Back to Journals » Infection and Drug Resistance » Volume 16
Impact of Positive Culture Reports of E. coli or MSSA on De-Escalation of Antibiotic Use in a Teaching Hospital in Pakistan and the Implications
Authors Haseeb A , Saleem Z , Altaf U, Batool N, Godman B, Ahsan U, Ashiq M, Razzaq M, Hanif R, E-Huma Z, Amir A , Hossain MA, Raafat M , Radwan RM, Iqbal MS, Kamran SH
Received 29 September 2022
Accepted for publication 23 December 2022
Published 5 January 2023 Volume 2023:16 Pages 77—86
DOI https://doi.org/10.2147/IDR.S391295
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Abdul Haseeb,1 Zikria Saleem,2 Ummara Altaf,3 Narjis Batool,4 Brian Godman,5– 7 Umar Ahsan,8 Mehreen Ashiq,9 Mutiba Razzaq,9 Rabia Hanif,9 Zill E-Huma,9 Afreenish Amir,10 Mohammad Akbar Hossain,11 Mohamed Raafat,12 Rozan Mohammad Radwan,13 Muhammad Shahid Iqbal,14 Sairah Hafeez Kamran15
1Department of Clinical Pharmacy, College of Pharmacy, Umm AL-Qura University, Makkah, Saudi Arabia; 2Department of Pharmacy Practice, Faculty of Pharmacy, Bahauddin Zakariya University, Multan, Pakistan; 3Department of Pharmacy, Ghurki Trust Teaching Hospital, Lahore, Pakistan; 4Australian Institute of Health Innovation, Center of Health Systems and Safety Research, Faculty of Medicine, Health and Human Sciences, Macquarie University, Sydney, NSW, Australia; 5Strathclyde Institute of Pharmacy and Biomedical Sciences, Strathclyde University, Glasgow, UK; 6School of Pharmacy, Sefako Makgatho Health Sciences University, Pretoria, South Africa; 7Centre of Medical and Bio-Allied Health Sciences Research, Ajman University, Ajman, United Arab Emirates; 8Department of Infection Prevention and Control, Al Noor Specialist Hospital, Ministry of health, Makkah, Kingdom of Saudi Arabia; 9Faculty of Pharmacy, The University of Lahore, Lahore, Pakistan; 10Department of Microbiology, National University of Medical Sciences, Rawalpindi, Pakistan; 11Department of Pharmacology and Toxicology, Faculty of Medicine in Al-Qunfudah, Umm Al-Qura University, Makkah, Kingdom of Saudi Arabia; 12Department of Pharmacology and Toxicology, College of Pharmacy, Umm AL-Qura University, Makkah, Saudi Arabia; 13Pharmaceutical Care Department, Al Noor Specialist Hospital, Ministry of Health, Makkah, Saudi Arabia; 14Department of Clinical Pharmacy, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Alkharj, Saudi Arabia; 15Institute of Pharmacy, Lahore College for Women University, Lahore, Pakistan
Correspondence: Zikria Saleem, Department of Pharmacy Practice, Faculty of Pharmacy, Bahauddin Zakariya University, Multan, Pakistan, Email [email protected]
Background: Antibiotic de-escalation is a key element of antimicrobial stewardship programs that restrict the spread and emergence of resistance. This study was performed to evaluate the impact of positive culture sensitivity reports of E. coli or Methicillin sensitive Staphylococcus aureus (MSSA) on de-escalation of antibiotic therapy.
Methods: This prospective observational study was performed on 256 infected patients. The samples were obtained principally from the pus of infected sites for the identification of pathogens and culture-sensitivity testing. The data were collected from patient medical files, which included their demographic data, sample type, causative microbe and antimicrobial treatment as empiric or definitive treatment based on cultures. Data were analyzed using SPSS.
