
 |
Table 7 Frequency Distribution of Olfactory Dysfunction Types in Relation to the Comorbidities |
Discussion
The CDC (Center for Disease Control and Prevention) has highlighted the loss of smell as a significant symptom of COVID-19. In addition, recent research has indicated that OD may serve as an early clinical manifestation of this contagious.15–17
The current study was conducted to study the prevalence of different types of olfactory disorders in patients with laboratory-confirmed COVID-19 infection and its relationship with preexisting systemic comorbidities. Handling the effects of systemic comorbidities on olfactory manifestations in Covid-19 patients is poorly discussed in the literature. This poses a strong point in favor of our study.
We included a total of 308 patients confirmed with Covid-19 infection, 223 patients from them developed olfactory dysfunction (72.4%). When analyzing OD encountered in our research, it was distributed as follows; complete anosmia (57.8%), troposmia (8.4%), hyposmia (2.9%), partial anosmia (2.6%) and euosmia (0.6%). This is in line with multiple previous studies which reported that smell alternations are frequent manifestations of Covid-19 infection, with a prevalence ranging from 19.4% to 88%.3,12,13
This prevalence appears to be widely different between different studies. Mao et al reported lower prevalence (5%) in China18 and Marzano et al (18%) in Italy.19 Others reported much higher prevalence, reaching up to 98% in the study of Moein et al20 and 100% in the study of Heidari et al21 in Iran. This great heterogenicity could be explained by different sample sizes, patient characteristics, and methods of evaluating OD. In addition, Meng et al22 reported the difference of incidence in different countries as COVID-19 has three central variants A, B and C. Variants A and C which affect the nasal cavity causing OD were prevalent in Europe and America. Beside, human species affects significantly the susceptibility for infection.
Brann et al23 suggested that OD associated with Covid-19 infection is due to viral invasion of olfactory epithelial cells and vascular pericytes, which will negatively impact olfactory neuronal function. Additionally, nasal inflammation, congestion, and swelling may prevent olfactory molecules from reaching the olfactory cleft. Therefore, this conductive malfunction may play a role in developing OD.24
Lechien et al25 study handling the same perspective, the encountered OD was distributed as follows; anosmia (79.6%), while the remaining cases had hyposmia (20.4%). In another study, Vaira et al26 reported that among the Covid patients diagnosed with olfactory dysfunction, mild, moderate, and severe hyposmia was detected in 76, 59, and 45 patients, respectively. In addition, the remaining 61 cases had anosmia. It is expected to find some differences between different studies regarding the type of olfactory function diagnosed, according to the sample population included and criteria used to define each type.
In the current study, a significant difference was noted between patients with and without OD regarding patient age (p < 0.001), which tended to be significantly younger in the OD group. On the contrary a previous meta-analysis by Desiato et al17 has against this relationship. Several mechanisms could explain this association between advancing age and declining olfactory function including, nasal epithelial atrophy, olfactory bulb shrinkage, cribriform plate changes, in addition to age-associated cortical degeneration.27–29,30
However, another study by Mercante et al31 reported that the severity of OD was significantly increased in younger individuals, while older ones expressed mild or no symptoms. This confirms our findings.
Our findings showed no significant impact of gender on the development of this complication (p = 0.167). Thakur et al25 confirmed the previous findings regarding the insignificant association between gender and OD (p = 0.59). On the other hand, a recent meta-analysis by Saniasiaya et al32 had shown that the female gender is a risk factor for this manifestation, as it showed higher predominance compared to men. Researchers attributed that finding to the sex-related difference in the inflammatory process.33 Additionally, female patients were more sensitive than males to detect small alternations.32
Our findings showed a significant association between OD and fever, which is more prevalent in patients with this complication. In accordance with the previous results, Lechien et al25 reported a significant positive association between OD and fever (p < 0.001).
In the current study, the headache was significantly more prevalent in patients with OD (p < 0.001). This coincides with multiple previous studies which confirmed the association between headache and olfactory disturbances.34,35 This association was explained by either CNS involvement by the virus itself or hypoxic headache, which results from nasal congestion, which is associated with a decrease in olfactory function.36,37
In our study, taste dysfunction was significantly more encountered in patients with olfactory disturbances. This was confirmed before; as Lechien et al26 reported a significant positive association between both olfactory and gustatory functions (p < 0.001). Also Speth et al38 confirmed the previous findings.
