Back to Journals » Patient Preference and Adherence » Volume 17
Impact of Potential Symptoms and Risks Associated with Acid Sphingomyelinase Deficiency on Patients and Caregivers: A Best-Worst Scaling Study
Authors Mansfield C , Nalysnyk L, Joshi D, Coulter J , Pulikottil-Jacob R
Received 20 July 2022
Accepted for publication 25 February 2023
Published 30 March 2023 Volume 2023:17 Pages 927—939
DOI https://doi.org/10.2147/PPA.S381371
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Johnny Chen
Carol Mansfield,1 Lubomyra Nalysnyk,2 Dhaivat Joshi,2 Joshua Coulter,1 Ruth Pulikottil-Jacob3
1Health Preference Assessment, RTI Health Solutions, Research Triangle Park, North Carolina, USA; 2Health Economics and Value Assessment, Sanofi, Cambridge, MA, USA; 3Health Economics and Value Assessment, Sanofi, Thames Valley Park, Reading, UK
Correspondence: Ruth Pulikottil-Jacob, Sanofi, Thames Valley Park, Reading, RG6 1AD, UK, Tel +44-7525594087, Email [email protected]
Purpose: Acid sphingomyelinase deficiency (ASMD) is a rare, progressive, and potentially fatal disease affecting major organs; its symptoms present heterogeneously. Data on the most bothersome symptoms for patients with ASMD types B or A/B and their caregivers or parents are limited. We conducted a survey to quantify the relative impact of potential ASMD symptoms and risks for patients and parents/caregivers.
Patients and Methods: Twenty respondents, recruited via National Niemann-Pick Disease Foundation (United States) and Niemann-Pick United Kingdom, took a preference survey: 11 patients who had a self-reported diagnosis of ASMD types B or A/B and 9 parents who had a child with ASMD types B or A/B. Using object-case best-worst scaling, we explored the most and least bothersome among a set of 15 ASMD symptoms/risks selected based on clinical input and qualitative research with patients and caregivers. In 15 experimentally designed questions containing five items each, respondents ranked the symptoms/risks, irrespective of their experiences with them. Data were analyzed using a conditional multinomial logit model.
Results: Patients reported constant abdominal pain, severe pain in bones and joints, and severe fatigue to be the most bothersome potential symptoms or risks, followed by a chance of bleeding in the spleen. The next most bothersome potential symptom was constant shortness of breath. Easy bruising and noticeable abdominal enlargement were among the least bothersome symptoms. The most bothersome symptom for parents was bleeding in the spleen.
Conclusion: Patients and parents had similar perceptions of the most bothersome potential symptoms/risks. Despite the small sample size typical of rare disease studies, understanding patient preferences is important for such diseases and can inform shared decision-making.
Keywords: best-worst scaling, patient, caregiver, rare disease
Introduction
Acid sphingomyelinase deficiency (ASMD), historically known as Niemann-Pick disease type A, type A/B, and type B, is a rare, progressive, potentially fatal lysosomal storage disease caused by pathogenic variants in the SMPD1 gene that affect multiple organ systems.1 ASMD varies in severity, and symptom presentation is heterogeneous.2 The most severe form of ASMD, type A (infantile neurovisceral ASMD), is characterized by extensive central nervous system (CNS) involvement along with visceral manifestations,1 whereas ASMD type B (chronic visceral ASMD) has little to no neurological involvement but affects the lungs, liver, spleen, heart, and other organs, potentially leading to serious and life-threatening complications.2 ASMD type A/B, an intermediate form, involves more significant neurological symptoms than ASMD type B.2 Historically, patients with ASMD have relied on supplemental breathing aides, occupational and physical therapy, and equipment such as spleen-guards to address the symptoms and risks of the disease. Recently, the first disease-modifying treatment for non-neurologic symptoms of ASMD (olipudase alfa, an acid sphingomyelinase enzyme replacement therapy) was approved in the European Union, the United States (US), and other countries.3–6
