Back to Journals » Infection and Drug Resistance » Volume 16
Impact of Nosocomial Infection on in-Hospital Mortality Rate in Adult Patients Under Venoarterial Extracorporeal Membrane Oxygenation After Cardiac Surgery
Authors Li X, Wang X, Wang L, Li C, Hao X, Du Z, Xie H, Yang F, Wang H, Hou X
Received 26 September 2022
Accepted for publication 30 May 2023
Published 28 June 2023 Volume 2023:16 Pages 4189—4200
DOI https://doi.org/10.2147/IDR.S390599
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Xiyuan Li,1,2 Xiaomeng Wang,1 Liangshan Wang,1 Chenglong Li,1 Xing Hao,1 Zhongtao Du,1 Haixiu Xie,1 Feng Yang,1 Hong Wang,1 Xiaotong Hou1
1Center for Cardiac Intensive Care, Beijing Institute of Heart, Lung, and Blood Vessel Diseases, Beijing Anzhen Hospital, Capital Medical University, Beijing, 100029, People’s Republic of China; 2Department of Intensive Care Unit, Aviation General Hospital of China Medical University, Beijing, 100012, People’s Republic of China
Correspondence: Xiaotong Hou; Hong Wang, Center for Cardiac Intensive Care, Beijing Institute of Heart, Lung and Blood Vessel Diseases, Beijing Anzhen Hospital, Capital Medical University, No. 2 Anzhen Road, Chaoyang District, Beijing, 100029, People’s Republic of China, Tel +86 10 64456631 ; +86 15010516438, Email [email protected]; [email protected]
Objective: There was no consensus on the impact of nosocomial infection on In-hospital mortality rate in patients receiving ECMO. This study aimed to investigate the impact of nosocomial infection (NI) on In-hospital mortality rate in adult patients receiving venoarterial extracorporeal membrane oxygenation (VA-ECMO) after cardiac surgery.
Materials and Methods: This retrospective study included 503 adult patients who underwent VA-ECMO after cardiac surgery. The impact of time-dependent NIs on In-hospital mortality rate within 28 days of ECMO initiation was investigated using a Cox regression model. The cumulative incidence function for death was compared between patients with NIs and those without NIs using a competing risk model.
Results: Within 28 days after ECMO initiation, 206 (41.0%) patients developed NIs, and 220 (43.7%) patients died. The prevalence rates of NIs were 27.8% and 20.3% during and after ECMO therapy, respectively. The incidence rates of NIs during and after ECMO therapy were 49‰ and 25‰, respectively. Time-dependent NI was an independent risk factor for predicting death (hazard ratio = 1.05, 95% confidence interval = 1.00– 1.11). The cumulative incidence of death in patients with NI was significantly higher than that in patients without NI at each time point within 28 days of ECMO initiation. (Z = 5.816, P = 0.0159).
Conclusion: NI was a common complication in adult patients who received VA-ECMO after cardiac surgery, and time-dependent NI was an independent risk factor for predicting mortality in these patients. Using a competing risk model, we confirmed that NIs increased the risk of In-hospital mortality rate in these patients.
Keywords: venoarterial extracorporeal membrane oxygenation, nosocomial infection, in-hospital mortality rate, Cox regression, competing risk model
Introduction
Data adapted from the Extracorporeal Life Support Organization indicated that more than 2000 extracorporeal membrane oxygenation (ECMO) runs were performed per year from 2014 to 2016 in adult patients.1 The use of ECMO post-cardiotomy plays an important role in clinical practice. The rate of venoarterial extracorporeal membrane oxygenation (VA-ECMO) applications has increased in recent decades.1 However, even in developed countries with the most advanced medical care, the In-hospital mortality rate of patients receiving VA-ECMO therapy is as high as 57.2%.2 Improving the survival of patients receiving ECMO has become a hot topic in various medical units.
Nosocomial infection (NI) is a common complication in adult patients on ECMO. There is no consensus on whether NI increases In-hospital mortality rate in these patients. Several studies have presented similar opinions,3–10 whereas others have reported contradictory views.11–25 Traditional research methods have difficulty removing the time-dependent bias and competing risk bias;26–30 therefore, the adverse effects of NIs in patients receiving VA-ECMO could not be accurately disclosed. Several studies have pointed out29,31,32 that using competitive risk models instead of traditional methods to explore the impact of NI on In-hospital mortality rate is a key factor in reducing the competing bias.
This study aimed to clarify the impact of NI on In-hospital mortality rate within 28 days of ECMO initiation in patients receiving VA-ECMO after cardiac surgery.
Materials and Methods
Setting and Study Design
This retrospective observational study was conducted in the surgical intensive care unit (ICU) of a single cardiac center in Beijing Anzhen Hospital, Capital Medical University, Beijing, China. Since the initiation of ECMO therapy in 2004, the complete data of more than 1100 patients receiving ECMO therapy have been available in the electronic medical records system. This study included adult patients who received VA-ECMO after cardiac surgery between January 1, 2011, and December 31, 2020. Ethical approval was obtained from the Medical Ethics Committee of the Anzhen Hospital (approval number:2022064X).
Inclusion/Exclusion Criterion
Patients (1) aged ≥18 years, (2) who received VA-ECMO support after cardiac surgery from December 31, 2011, to January 1, 2020, and (3) who were under VA-ECMO support for ≥48 h were included in the study. By contrast, patients (1) with any infection before receiving ECMO support, (2) on VA-ECMO who were transferred from another hospital were excluded.