In the current study, one could notice the significantly higher prevalence of other clinical findings (including sore throat, cough, diarrhea, nausea, vomiting, arthralgia, and myalgia) in association with OD.
Likewise, Talavera et al39 also reported the significant relationship between anosmia, myalgia, and cough in patients with Covid disease (p = 0.006). Nevertheless, other manifestations did not express a significant association with olfactory disturbances (p > 0.05).
Conversely, Yan et al12 reported that olfactory dysfunction was associated with a mild disease form. Moreover, another study Izquierdo-Domínguez et al40 reported that the same dysfunction was associated with lower C-reactive protein levels and a lower need for hospitalization.
Our findings showed significantly increased diabetes prevalence in the group with OD (p < 0.001). Although there is a paucity of studies handling the link between diabetes and OD in Covid-19 patients, the association between diabetes and the development of such dysfunction is well documented in a recent meta-analysis by Kim et al.41
Multiple mechanisms could induce this, including olfactory neurodegeneration and diabetes-associated microvascular disease.22,42,43 Of course, with the presence of these diabetes-associated factors, catching Covid-19 infection will increase the chance of having that dysfunction, especially in diabetic personnel. It was previously reported that the diabetic population is at high risk of having OD compared to healthy controls (OR = 1.58).41 In contrast to the previous findings, Talavera et al39 noted no significant impact of diabetes on the development of anosmia (p = 0.448). It was present in 17.1% and 20.5% of patients with and without anosmia, respectively.
Our findings showed that olfactory disturbances were significantly associated with hypertension (p < 0.001). Hypertension was present in 23.3% and 3.5% of patients with and without this dysfunction. We are the first researchers to report that finding in Covid-19 patients to the best of our knowledge. Our finding is supported by the accumulating evidence supporting the association between OD and cardiovascular disease.44,45 Several theories could explain this association; cardiovascular disease is common in the elderly, which is associated with degenerative neuronal changes, as discussed before. Also, the proinflammatory cytokines present with atherosclerosis could decrease olfactory function. Furthermore, some cardiovascular medications have a negative impact on hearing.46,47,48
In a recent study conducted in 2021, hypertensive patients expressed a lower prevalence of OD (p < 0.001), which was present in 74.9% and 88% of patients without and with hypertension, respectively.3 This is in contrast with our findings. In fact, the role of hypertension and the potential intake of angiotensin-converting enzyme inhibitors in the development of OD need to be well discussed in the upcoming studies.
In the current study, the prevalence of asthma showed a significant increase in patients with OD (p < 0.001). Asthma and olfactory impairment have never been linked, according to a recent report published in 2021 by Rhyou et al.48 However, the presence of allergic rhinitis or sinusitis in association with asthma surely decreases the olfactory sensation.29,49
Another study negated any significant difference between the anosmia and non-anosmia groups regarding the prevalence of respiratory diseases, which was present in 19.9% and 27% of patients in the same groups, respectively (p = 0.109).39
Our findings showed a higher prevalence of chronic liver disease in association with anosmia (p = 0.003). Previously, Heiser et al50 reported that olfactory deficits are frequently encountered in patients with cirrhosis. This functional decline is the result of calorie, protein, and micronutrient deficiency in such patients.51 This evidence was supported by the improvement of this function after liver transplantation, as reported by Bloomfield et al.52
In the current study, the prevalence of chronic kidney disease was significantly higher in association with OD (p = 0.005). In fact, patients with such comorbidities often complain of olfactory impairment, which could be the consequence of malnutrition and decreased fluid intake.53,54 Uremia itself could induce neuropathy and decreased smell sensation.54
Our findings showed that hypothyroidism was significantly more common in the OD group (p = 0.03). In line with the previous findings, Tsivgoulis et al55 have reported that hypothyroidism is associated with more prolonged Covid-19 induced anosmia. Sorrily, there is no clear data about whether hypothyroidism can induce OD in adult humans.56
Our study has some limitations; we should have evaluated the impact of OD on patient outcome and long-term nasal function. In addition to this retrospective study may together have some bias to mention. This study did not perform an objective olfactory test on the patients but was based on an electronic questionnaire, which may affect the accuracy of the survey.