Results: Of 256 isolated microbes, 138 (53.9%) were MSSA and 118 were E. coli (46.1%). MSSA showed 100% sensitivity to cefoxitin, oxacillin, vancomycin, fosfomycin, colistin and more than 90% to linezolid (95.3%), tigecycline (93.1%), chloramphenicol (92.2%) and amikacin (90.2%). E. coli showed 100% sensitivity to only fosfomycin and more than 90% to colistin (96.7%), polymyxin-B (95.1%) and tigecycline (92.9%). The high use of cefoperazone+sulbactam (151), amikacin (149), ceftriaxone (33), metronidazole (30) and piperacillin + tazobactam (22) was seen with empiric prescribing. Following susceptibility testing, the most common antibiotics prescribed for E. coli were meropenem IV (34), amikacin (34), ciprofloxacin (29) and cefoperazone+sulbactam (25). For MSSA cases, linezolid (48), clindamycin (30), cefoperazone+ sulbactam IV (16) and amikacin (15) was used commonly. Overall, there was 23% reduction in antibiotic use in case of E. coli and 43% reduction in MSSA cases.
Conclusion: Culture sensitivity reports helped in the de-escalation of antimicrobial therapy, reducing the prescribing of especially broad-spectrum antibiotics. Consequently, it is recommended that local hospital guidelines be developed based on local antimicrobial susceptibility patterns while preventing the unnecessary use of broad-spectrum antibiotics for empiric treatment.
Keywords: antimicrobial resistance, antimicrobial stewardship, culture sensitivity reports, definitive treatment, empirical treatment, de-escalation, Pakistan
Introduction
According to the 10th International Conference on Emerging Infectious Diseases (EID), emerging infections are responsible for approximately 15% of all human pathogens.1 In most cases, antibiotic-resistant pathogens are responsible for nosocomial infections, which have risen due to excessive use of broad-spectrum antibiotics.2–6 This is a concern as the development of new antibiotics and repositioning of old antibiotics has slowed.3,7 Alongside this, the Centers for Disease Control and Prevention (CDC) recognized that 20–50% of all antibiotics prescribed to patients in critical care hospitals in the US are either redundant or inappropriate.8 Consequently, optimizing the use of antibiotics is essential to minimize the increasing trend of nosocomial infections due to antibiotic-resistant pathogens.9 The choice of appropriate antibiotics must be based on antibiotic susceptibility and microbiology profiling to optimize appropriate antibiotic prescribing within hospitals.10,11 Consequently, any empirical use of broad-spectrum antibiotics must be based on local antibiograms and adjusted based on subsequent culture sensitivity reports.12–14 The two approaches reported for the optimization of antibiotic therapy include de-escalation and surveillance of effective antibiotic treatment, with the potential to reduce costs.15 Such monitoring can assist the hospital antibiotic stewardship team in deciding how to provide effective therapy and minimize the emergence and spread of antibiotic-resistant organisms.8
De-escalation of antibiotics is a key component of antibiotic stewardship to promote the rational use of antibiotics by narrowing the spectrum of suggested antibiotics or by selecting antibiotics for specific microorganism, thereby decreasing the possibility of antimicrobial resistance (AMR).16–18 This approach also helps reduce inappropriate antibiotics prescribing, cost of hospitalization, length of stay and mortality.19 Antibiotic de-escalation is considered a safe and effective strategy in many infectious diseases (ID) including pneumonia (hospital-acquired or community-acquired pneumonia), bacteremia, urinary tract infections (UTIs), sepsis with bloodstream infection (BSI), severe sepsis among neutropenic patients, and pneumococcal bacteremia.20–25 However, due to multiple barriers, the practice of antibiotic de-escalation is not consistent among hospitals across countries. These barriers include a lack of diagnostic facilities, lack of education, lack of multidisciplinary collaboration, and hesitancy to de-escalate antibiotics in critically ill patients who are improving with broad-spectrum antibiotic therapy.26–28