No ASMD-specific patient-reported outcome (PRO) measure exists, and limited data exist regarding ASMD-related disease burdens and associated symptoms.2,7 Research evaluating patient and caregiver (ie, parent, relative, or guardian) experiences has suggested that the most bothersome symptoms of ASMD are fatigue, shortness of breath, bruising/bleeding, lower limb/joint pain, sleep disturbances, headaches, and diarrhea.7 However, the relative impact of ASMD’s symptoms has not been measured. To help fill this knowledge gap, preference surveys, using object-case best-worst scaling (BWS) methodology, were conducted to quantify the relative impact of potential ASMD symptoms and risks among patients and parents of patients with ASMD types B or A/B and inform shared decision-making and treatment improvements. BWS methods are used in a variety of contexts to gather information on the relative importance, burden, or bothersomeness of disease impacts and adverse events.8–11
Materials and Methods
Survey Development
Two online surveys containing object-case BWS questions were used to explore the relative importance of 15 actual or potential ASMD symptoms and risks. The symptoms and risks were selected on the basis of qualitative research with patients and caregivers and after consultation with clinical experts.2,7 The surveys, one for patients and one for parents, contained demographic and background questions about ASMD symptoms and treatments and patient-friendly descriptions of the 15 symptoms and risks. The survey instruments were also reviewed by the panel members of the National Niemann-Pick Disease Foundation, Inc. (NNPDF), and Niemann-Pick United Kingdom (NPUK) advocacy organizations; their input was incorporated into the final surveys.
Object-case BWS has been used extensively in healthcare settings.8–14 Prior studies have used object-case BWS to assess the potential benefits and risks of treatments, rank the relative importance of adverse events that patients might experience from treatments, and rank preferences for different modes of treatment administration.11–13 In object-case BWS, respondents answer a series of questions, each question presenting a subset of features or outcomes from a longer list of such items. Respondents identify the best or most preferred item and the worst or least preferred item in each subset. This yields the relative importance of each item included in the BWS exercise.
Table 1 lists the 15 items (symptoms and risks) included in the BWS exercise, some of which are different levels of severity of the same symptom. Respondents were shown a series of 15 questions, each question containing a varying subset of five items from the complete list of 15 items shown in Table 1. Figure 1 presents an example question. In each question, respondents were asked to rank the symptom or risk from most bothersome to least bothersome, even if the respondents had not experienced the symptom or risk. The BWS method requires all respondents to rank all 15 items, and each respondent expresses preferences based on their own experience and circumstances.
 |
Table 1 Best-Worst Scaling Symptoms and Risks and Number of Times Each Symptom or Risk Selected as Most or Least Bothersome |
A total of 30 BWS questions each containing 5 items were created using a balanced incomplete block design created in SAS.14–17 The questions were split into 2 blocks of 15 questions, and respondents were randomly assigned to view one block of 15 questions. Appendix A presents the 5 specific items that appeared in each of the 30 questions.
For the four symptoms with multiple levels of severity, the surveys first described each of the four symptoms and provided definitions of the levels of severity (eg, constant, frequent, occasional) in patient-friendly language, followed by questions about the respondents’ experiences with each of the symptoms (Table 2).
 |
Table 2 Symptoms/Risks and Levels of Severity |
To test the language and questions in the surveys, four pretest interviews were completed (two interviews were with caregivers who have a child of any age diagnosed with chronic ASMD, and the remaining two interviews were with adult ASMD patients aged ≥18 years). One patient and one parent each were recruited from the US (NNPDF) and the UK (NPUK).