All in all, based on our findings, complete anosmia was the most presented modality of OD. Fever, headache, taste dysfunction, sore throat, cough, diarrhea, nausea, vomiting, arthralgia, and myalgia were common symptoms associated with OD. Mild disease form, low C-reactive protein and lower need for hospitalization were common association with OD. Significant increases in incidence of OD in diabetes mellitus, hypertension, bronchial asthma, chronic liver disease, chronic kidney disease and hypothyroidism. Lower incidence of respiratory symptoms in anosmia compared to non-anosmia group.
Funding
The authors afforded all sources of funding for this research.
Disclosure
The authors report no conflicts of interest in relation to this work.
References
1. European Centre for Disease Prevention and Control (ECDC). COVID-19 situation update worldwide, as of 27 May 2020. Available from: https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases.
2. Ramanathan K, Antognini D, Combes A, et al. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med. 2020;8:518–526. doi:10.1016/S2213-2600(20)30121-1
3. Lechien JR, Chiesa-Estomba CM, De Siati DR. Epidemiological, otolaryngological, olfactory and gustatory outcomes according to the severity of COVID-19: a study of 2579 patients. Eur Arch Otorhinolaryngol. 2021;278(8):2851–2859. doi:10.1007/s00405-020-06548-w
4. Ibekwe TS, Fasunla AJ, Orimadegun AE. Systematic review and meta-analysis of smell and taste disorders in COVID-19. OTO Open. 2020;4(3):2473974X20957975. doi:10.1177/2473974X20957975
5. Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323:1488. doi:10.1001/jama.2020.3204
6. Sohrabi C, Alsafi Z, O’Neill N, et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19). Int J Surg. 2020;76:71–76. doi:10.1016/j.ijsu.2020.02.034
7. Van Riel D, Verdijk R, Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol. 2015;235(2):277–287. doi:10.1002/path.4461
8. World Health Organization. Health topic: coronavirus. Available from: https://www.who.int/health-topics/coronavirus#tab=tab_3.
9. American Academy of Otolaryngology–Head and NeckSurgery. Anosmia, hyposmia, and dysgeusia symptoms of coronavirus disease. Available from: https://www.entnet.org/content/aao-hns-anosmia-hyposmia-and-dysgeusia-symptoms-coronavirus-disease.
10. Spinato G, Fabbris C, Polesel J, et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA. 2020;323:2089–2090. doi:10.1001/jama.2020.6771
11. Castillo-López IY, Govea-Camacho LH, Rodríguez-Torres IA, Recio-Macías DA, Alobid I, Mullol J. Olfactory dysfunction in a mexican population outside of COVID-19 pandemic: prevalence and associated factors (the OLFAMEX Study). Curr Allergy Asthma Rep. 2020;20(12):78. doi:10.1007/s11882-020-00975-9
12. Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020;10(7):806–813. doi:10.1002/alr.22579
13. Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020;130(7):1787. doi:10.1002/lary.28692
14. Al-Ani RM, Acharya D. Prevalence of anosmia and ageusia in patients with COVID-19 at a primary health center, Doha, Qatar. Indian J Otolaryngol Head Neck Surg. 2020. doi:10.1007/s12070-020-02064-9
15. Lao WP, Imam SA, Nguyen SA. Anosmia, hyposmia, and dysgeusia as indicators for positive SARS-CoV-2 infection. World J Otorhinolaryngol Head Neck Surg. 2020;6(1):S22–s25. doi:10.1016/j.wjorl.2020.04.001
16. Whitcroft KL, Hummel T. Olfactory dysfunction in COVID-19: diagnosis and management. JAMA. 2020;323(24):2512–2514. doi:10.1001/jama.2020.8391
17. Desiato VM, Levy DA, Byun YJ, Nguyen SA, Soler ZM, Schlosser RJ. The prevalence of olfactory dysfunction in the general population: a systematic review and meta-analysis. Am J Rhinol Allergy. 2021;35(2):195–205. doi:10.1177/1945892420946254
18. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi:10.1001/jamaneurol.2020.1127