There is generally high resistance to Methicillin Sensitive Staphylococcus Aureus (MSSA), the most common pathogen responsible for different infections in hospitals, making infections caused by these bacteria difficult to treat.29 MSSA and Escherichia coli (E. coli) have been most common microbes isolated in orthopedic infections.30 MSSA is also responsible for causing hospital acquired infections and community outbreak infections, with multidrug resistance making these infections difficult to treat.31,32 E. coli is also a common pathogen among animals.33 Different pathogenic strains of E. coli are responsible for different infections including UTIs and blood stream infections, wounds and other complications.30 E. coli also causes nosocomial infections. Consequently, any de-escalation following empiric use based on local antibiograms needs to be implemented if pertinent after culture reports in order to prevent complications associated with resistant infections of E. coli and MSSA.34 However, among health-care settings of Pakistan, there is currently excessive use of broad-spectrum antibiotics as empiric therapy.35 This is a concern, especially as we believe there are currently no published data regarding the de-escalation of antibiotic therapy among hospitals in Pakistan. Having said this, there have been initiatives to improve antibiotic use using antibiotic stewardship program (ASP) in Pakistan.36,37 In view of this, we sought to evaluate the impact of positive culture sensitivity reports of E. coli or MSSA on subsequent antibiotics use pattern within a leading teaching hospital in Pakistan. We believe the findings will not only be useful in this hospital but also in hospitals throughout Pakistan and wider as they seek to reduce AMR to these two leading organisms within hospitals as part of the National Action Plan to reduce AMR in Pakistan.38
Methods
Study Design and Study Setting
This prospective observational study was undertaken in the orthopedic ward of the Ghurki Trust Teaching Hospital (GTTH) to check the impact of positive culture reports of MSSA & E. coli on de-escalation of antimicrobial therapy from September 2020 to December 2020.
The Ghurki Trust Teaching Hospital, which is a 600 bed teaching hospital, is one of the largest charity hospitals in Pakistan certified by the Pakistan Center of Philanthropy and has a specialty in orthopedics. Consequently, if stewardship programmes can be introduced in this hospital to enhance de-escalation, this can serve as an exemplar across Pakistan.
Ethics approval was taken from the administration department and ethical board of hospital Research Ethics Committee, Discipline of Pharmacy Practice, Faculty of Pharmacy, University of Lahore (REC/DPP/FOP/8-2020) before conducting the study. Informed consent was taken from patients or parents or legal guardians of patients under 18 years of age before collecting the data. The study was conducted in compliance with the Declaration of Helsinki.
Inclusion and Exclusion Criteria
Patients with positive culture reports of E. coli and MSSA microbes during their stay in the orthopedic ward were included in this study. All patients had already been started on antibiotics empirically, and their therapies were changed if pertinent after the availability of culture reports. Patients with incomplete medical information, patients who died before the results of culture reports were known, patients who were discharged from the hospital before data collection, patients with positive culture reports of other microbes, patients with negative culture reports and patients with contaminated culture samples were excluded from the study. Patients who were admitted into other wards of the hospital were also excluded from the study.
Sample for Culture Sensitivity
Samples for the identification of microbes and for culture-sensitivity testing were obtained from infected sites of patients. The samples were subsequently enclosed within specialized containers and transported to the microbiological laboratory immediately at room temperature. Each culture-positive patient was followed regularly to determine their antibiotic prescribing pattern, and total length of hospital stay, as well as to check the sensitivity patterns of the prescribed antibiotics. All patients had only one culture report rather than multiple reports.
Data Collection
Data were collected on a pre-designed Performa from the patients in the orthopedic ward of the hospital. The data collection form included demographic characteristics, past surgical history, diagnosis, microbiological profiles and prescribed antibiotics empirically and for definitive treatment following the results of the sensitivity analysis. The data was collected by in-hospital staff following patient consent. The pre-designed Performa was based on the published literature and the considerable knowledge of the co-authors working in this area.
Antibiotic de-escalation was defined as switching from a broad-spectrum empiric antibiotic regimen to streamlining to a narrow-spectrum antibiotic regimen after the re-evaluation of the patient’s condition combined with microbiological data and inflammatory markers.39
Statistical Analysis
The data were analyzed using Statistical Package for Social Sciences (SPSS) version 21.0. Results were presented in the form of frequency and percentages as graphical and tabular presentations.