Sample
The study sample was recruited from the memberships of two advocacy organizations: NNPDF, based in the US with a membership of 450 families, and NPUK, based in the UK with approximately 500 members. Recruitment was conducted via emails, social media, and publicity at conferences. To be eligible to complete the survey, individuals were required to be a resident of the US or UK, be aged 18 years or older, have a self-reported diagnosis of ASMD types B or A/B or have a child diagnosed with ASMD types B or A/B, and not be using an investigational therapy as part of a clinical trial for ASMD. The study design was reviewed by RTI International’s institutional review board (IRB) and determined to be exempt from full review (IRB ID STUDY00020258). All pretest participants and survey respondents were required to provide informed consent; participation in the survey was voluntary.
Analysis
Responses to all questions in the survey were summarized using descriptive statistics, except for the BWS questions. The patient sample was analyzed separately from the parent sample.
The BWS data were analyzed using a conditional multinomial logit (MNL) model that accounted for correlation in the responses from each respondent.13,18 Log-odds importance weights were estimated using an MNL model that related respondents’ choices for the most and least preferred items to the item-specific variables; items were effects coded.19 The log-odds importance weights represented the relative weights respondents placed on each item when selecting the most bothersome item. Larger coefficients indicated that the symptoms or risks were considered more important to avoid. Relative importance weights from the log-odds importance weights were then calculated, using a probability-based rescaling procedure.20–22 A Wald χ2 test was used to calculate the statistical significance of the difference between each pair of items; differences were considered statistically significant if P values were ≤0.05. The analyses were independently replicated by two analysts. The size of the sample precluded analysis of differences across subgroups of respondents.
Results
Cognitive Pretesting Interviews
In the four cognitive pretesting interviews, participants stated that they generally understood the survey and felt it was appropriate for patients with ASMD or their caregivers. Patients and parents noted that it was sometimes difficult to know whether a symptom was associated with ASMD. Parents also mentioned not knowing their child was experiencing a symptom until the child told them. To more comprehensively identify the range of patients’ and parents’ experiences, the question that asked respondents to identify the symptoms they had experienced was expanded to contain additional symptoms, including frequent respiratory infections, psychiatric or mental health problems, psoriasis, vision problems, insomnia/difficulty sleeping, asthma, eating disorders, and chance of bleeding from a light hit to the abdomen. Some participants mentioned that it was difficult to know how to respond when the questions included symptoms or risks that they had never experienced. Instructions were clarified to ask respondents to imagine that they (or their child) would eventually experience the symptom or risk, even if they had not yet experienced it, when ranking the items as most or least bothersome.
Sample Characteristics
The survey sample consisted of 20 respondents: 16 respondents in the US (10 patients and 6 parents) and 4 respondents in the UK (1 patient and 3 parents) (Table 3). Of the 11 patients who completed the survey, 8 (72.7%) were female. Of the 9 parents who completed the survey, 4 (44.4%) reported that their child with ASMD was female. Patients in the US had a median age of 5 years (range, 2–51 years) when they were diagnosed with ASMD. The UK patient was 52 years old when diagnosed with ASMD. The median age at diagnosis for children of parents in the UK and the US was 3 years (range, 3–14 years) and 2.3 years (range, 1–6 years), respectively. All patients and parents in our sample reported that they or their child had experienced most of the symptoms of ASMD presented in the survey. Most respondents reported that they or their child had experienced abdominal enlargement (80%), fatigue (75%), abdominal pain (75%), and shortness of breath (60%) (Table 3).
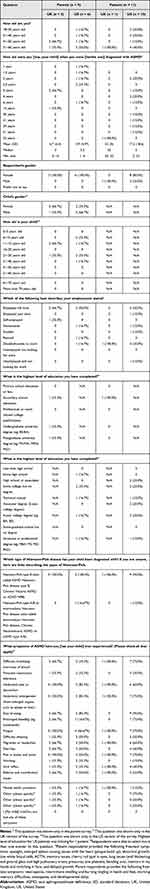 |
Table 3 Sample Characteristics |
Best-Worst Scaling
The BWS results indicated heterogeneity in respondents’ preferences pertaining to items of potential symptoms or risks presented in the survey. Among patients, only constant abdominal pain and severe fatigue were never selected as least bothersome; noticeable abdominal enlargement and easy bruising were the only items never selected as most bothersome. Among parents, every item was selected as least bothersome by at least one parent across all the questions (Table 1). Frequent headaches, mild-to-moderate fatigue, and easy bruising were the only items never selected by parents as most bothersome. Patients’ and parents’ preferences for ASMD symptoms and risks were generally aligned, with slight differences in the order of ranking.