19. Marzano AV, Genovese G, Fabbrocini G, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: multicenter case series of 22 patients. J Am Acad Dermatol. 2020;83(1):280–285. doi:10.1016/j.jaad.2020.04.044
20. Moein ST, Hashemian SM, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020;10(8):944–950. doi:10.1002/alr.22587
21. Heidari F, Karimi E, Firouzifar M, et al. Anosmia as a prominent symptom of COVID-19 infection. Rhinology. 2020;58(3):302–303. doi:10.4193/Rhin20.140
22. Meng X, Deng Y, Dai Z, Meng Z. COVID-19 and anosmia: a review based on up-to-date knowledge. Am J Otolaryngol. 2020;41(5):102581. doi:10.1016/j.amjoto.2020.102581
23. Brann DH, Tsukahara T, Weinreb C, et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv. 2020;6(31). doi:10.1126/sciadv.abc5801
24. Thakur K, Sagayaraj A, Prasad KC, Gupta A. Olfactory dysfunction in COVID-19 patients: findings from a tertiary rural centre. Indian J Otolaryngol Head Neck Surg. 2021;1–7. doi:10.1007/s12070-021-02364-8
25. Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251–2261. doi:10.1007/s00405-020-05965-1
26. Vaira LA, Hopkins C, Salzano G, et al. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck. 2020;42(7):1560–1569. doi:10.1002/hed.26269
27. Kalmey JK, Thewissen JG, Dluzen DE. Age-related size reduction of foramina in the cribriform plate. Anat Rec. 1998;251(3):326–329. doi:10.1002/(SICI)1097-0185(199807)251:3<326::AID-AR7>3.0.CO;2-T
28. Sama Ul H, Tahir M, Lone KP. Age and gender-related differences in mitral cells of olfactory bulb. J Coll Physicians Surg Pak. 2008;18(11):669–673.
29. Segura B, Baggio HC, Solana E, et al. Neuroanatomical correlates of olfactory loss in normal aged subjects. Behav Brain Res. 2013;246:148–153. doi:10.1016/j.bbr.2013.02.025
30. Doty RL, Kamath V. The influences of age on olfaction: a review. Front Psychol. 2014;5:20. doi:10.3389/fpsyg.2014.00020
31. Mercante G, Ferreli F, De Virgilio A, et al. Prevalence of taste and smell dysfunction in coronavirus disease 2019. JAMA Otolaryngol Head Neck Surg. 2020;146(8):723–728. doi:10.1001/jamaoto.2020.1155
32. Saniasiaya J, Islam MA, Abdullah B. Prevalence of olfactory dysfunction in coronavirus disease 2019 (COVID-19): a meta-analysis of 27,492 patients. Laryngoscope. 2021;131(4):865–878. doi:10.1002/lary.29286
33. Lefèvre N, Corazza F, Valsamis J, et al. The number of X chromosomes influences inflammatory cytokine production following toll-like receptor stimulation. Front Immunol. 2019;10:1052. doi:10.3389/fimmu.2019.01052
34. Sedaghat AR, Gengler I, Speth MM. Olfactory dysfunction: a highly prevalent symptom of COVID-19 with public health significance. Otolaryngol Head Neck Surg. 2020;163(1):12–15. doi:10.1177/0194599820926464
35. Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi:10.1016/j.bbi.2020.03.031
36. Hatton CF, Duncan CJA. Microglia are essential to protective antiviral immunity: lessons from mouse models of viral encephalitis. Front Immunol. 2019;10:2656. doi:10.3389/fimmu.2019.02656
37. Ralli M, Di Stadio A, Greco A, de Vincentiis M, Polimeni A. Defining the burden of olfactory dysfunction in COVID-19 patients. Eur Rev Med Pharmacol Sci. 2020;24(7):3440–3441. doi:10.26355/eurrev_202004_20797
38. Speth MM, Singer-Cornelius T, Oberle M, Gengler I, Brockmeier SJ, Sedaghat AR. Olfactory dysfunction and sinonasal symptomatology in COVID-19: prevalence, severity, timing, and associated characteristics. Otolaryngol Head Neck Surg. 2020;163(1):114–120. doi:10.1177/0194599820929185
39. Talavera B, García-Azorín D, Martínez-Pías E, et al. Anosmia is associated with lower in-hospital mortality in COVID-19. J Neurol Sci. 2020;419:117163. doi:10.1016/j.jns.2020.117163