Results
Demographic Characteristics of Patients
The patient’s base-line demographics are shown in Table 1. In this study, 256 patients were included, the majority were males (N=159, 62.1%) with a mean age of 65 years old. The majority of the patients suffered from surgical site infections (59.4%), and the co-morbid condition present in majority of patients was diabetes mellitus (N=57, 22%). Different samples were taken from patient infected site with most of samples taken from pus (N=171, 66.8%). Of 256 isolated microbes, 118 were E. coli (46.1%) and 138 were MSSA (53.9%).
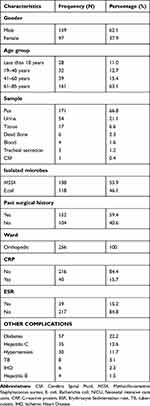 |
Table 1 Demographic Characteristics of Selected Patients |
Sensitivity Pattern of Antibiotics
The sensitivity to prescribed antibiotics is shown in Table 2. Fosfomycin (100%) was the most effective antibiotic against E. coli, followed by colistin (96.7%), polymyxin-B (95.1%), tigecycline (92.9%) and chloramphenicol (80.5%). However, MSSA showed 100% sensitivity to cefoxitin, oxacillin, fosfomycin, vancomycin and colistin. Resistant strains of linezolid were identified against MSSA, which is a concern.
 |
Table 2 Antibiotic Sensitivity Pattern of E. coli and MSSA Isolates |
Antibiotic Prescribing Trend
In total, for E. coli and MSSA, there was high use of cefoperazone+sulbactam (151), amikacin (149), ceftriaxone,33 metronidazole30 and piperacillin + tazobactam.22 After getting the results of susceptibility test, the most common antibiotics prescribed for E. coli were meropenem IV,34 amikacin,34 ciprofloxacin29 and cefoperazone+sulbactam.25 In MSSA cases, linezolid,48 clindamycin,30 cefoperazone+ sulbactam IV16 and amikacin15 were the most commonly prescribed antibiotics following sensitivity analyses. Overall, there was 23% reduction in antibiotic use in case of E. coli and 43% reduction in MSSA cases. Details of antibiotics use in empiric and definitive treatment following susceptibility testing are described in Table 3.
 |
Table 3 De-Escalation of Antibiotics |
Discussion
Prompt diagnosis and appropriate antibiotic administration are essential to reduce morbidity and mortality with infectious diseases.40 Despite recommendations, clinical practice regarding requesting culture reports are often inadequate, exacerbated by habit, clinical judgement, available personnel, facilities and costs, especially among low- and middle-income countries.41–44 This is a concern as inappropriate utilization of antibiotics will increase resistant strains and associated morbidity, mortality and costs.45–47 Alongside this, due to increasing resistance rates, broad-spectrum antibiotics are preferred in definitive treatment.48 However, this is not always the case with a study in South Africa reporting that 83% of antibiotic therapy was changed following culture sensitivity results where these data were recorded.49 Implementation of antibiotic stewardship programs is considered as an essential approach to restrict inappropriate use of antibiotics.50,51 Therefore, there is an urgent need for the implementation of antibiotic stewardship programs in Pakistan,52 building on the goals of the NAP in Pakistan.38
We believe this is the first study in Pakistan to estimate the effect of E. coli or MSSA positive culture sensitivity reports on antibiotic de-escalation. The culture reports of most of the patients in our study were positive for E. coli and MSSA for orthopedic infections, which is contrary to a study conducted in South India where Methicillin-resistant Staphylococcus aureus (MRSA) was the predominant cause of orthopedic infection.53 These differences may exist due to the prevalence of different pathogens and their resistance in different geographical regions.