Figure 2 presents the BWS rescaled relative importance estimates from the MNL model for patients and parents so that the most important item to patients—constant abdominal pain—is set to 10. The scaled relative importance weight for each of the remaining items can be interpreted as the effect of that item on utility relative to the utility of constant abdominal pain for that sample. Tables B1 and B2 in Appendix B present the statistical significance of the difference between each pair of items. For patients, constant abdominal pain was rated as most bothersome of all items included in the BWS exercise, although there were no statistically significant differences (at a threshold of P ≤ 0.05) between constant abdominal pain and severe pain in bones or joints and severe fatigue. The next most bothersome item was chance of bleeding in the spleen (which was statistically significantly different from constant abdominal pain [P = 0.046]). Constant abdominal pain (P = 0.001), severe pain in bones and joints (P = 0.004), and severe fatigue (P = 0.014) were statistically significantly more bothersome to patients than constant shortness of breath. For patients, the relative weights for constant shortness of breath, frequent abdominal pain, chance of bleeding in the spleen (defined herein as caused by a light hit to the abdomen), headaches, and diarrhea were not statistically different from one another. The only items statistically significantly less bothersome than frequent shortness of breath were mild-to-moderate fatigue (P = 0.017), both levels of abdominal enlargement (very noticeable enlargement [P = 0.002] and noticeable enlargement [P ≤ 0.000]), and easy bruising (P ≤ 0.000).
Parents viewed chance of bleeding in the spleen as the most bothersome item relative to all other BWS items, although there were no statistically significant differences between the chance of bleeding and severe pain in bones or joints, constant abdominal pain, and constant moderate shortness of breath. Further, there were no statistically significant differences between constant shortness of breath and severe pain in bones or joints, severe fatigue, constant abdominal pain, chance of bleeding in the spleen, frequent abdominal pain, or frequent moderate shortness of breath. However, frequent moderate shortness of breath was statistically significantly more bothersome than difficulty sleeping (P = 0.006), mild-to-moderate fatigue (P ≤ 0.000), either level of abdominal enlargement (very noticeable enlargement [P = 0.037] or noticeable enlargement [P = 0.001]), and frequent headaches (P = 0.039).
For both patients and parents, the largest differences in severity levels were observed for fatigue, joint pain, and abdominal pain. Parents did not statistically significantly differentiate between either level of abdominal enlargement, shortness of breath, and abdominal pain.
Discussion
Patients with ASMD experience a wide range of symptoms and symptom severity, reflecting the heterogeneity of this rare disease. Limited data exist regarding patient and parent perceptions of how bothersome potential ASMD symptoms and risks may be. To fill this gap, we conducted a preference survey in the US and UK to quantify patients’ and parents’ perceptions of the most and least burdensome ASMD symptoms and risks from a set of 15 potential symptoms and risks. Object-case BWS methodology was used to explore patient and parent preferences for these 15 potential symptoms and risks.