40. Izquierdo-Domínguez A, Rojas-Lechuga MJ, Chiesa-Estomba C, et al. Smell and taste dysfunction in COVID-19 is associated with younger age in ambulatory settings: a multicenter cross-sectional study. J Investig Allergol Clin Immunol. 2020;30(5):346–357. doi:10.18176/jiaci.0595
41. Kim SJ, Windon MJ, Lin SY. The association between diabetes and olfactory impairment in adults: a systematic review and meta-analysis. Laryngoscope Investig Otolaryngol. 2019;4(5):465–475. doi:10.1002/lio2.291
42. Brady S, Lalli P, Midha N, et al. The presence of neuropathic pain may explain poor performances on olfactory testing in diabetes mellitus patients. Chem Senses. 2013;38(6):497–507. doi:10.1093/chemse/bjt013
43. Gouveri E, Katotomichelakis M, Gouveris H, Danielides V, Maltezos E, Papanas N. Olfactory dysfunction in type 2 diabetes mellitus: an additional manifestation of microvascular disease? Angiology. 2014;65(10):869–876. doi:10.1177/0003319714520956
44. Liu B, Luo Z, Pinto JM, et al. Relationship between poor olfaction and mortality among community-dwelling older adults: a cohort study. Ann Intern Med. 2019;170(10):673–681. doi:10.7326/M18-0775
45. Siegel JK, Wroblewski KE, McClintock MK, Pinto JM. Olfactory dysfunction persists after smoking cessation and signals increased cardiovascular risk. Int Forum Allergy Rhinol. 2019;9(9):977–985. doi:10.1002/alr.22357
46. Thiebaud N, Johnson MC, Butler JL, et al. Hyperlipidemic diet causes loss of olfactory sensory neurons, reduces olfactory discrimination, and disrupts odor-reversal learning. J Neurosci. 2014;34(20):6970–6984. doi:10.1523/JNEUROSCI.3366-13.2014
47. Schubert CR, Cruickshanks KJ, Fischer ME, et al. Carotid intima-media thickness, atherosclerosis, and 5-year decline in odor identification: the beaver dam offspring study. J Gerontol a Biol Sci Med Sci. 2015;70(7):879–884. doi:10.1093/gerona/glu158
48. Roh D, Lee DH, Kim SW, et al. The association between olfactory dysfunction and cardiovascular disease and its risk factors in middle-aged and older adults. Sci Rep. 2021;11(1):1248. doi:10.1038/s41598-020-80943-5
49. Jung AY, Kim YH. Reversal of olfactory disturbance in allergic rhinitis related to omp suppression by intranasal budesonide treatment. Allergy Asthma Immunol Res. 2020;12(1):110–124. doi:10.4168/aair.2020.12.1.110
50. Heiser C, Haller B, Sohn M, et al. Olfactory function is affected in patients with cirrhosis depending on the severity of hepatic encephalopathy. Ann Hepatol. 2018;17(5):822–829. doi:10.5604/01.3001.0012.3143
51. Gundling F, Seidl H, Pehl C, Schmidt T, Schepp W. How close do gastroenterologists follow specific guidelines for nutrition recommendations in liver cirrhosis? A survey of current practice. Eur J Gastroenterol Hepatol. 2009;21(7):756–761. doi:10.1097/MEG.0b013e328311f281
52. Bloomfeld RS, Graham BG, Schiffman SS, Killenberg PG. Alterations of chemosensory function in end-stage liver disease. Physiol Behav. 1999;66(2):203–207. doi:10.1016/S0031-9384(98)00266-2
53. Frasnelli JA, Temmel AF, Quint C, Oberbauer R, Hummel T. Olfactory function in chronic renal failure. Am J Rhinol. 2002;16(5):275–279. doi:10.1177/194589240201600511
54. Koseoglu S, Derin S, Huddam B, Sahan M. The effect of non-diabetic chronic renal failure on olfactory function. Eur Ann Otorhinolaryngol Head Neck Dis. 2017;134(3):161–164. doi:10.1016/j.anorl.2016.04.022
55. Tsivgoulis G, Fragkou PC, Karofylakis E, et al. Hypothyroidism is associated with prolonged COVID-19-induced anosmia: a case-control study. J Neurol Neurosurg Psychiatry. 2021;92:911–912. doi:10.1136/jnnp-2021-326587
56. Günbey E, Karlı R, Gökosmanoğlu F, et al. Evaluation of olfactory function in adults with primary hypothyroidism. Int Forum Allergy Rhinol. 2015;5(10):919–922. doi:10.1002/alr.21565
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