In our study, fosfomycin exhibited 100% sensitivity to E. coli isolates. This compares to a study conducted in Ethiopia documented that E. coli isolates exhibited high level of sensitivity to nitrofurantoin (96.4%), norfloxacin (90.6%) and gentamicin (79.6%).54 Alongside this, MSSA showed a high level of sensitivity in our study to cefoxitin, oxacillin, fosfomycin and colistin. However, in a previous study in Pakistan, MSSA isolates were highly sensitive to vancomycin (100%), linezolid (98.9%), rifampicin (95.7%) and chloramphenicol (94.7%), respectively.55 Oxacillin was 100% sensitive in our study with comparison to the national data (91.7%), which may be because of the decreased use of oxacillin nowadays.55,56 Co-trimoxazole and nitrofurantoin showed the least sensitivity for MSSA.57
The most common antibiotics prescribed as empiric therapy in our study were cefoperazone + sulbactam, amikacin and ceftriaxone. However, a study conducted in India documented the utilization of piperacillin + Tazobactam in orthopedic patients as empiric therapy.41 This difference may be due to the high availability of cefoperazone + sulbactam in GTTH and sensitivity against microbes, and the use of amikacin has good therapeutic coverage against E. coli. For definitive therapy, the most prescribed antibiotic in our study was meropenem and amikacin. Similar findings have been documented in another study.41
We are aware that this study has some limitations. Firstly, as this was an observational study, the pharmacist could not intervene with physician prescribing trends in the selection of antibiotics. Secondly, we included patients with positive culture reports of only MSSA and E. coli so our sample size was limited. Third, there are no guidelines currently available in Pakistan with a special focus on culture sensitivity reports and antibiotic prescribing. Consequently, we were unable to compare our results with any such guidelines. However, we believe that our findings are robust as this study documented the impact of culture sensitivity reports on de-escalation of therapy and has also reduced antibiotic prescribing and hospital stay. Furthermore, this study also accentuates the need for antibiotic guidelines for initiating effective treatment and reducing unnecessary antibiotic prescribing.
Conclusion
Our study reported that sensitivity reports help narrow antibiotic therapy. Consequently, antibiotic de-escalation practice is increasingly required for all medical and surgical patients to reduce the overuse of antibiotics and minimize the spread of resistance. Guidelines for appropriate use of antibiotics and narrowing the level of health-care professionals who can prescribe antibiotics are also needed. We will be following this up in future projects.
Acknowledgments
Authors acknowledge the contributions of all the participants.
Funding
The authors would like to thank the Deanship of Scientific Research at the Umm Al-Qura University for supporting this work by grant code 22UQU4290073DSR08.
Disclosure
Authors do not have any conflict of interest.
References
1. CDC International.
2. Saleem Z, Saeed H, Hassali MA, et al. Pattern of inappropriate antibiotic use among hospitalized patients in Pakistan: a longitudinal surveillance and implications. Antimicrob Resist Infect Control. 2019;8(1):188. doi:10.1186/s13756-019-0649-5
3. Saleem Z, Hassali MA. Travellers take heed: outbreak of extensively drug resistant (XDR) typhoid fever in Pakistan and a warning from the US CDC. Travel Med Infect Dis. 2019;27:127. doi:10.1016/j.tmaid.2018.10.013
4. Almangour TA, Alenazi B, Ghonem L, Alhifany AA, Aldakheel BA, Alruwaili A. Inhaled colistin for the treatment of nosocomial pneumonia due to multidrug-resistant Gram-negative bacteria: a real-life experience in tertiary care hospitals in Saudi Arabia. Saudi Pharma J. 2020;28(8):1009–1013. doi:10.1016/j.jsps.2020.06.023