The MNL results, when corrected to account for correlation in the responses within each respondent, indicated that, on average, patients and parents had similar views on what were the most and least bothersome ASMD symptoms or risks presented in the survey. Patients ranked constant abdominal pain, severe pain in bones or joints, and severe fatigue as the most bothersome potential symptoms or risks, followed by a chance of bleeding in the spleen (although it was only statistically significantly different from constant abdominal pain [P = 0.046]). It should be noted that severe fatigue was defined as fatigue so severe that the respondent might need help taking care of themselves and doing daily activities. The next most bothersome potential symptom or risk, constant shortness of breath, was statistically significantly less bothersome to patients than severe pain in bones and joints (P = 0.004), severe fatigue (P = 0.014), and constant abdominal pain (P = 0.001). Parents had similar ratings, but in a somewhat different order. Parents viewed the chance of bleeding in the spleen as the most bothersome potential symptom or risk, although there was no statistically significant difference between the chance of bleeding and the next most bothersome items: severe pain in bones or joints, constant abdominal pain, and constant moderate shortness of breath. Irrespective of the findings from the BWS exercise, all 15 items constituted moderate-to-severe potential symptoms and risks that should receive prompt medical attention.
Our findings are broadly consistent with qualitative evidence indicating fatigue, shortness of breath, bleeding, and pain to be among the most bothersome ASMD symptoms for patients and caregivers.7 By evaluating and quantifying the relative impact of potential ASMD symptoms and risks, our study provides novel insights into the experiences of patients with ASMD and their caregivers that can inform shared decision-making with their physicians. Our results suggested some heterogeneity between patients and parents in the perceived burden of potential symptoms and risks. The chance of bleeding in the spleen was frequently selected as the most bothersome potential symptom or risk, chosen 22 times by patients and 26 times by parents. However, among the items that were selected as most bothersome at least 20 times by either patients or parents, the chance of bleeding in the spleen was the item most often selected as the least bothersome potential symptom or risk, indicating differing views about the impact of this item. Heterogeneity in our findings is not unexpected, given that ASMD affects multiple organ systems and has a heterogeneous presentation,2 and patients’ individual impressions of the bothersomeness of ASMD symptoms and risks reflect their unique experiences and circumstances.
Prior research supports the patient-perceived burden of respiratory symptoms and abdominal symptoms;23 the burden of other factors, such as ASMD-related fatigue and pain, is less well known. Our results suggested that several ASMD-related symptoms or risks carry a similar degree of bothersomeness. Although reductions in abdominal enlargement were less important overall than severe pain, fatigue, and constant shortness of breath, some respondents in our study prioritized spleen size over frequency of shortness of breath. A possible explanation for the importance of this item is that abdominal enlargement can contribute to fatigue, shortness of breath, and early satiety.
As with many rare diseases, generic measures may not comprehensively capture patients’ experiences with the disease, and no ASMD-specific PRO measure exists to capture ASMD-specific disease burdens and symptom severity.2 There are challenges associated with adapting existing PRO measures to rare diseases, particularly diseases with a heterogeneous presentation such as ASMD.24–26 For example, although symptoms of Gaucher disease may seem similar to those of ASMD, adapting a Gaucher disease measure for use in ASMD would not be suitable because symptoms of Gaucher disease are not always similar to those observed in patients with ASMD.27 Another difficulty in adapting a PRO measure is testing and demonstrating the validity of the adapted measure and its items, due to the small size of the patient population.28 Nevertheless, the results of this survey provided a potential foundation for the future development of a specific ASMD PRO measure. Such a measure could provide data on patient quality of life, help prioritize the symptoms of ASMD, and inform treatment improvements.
Limitations
Some limitations of this study should be considered. The survey presented 15 actual or potential symptoms and risks associated with ASMD types B or A/B identified as important to patients with ASMD in prior qualitative research;7 not all symptoms and risks associated with ASMD were presented. Not all respondents had experienced all symptoms presented in the surveys, and the symptoms and risks were separated from their pathophysiologic basis. Whether experience with a symptom or risk would have led a respondent to rank it as more or less bothersome would depend on the individual and the symptom or risk. Because of the small sample size and because most respondents had experienced most of the symptoms included in the BWS exercise, we were unable to analyze whether having experienced a given symptom or risk influenced the respondent’s perception of its impact. Further, many ASMD symptoms and risks are interrelated. Splenomegaly, for example, may be associated with abdominal enlargement, fatigue, difficulty sleeping, easy bruising, shortness of breath, abdominal pain, or chance of bleeding in the spleen. Because of splenomegaly’s association with other clinical manifestations of ASMD, multiple items in the surveys could be related to splenomegaly, potentially confounding the results. Likewise, it is unclear whether patients consistently interpreted fatigue as persistent overwhelming tiredness or whether they confounded the experience of fatigue from other symptoms such as shortness of breath; however, pretest interviews did not observe this issue.