5. Alghamdi M, Alotaibi F, Ahmed H, Alharbi F, Bukhari O, Youssef A-R. Effect of medical education on the knowledge, attitude and compliance regarding infection control measures among dental students in Makkah. J Umm Al-Qura Univ Med Sci. 2021;7(1):14–17. doi:10.54940/ms31191227
6. Almeleebia TM, Alhifany AA, Almutairi F, Alshibani M, Alhossan AM. Regulating antimicrobial sales in Saudi Arabia: achievements and challenges. Int J Clin Pract. 2021;75(4):e13833. doi:10.1111/ijcp.13833
7. Rolain J-M, Abat C, Jimeno M-T, Fournier P-E, Raoult D. Do we need new antibiotics? Clin Microbiol Infect. 2016;22(5):408–415. doi:10.1016/j.cmi.2016.03.012
8. Pollack LA, Srinivasan A. Core elements of hospital antibiotic stewardship programs from the Centers for Disease Control and Prevention. Clin Infect Dis. 2014;59(suppl_3):S97–S100. doi:10.1093/cid/ciu542
9. Brodt H-R, Brodt H-R, Brodt H-R, et al. Strategies to enhance rational use of antibiotics in hospital: a guideline by the German Society for Infectious Diseases. Infection. 2016;44(3):395–439. doi:10.1007/s15010-016-0885-z
10. Jacobs MR, Felmingham D, Appelbaum PC, Grüneberg RN. The Alexander Project 1998–2000: susceptibility of pathogens isolated from community-acquired respiratory tract infection to commonly used antimicrobial agents. J Antimicrob Chemother. 2003;52(2):229–246. doi:10.1093/jac/dkg321
11. Agaba P, Tumukunde J, Tindimwebwa J, Kwizera A. Nosocomial bacterial infections and their antimicrobial susceptibility patterns among patients in Ugandan intensive care units: a cross sectional study. BMC Res Notes. 2017;10(1):1–12. doi:10.1186/s13104-017-2695-5
12. Morel J, Casoetto J, Jospé R, et al. De-escalation as part of a global strategy of empiric antibiotherapy management. A retrospective study in a medico-surgical intensive care unit. Crit Care. 2010;14(6):1–7.
13. Palavecino EL, Williamson JC, Ohl CA. Collaborative antimicrobial stewardship: working with microbiology. Infect Dis Clin North Am. 2020;34(1):51. doi:10.1016/j.idc.2019.10.006
14. Leeman HM, Chan BP, Zimmermann CR, et al. Creation of state antibiogram and subsequent launch of public health–coordinated antibiotic stewardship in new hampshire: small state, big collaboration. Public Health Rep. 2021;2021:0033354921995778.
15. Moraes RB, Guillén JAV, Zabaleta W, Borges FK. De-escalation, adequacy of antibiotic therapy and culture positivity in septic patients: an observational study. Revista Brasileira de terapia intensiva. 2016;28(3):315–322. doi:10.5935/0103-507X.20160044
16. Moehring RW, Ashley ED, Davis AE, et al. Development of an electronic definition for de-escalation of antibiotics in hospitalized patients. 2020.
17. Dellit TH, Owens RC, McGowan JE, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177. doi:10.1086/510393
18. Younis BB, Rozina A, Junaid M, Saima K, Farhan N, Maham T. A Study of Unnecessary Use of Antibiotics at a Tertiary care hospital: urgent need to implement antimicrobial stewardship programs. J Young Pharma. 2015;7(4):311. doi:10.5530/jyp.2015.4.5
19. Liu P, Ohl C, Johnson J, Williamson J, Beardsley J, Luther V. Frequency of empiric antibiotic de-escalation in an acute care hospital with an established Antimicrobial Stewardship Program. BMC Infect Dis. 2016;16(1):1–7.
20. Ohji G, Doi A, Yamamoto S, Iwata KJ. Is de-escalation of antimicrobials effective? A systematic review and meta-analysis. Int J Infect Dis. 2016;49:71–79. doi:10.1016/j.ijid.2016.06.002
21. Mokart D, Slehofer G, Lambert J, et al. De-escalation of antimicrobial treatment in neutropenic patients with severe sepsis: results from an observational study. Intensive Care Med. 2014;40(1):41–49. doi:10.1007/s00134-013-3148-9
22. Knaak E, Cavalieri SJ, Elsasser GN, Preheim LC, Gonitzke A, Destache CJ. Does antibiotic de-escalation for nosocomial pneumonia impact intensive care unit length of stay? Infect Dis Clin Pract. 2013;21(3):172–176.
23. Guo Y, Gao W, Yang H, Sui SJH, Sui S. De-escalation of empiric antibiotics in patients with severe sepsis or septic shock: a meta-analysis. Heart Lung. 2016;45(5):454–459. doi:10.1016/j.hrtlng.2016.06.001
24. Khasawneh FA, Karim A, Mahmood T, Ahmed S, Jaffri SF, Mehmood M. Safety and feasibility of antibiotic de-escalation in bacteremic pneumonia. Infect Drug Resist. 2014;7:177. doi:10.2147/IDR.S65928
25. Hadi MA, Karami NA, Al-Muwalid AS, et al. Community pharmacists’ knowledge, attitude, and practices towards dispensing antibiotics without prescription (DAwP): a cross-sectional survey in Makkah Province, Saudi Arabia. Int J Infect Dis. 2016;47:95–100. doi:10.1016/j.ijid.2016.06.003
26. Masterton RG. Antibiotic de-escalation. Crit Care Clin. 2011;27(1):149–162. doi:10.1016/j.ccc.2010.09.009
27. Bal AM, Gould IM. Antibiotic stewardship: overcoming implementation barriers. Curr Opin Infect Dis. 2011;24(4):357–362. doi:10.1097/QCO.0b013e3283483262
28. Gonzalez L, Cravoisy A, Barraud D, et al. Factors influencing the implementation of antibiotic de-escalation and impact of this strategy in critically ill patients. Crit Care. 2013;17(4):R140. doi:10.1186/cc12819
29. Almangour TA, Perry GK, Terriff CM, Alhifany AA, Kaye KS. Dalbavancin for the management of gram-positive osteomyelitis: effectiveness and potential utility. Diagn Microbiol Infect Dis. 2019;93(3):213–218. doi:10.1016/j.diagmicrobio.2018.10.007
30. Vila J, Sáez-López E, Johnson JR, et al. Escherichia coli: an old friend with new tidings. FEMS Microbiol Rev. 2016;40(4):437–463. doi:10.1093/femsre/fuw005
31. Lindsay JA. Hospital-associated MRSA and antibiotic resistance—What have we learned from genomics? Int J Med Microbiol. 2013;303(6–7):318–323. doi:10.1016/j.ijmm.2013.02.005
32. Otto M. Community-associated MRSA: what makes them special? Int J Med Microbiol. 2013;303(6–7):324–330. doi:10.1016/j.ijmm.2013.02.007
33. Harada K, Asai T. Role of antimicrobial selective pressure and secondary factors on antimicrobial resistance prevalence in Escherichia coli from food-producing animals in Japan. Biomed Res Int. 2010;2010:115.
34. Ibrahim EH, Ward S, Sherman G, Schaiff R, Fraser VJ, Kollef MH. Experience with a clinical guideline for the treatment of ventilator-associated pneumonia. Crit Care Med. 2001;29(6):1109–1115. doi:10.1097/00003246-200106000-00003
35. Saleem Z, Hassali MA, Versporten A, Godman B. A multicenter point prevalence survey of antibiotic use in Punjab, Pakistan: findings and implications. Expert Rev Anti Infect Ther. 2019;17(4):285–293. doi:10.1080/14787210.2019.1581063
36. Saleem Z, Hassali MA, Hashmi FK, Godman B, Ahmed Z. Snapshot of antimicrobial stewardship programs in the hospitals of Pakistan: findings and implications. Heliyon. 2019;5(7):e02159. doi:10.1016/j.heliyon.2019.e02159
37. Mubarak N, Khan AS, Zahid T, et al. Assessment of adherence to the core elements of hospital antibiotic stewardship programs: a survey of the tertiary care hospitals in Punjab, Pakistan. Antibiotics. 2021;10(8):906. doi:10.3390/antibiotics10080906
38. Saleem Z, Godman B, Azhar F, et al. Progress on the national action plan of Pakistan on antimicrobial resistance (AMR): a narrative review and the implications. Expert Rev Anti Infect Ther. 2022;20(1):71–93. doi:10.1080/14787210.2021.1935238
39. Khan RA, Aziz Z. Antibiotic de-escalation therapy in neurosurgical patients with ventilator-associated pneumonia in Intensive Care Unit: a retrospective observational study. Indian J Pharm Educ Res. 2017;51:144–149.