The BWS approach has been widely used in healthcare settings to rank the importance of symptoms and outcomes. Patients and caregivers who participated in the pretesting of our surveys were able to understand the exercise and were able to select the most and least bothersome potential symptoms and risks. Future research should explore the impact of other known features of ASMD, including hepatomegaly, gastrointestinal symptoms, cardiac disease (eg, atherogenic dyslipidemia, coronary artery disease, or heart valve disease),2 and musculoskeletal manifestations, which will aid in understanding the burden of ASMD’s clinical presentation. Future research should also explore whether patients’ prior experiences with the symptoms and risks of ASMD have an influence on their perceptions of their impact.
The samples were recruited through patient advocacy organizations, whose preferences may not be representative of the broader population of ASMD patients or their caregivers. All clinical information was self-reported by the respondents, including ASMD type, and was not verified in patient health records, although the survey described the corresponding ASMD and Niemann-Pick classifications and contained links to websites that described the ASMD classifications. The sample size for this study was small, resulting in potential uncertainty in the findings. In addition, there is the potential that some respondents may have been relatives (eg, a sibling or parent of another respondent), possibly influencing the results. Recruiting patients with rare diseases into patient preference studies is a challenge, and studies of rare diseases often lack data on patient preferences, although such data can be important for decision-makers. In future quantitative preference studies of rare diseases with small sample sizes, researchers could consider mixed-methods studies that capture quantitative rankings via qualitative interviews. Alternatively, patient preference methods that produce individual-level data, such as the threshold technique, could be considered.
Conclusions
ASMD is a rare disease with a heterogeneous set of symptoms, which makes it challenging to identify priorities for research on treatments or develop disease-specific PROs. Little quantitative data exist on the preferences of patients and caregivers regarding the burden of potential ASMD symptoms and its impact on quality of life. Recruiting for studies of rare disease populations is challenging; however, despite this study’s small sample size, results provide important insights into patient views and concerns. This study, designed based on research and input from patients and caregivers, provides evidence on the relative impact of potential ASMD symptoms and risks that adds to the base of knowledge about the preferences of ASMD patients. Patients and parents had similar relative preferences when ranking potential symptoms and risks, although their responses displayed heterogeneity across the sample. Understanding patients’ concerns will help clinicians, researchers, and other stakeholders better support patient communities.
Abbreviations
ASMD, Acid sphingomyelinase deficiency; BWS, Best worst scaling; CNS, Central nervous system; MNL, Multinomial logit; NNPDF, National Niemann-Pick Disease Foundation; NPUK, Niemann-Pick United Kingdom; PRO, Patient reported outcome; SAS, Statistical Analysis System; SD, Standard deviation; UK, United Kingdom; US, United States
Ethics Approval and Informed Consent
The study design was reviewed by RTI International’s institutional review board and determined to be exempt from full review. All pretest participants and survey respondents were required to provide informed consent; participation in the survey was voluntary.
Acknowledgments
Kimberly Moon of RTI Health Solutions provided project management for this study. Kate Lothman of RTI Health Solutions provided medical writing services, which were funded by Sanofi. The authors thank Joslyn Crowe of the National Niemann-Pick Disease Foundation and Toni A. Mathieson of Niemann-Pick UK for their valuable insights during the design of the study and their support recruiting the respondents.
Parts of this work were presented in preliminary form as a poster at the Virtual ISPOR 2021 conference (Mansfield C, Nalysnyk L, Joshi D, Coulter J, Pulikottil-Jacob R. Patient and parent preferences for symptom control in ASMD Type B and A/B using best-worst scaling methodology; Value Health. 2021; 24(5)). Congress URL, https://www.valueinhealthjournal.com/article/S1098-3015(21)01261-4/fulltext.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was conducted under a research contract between Sanofi and RTI Health Solutions and was funded by Sanofi.
Disclosure
CM is an employee of RTI Health Solutions and receives funding from commercial and federal entities to conduct studies on healthcare, outside the submitted work. JC was an employee of RTI Health Solutions at the time of the study conduct. RPJ is an employee of Sanofi and may hold shares and/or stock options in the company. LN and DJ were full-time employees of Sanofi when this study was conducted. The authors report no other conflicts of interest in this work.
References
1. Schuchman EH, Desnick RJ. Types A and B Niemann-Pick disease. Mol Genet Metab. 2017;120(1–2):27–33. doi:10.1016/j.ymgme.2016.12.008
2. McGovern MM, Avetisyan R, Sanson BJ, Lidove O. Disease manifestations and burden of illness in patients with acid sphingomyelinase deficiency (ASMD). Orphanet J Rare Dis. 2017;12(1):41. doi:10.1186/s13023-017-0572-x
3. Wasserstein MP, Diaz GA, Lachmann RH, et al. Olipudase alfa for treatment of acid sphingomyelinase deficiency (ASMD), safety and efficacy in adults treated for 30 months. J Inherit Metab Dis. 2018;41(5):829–838. doi:10.1007/s10545-017-0123-6
4. Diaz GA, Jones SA, Scarpa M, et al. One-year results of a clinical trial of olipudase alfa enzyme replacement therapy in pediatric patients with acid sphingomyelinase deficiency. Genet Med. 2021;23(8):1543–1550. doi:10.1038/s41436-021-01156-3
5. Diaz GA, Giugliani R, Guffon N, et al. Long-term safety and clinical outcomes of olipudase alfa enzyme replacement therapy in pediatric patients with acid sphingomyelinase deficiency, two-year results. Orphanet J Rare Dis. 2022;17(1):437. doi:10.1186/s13023-022-02587-0.
6. Wasserstein M, Lachmann R, Hollak C, et al. A randomized, placebo-controlled clinical trial evaluating olipudase alfa enzyme replacement therapy for chronic acid sphingomyelinase deficiency (ASMD) in adults: One-year results. Genet Med. 2022;24(7):1425–1436. doi:10.1016/j.gim.2022.03.021
7. Avetisyan R, Hareendran A, Stringer S, Tan S, Sanson B, Hass S. Qualitative research approaches in rare diseases, Acid Sphingomyelinase Deficiency (ASMD) symptoms and impact as reported by patients and caregivers. Value Health. 2016;19(7):A388. doi:10.1016/j.jval.2016.09.235
8. Hollin IL, Paskett J, Schuster ALR, et al. Best-worst scaling and the prioritization of objects in health, a systematic review. Pharmacoeconomics. 2022;40(9):883–899. doi:10.1007/s40273-022-01167-1
9. Yuan Z, Levitan B, Burton P, et al. Relative importance of benefits and risks associated with antithrombotic therapies for acute coronary syndrome, patient and physician perspectives. Curr Med Res Opin. 2014;30(9):1733–1741. doi:10.1185/03007995.2014.921611
10. Paquin RS, Fischer R, Mansfield C, et al. Priorities when deciding on participation in early-phase gene therapy trials for Duchenne muscular dystrophy, a best–worst scaling experiment in caregivers and adult patients. Orphanet J Rare Dis. 2019;14(1):102. doi:10.1186/s13023-019-1069-6
11. Mansfield C, Ndife B, Chen J, et al. Patient preferences for treatment of metastatic melanoma. Future Oncol. 2019;15(11):1255–1268.