40. Almangour TA, Ghonem L, Aljabri A, et al. Ceftazidime-avibactam versus colistin for the treatment of infections due to carbapenem-resistant Enterobacterales: a multicenter cohort study. Infect Drug Resist. 2022;15:211. doi:10.2147/IDR.S349004
41. Choudhary S, Yadav AK, Sharma S, Pichholiya M, Sharma P. Effect of blood culture reports on antibiotics use by physicians in septic patients of intensive care unit. Int J Res Med Sci. 2015;3(9):2425.
42. Afriyie DK, Sefah IA, Sneddon J, et al. Antimicrobial point prevalence surveys in two Ghanaian hospitals: opportunities for antimicrobial stewardship. JAC Antimicrob Resist. 2020;2(1):dlaa001. doi:10.1093/jacamr/dlaa001
43. Ogunleye OO, Oyawole MR, Odunuga PT, et al. A multicentre point prevalence study of antibiotics utilization in hospitalized patients in an urban secondary and a tertiary healthcare facilities in Nigeria: findings and implications. Expert review of anti-infective therapy. Expert Rev Anti-Infect Ther. 2022;20(2):297–306. doi:10.1080/14787210.2021.1941870
44. Kurdi A, Hasan AJ, Baker KI, et al. A multicentre point prevalence survey of hospital antibiotic prescribing and quality indices in the Kurdistan Regional Government of Northern Iraq: the need for urgent action. Expert Rev Anti Infect Ther. 2021;19(6):805–814. doi:10.1080/14787210.2021.1834852
45. Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. Pharma Therap. 2015;40(4):277.
46. Murray CJ, Ikuta KS, Sharara F, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–655. doi:10.1016/S0140-6736(21)02724-0
47. Hofer U. The cost of antimicrobial resistance. Nat Rev Microbiol. 2019;17(1):3. doi:10.1038/s41579-018-0125-x
48. Jabeen K, Zafar A, Hasan R. Frequency and sensitivity pattern of extended spectrum beta lactamase producing isolates in a tertiary care hospital laboratory of Pakistan. J Pak Med Assoc. 2005;55(10):436.
49. Dlamini NN, Meyer JC, Kruger D, Kurdi A, Godman B, Schellack N. Feasibility of using point prevalence surveys to assess antimicrobial utilisation in public hospitals in South Africa: a pilot study and implications. Hosp Pract. 2019;47(2):88–95. doi:10.1080/21548331.2019.1592880
50. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51–e77. doi:10.1093/cid/ciw118
51. Nathwani D, Varghese D, Stephens J, Ansari W, Martin S, Charbonneau C. Value of hospital antimicrobial stewardship programs [ASPs]: a systematic review. Antimicrob Resist Infect Control. 2019;8(1):1–13. doi:10.1186/s13756-019-0471-0
52. Khan EA, Owens RC, McGowan JE. An urgent need for national action plan for infection control and antibiotic stewardship in Pakistan. Clin Infect Dis. 2007;44:159–177.
53. Latha T, Anil B, Manjunatha H, et al. MRSA: the leading pathogen of orthopedic infection in a tertiary care hospital, South India. African Health Sci. 2019;19(1):1393–1401.
54. Kibret M, Abera B. Antimicrobial susceptibility patterns of E. Coli from Clinical Sources in Northeast Ethiopia. African Health Sci. 2011;11:40–45.
55. Mir F, Rashid A, Farooq M, Irfan M, Ijaz A. Antibiotic sensitivity patterns of staphylococcal skin infections. J Pak Assoc Dermatol. 2016;25(1):12–17.
56. Hanif MM, Butt T, Amjad M. Pathogens involved and antibiotic sensitivity pattern of isolates in community acquired common bacterial skin infections presenting in a tertiary care hospital. Pak Armed Forces Med J. 2006;56(3):289–294.
57. Onwubiko NE, Sadiq NM. Antibiotic sensitivity pattern of Staphylococcus aureus from clinical isolates in a tertiary health institution in Kano, Northwestern Nigeria. Pan African Med J. 2011;8(1). doi:10.4314/pamj.v8i1.71050
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