12. Hauber AB, Mohamed AF, Johnson FR, et al. Understanding the relative importance of preserving functional abilities in Alzheimer’s disease in the United States and Germany. Qual Life Res. 2014;23(6):1813–1821. doi:10.1007/s11136-013-0620-5
13. Peay HL, Hollin I, Fischer R, Bridges JF. A community-engaged approach to quantifying caregiver preferences for the benefits and risks of emerging therapies for Duchenne muscular dystrophy. Clin Ther. 2014;36(5):624–637. doi:10.1016/j.clinthera.2014.04.011
14. Cheung KL, Wijnen BF, Hollin IL, et al. Using best-worst scaling to investigate preferences in health care. Pharmacoeconomics. 2016;34(12):1195–1209. doi:10.1007/s40273-016-0429-5
15. Khare M, Federer WT. A simple construction procedure for resolvable incomplete block designs for any number of treatments. Biometrical J. 1981;23(2):121–132. doi:10.1002/bimj.4710230203
16. Parvin S, Wang P, Uddin J, Wright LT. Using best-worst scaling method to examine consumers’ value preferences, A multidimensional perspective. Cogent Bus Manag. 2016;3(1):1199110. doi:10.1080/23311975.2016.1199110
17. Street AP, Street DJ. Combinatorics of Experimental Design. Oxford University Press, Wiley; 1987.
18. Flynn TN, Marley AAJ. Best-Worst Scaling, Theory and Methods. In: Handbook of Choice Modelling. Cheltenham (UK): Edward Elgar Publishing; 2014:178–201.
19. Hensher DA, Rose JM, Greene WH. Applied Choice Analysis, a Primer. Cambridge University Press. Springer Science and Business Media LLC; 2005.
20. Flynn TN, Louviere JJ, Peters TJ, Coast J. Best-worst scaling, What it can do for health care research and how to do it. J Health Econ. 2007;26(1):171–189. doi:10.1016/j.jhealeco.2006.04.002
21. Marley AAJ, Flynn TN, Louviere JJ. Probabilistic models of set-dependent and attribute-level best–worst choice. J Math Psychol. 2008;52(5):281–296. doi:10.1016/j.jmp.2008.02.002
22. Marley AAJ, Louviere JJ. Some probabilistic models of best, worst, and best–worst choices. J Math Psychol. 2005;49(6):464–480. doi:10.1016/j.jmp.2005.05.003
23. Pokrzywinski R, Hareendran A, Nalysnyk L, et al. Impact and burden of acid sphingomyelinase deficiency from a patient and caregiver perspective. Sci Rep. 2021;11(1):20972. doi:10.1038/s41598-021-99921-6
24. Basch E, Bennett AV. Patient-reported outcomes in clinical trials of rare diseases. J Gen Intern Med. 2014;29(Suppl 3)):S801–3. doi:10.1007/s11606-014-2892-z
25. Lanar S, Acquadro C, Seaton J, Savre I, Arnould B. To what degree are orphan drugs patient-centered? A review of the current state of clinical research in rare diseases. Orphanet J Rare Dis. 2020;15(1):134. doi:10.1186/s13023-020-01400-0
26. Slade A, Isa F, Kyte D, et al. Patient reported outcome measures in rare diseases, a narrative review. Orphanet J Rare Dis. 2018;13(1):61. doi:10.1186/s13023-018-0810-x
27. Elstein D, Belmatoug N, Deegan P, et al. Development and validation of Gaucher disease type 1 (GD1)-specific patient-reported outcome measures (PROMs) for clinical monitoring and for clinical trials. Orphanet J Rare Dis. 2022;17(1):9. doi:10.1186/s13023-021-02163-y
28. Benjamin K, Vernon MK, Patrick DL, Perfetto E, Nestler-Parr S, Burke L. Patient-reported outcome and observer-reported outcome assessment in rare disease clinical trials, An ISPOR COA emerging good practices task force report. Value Health. 2017;20(7):838–855. doi:10.1016/j.jval.2017.05.015
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