Corresponding Definition
NI was defined based on the criteria issued by the National Health Commission of the People’s Republic of China in 2001 (the criterion referred to the definition of NI from the American Centers for Disease Control and Prevention in 1988).33 Ventilator associated pneumonia (VAP) was defined as the presence of ≥2 of the following criteria: temperature of >38.0°C or <36.0°C, white blood cell count of >12×109/L or less than 4×109/L, purulent excretion from the lower respiratory tract, new or consistent infiltration shown on chest imaging, positive sputum culture, and the initiation of antimicrobial therapy under the guidance of intensivists or infectious specialists.8,10,12,33,34 Bloodstream infection (BSI) was defined as the presence of any of the clinical manifestations (including fever (>38°C), chills, or hypotension) accompanied by positive blood culture from one or more blood samples.20,21,33,35,36 Urinary tract infection (UTI) was defined as the presence of positive symptoms, signs, or positive routine urine results, accompanied by a positive urine culture test.34 Other infections included surgical site infections (SSIs) and skin and soft tissue infections (SSTIs). SSI was defined as an infection involving the skin, subcutaneous tissue, or muscle layer occurring within 30 days after surgery, or any of the following features: purulent excretion from the drainage, positive results of drainage fluid culture, or infection identified by the surgeon.33,37 SSTI was defined as purulent drainage from the skin and soft tissue, an infection caused by any microorganism isolated from the culture of the skin or soft tissue, or a confirmed infection requiring surgical debridement.33
NI during ECMO was defined as infection occurring 24 h after ECMO initiation to 48 h after ECMO removal.10,12,14 NI after ECMO removal was defined as an infection occurring from 48 h after ECMO removal until hospital discharge. NI was defined as an infection that occurred throughout the hospital stay. Prevalence was defined as the ratio between the number of patients with NIs and the number of at-risk patients observed during the same period. The incidence of NI during ECMO was defined as the number of patients with NIs per 1000 ECMO-days (proportion of NI cases during the period of ECMO support). The incidence of NI after ECMO removal was defined as the number of patients newly diagnosed with NIs per 1000 patient-days (proportion of NI cases after ECMO removal). The overall incidence of NIs was defined as the number of patients with NIs per 1000 patient-days (throughout the entire hospitalization period). In-hospital mortality rate was defined as the ratio between the number of dead patients and the number of patients observed in the same period. When multiple infections occurred in the same patient, the first NI source was regarded as the etiology of the infection, which will be counted in the statistical analysis. Bleeding was defined hemorrhage in the surgical area with blood transfusion more than 2 units (concentrated red blood cells). Limb complications were defined as limb ischemic necrosis or thromboembolism during hospitalization. Cerebrovascular complications were defined as hypoxic ischemic encephalopathy, ischemic stroke, or cerebral hemorrhage.
ECMO Support and Infection Control Measures
The indications for VA-ECMO were jointly decided by the cardiac surgeon and extracorporeal life support physician.10 VA-ECMO cannulas were implanted by trained team members using the femoral vein-femoral artery approach. The ECMO flow rate titration was adjusted to ensure adequate tissue perfusion according to the lactic acid levels and organ function. A systematic heparin anticoagulation strategy was used to ensure that the activated clotting time is maintained within 180–220 s.
We used clustered preventive measures to reduce NIs. These measures included bed head elevation 30°~45°, waken-up plan daily, replace the condensate tank in time, replace the ventilator pipeline on demand, reduce gastroesophageal reflux. The central venous catheter was disinfected once a day. All patients received antibiotics to prevent NIs when VA-ECMO was initiated. Empirical antibiotics were routinely administered. The combination of ceftazidime and vancomycin was the most common prophylaxis selection in our center. When fungal infection was considered, fluconazole or voriconazole was added for antifungal therapy. When the results of the pathogenic culture were clear, the antibiotics were adjusted based on drug sensitivity test. All antibiotic doses were individually administered according to the patient’s liver and renal function. The dose of vancomycin was adjusted according to the drug concentration monitoring.
Data Collection
The following data were retrieved from the electronic medical records system (which contain information from the hospital information system (HIS), laboratory information system (LIS), and radiology information system (RIS)) according to the inclusion and exclusion criteria: demographic information (age, gender, race, past medical history, personal history, underlying disease, etc.), elaborate data involving VA-ECMO therapy (including the exact data prior to surgery, the duration of surgery, the duration of ECMO support, intra-aortic balloon pump (IABP), continuous renal replacement therapy (CRRT) and ventilator usage, ECMO placement site, and complication of ECMO treatment), the abnormal results of laboratory test within 48 h of ECMO initiation (such as counts of white blood cells and platelets, procalcitonin, liver and renal function, and microbiological data), maximal vasoactive-inotropic score (VIS),38,39 sequential organ failure assessment (SOFA) score,40,41 the date of NI diagnosis, the date of death and discharge from the ICU, and outcome (NI state and In-hospital mortality rate) during the entire hospitalization period.
Statistical Analysis
All numeric variables showed a skewed distribution after performing the Kolmogorov–Smirnov test. Continuous data were expressed as medians and interquartile ranges (25th and 75th quartiles). Categorical data were expressed as frequencies or ratios. Most of the continuous variables were divided into groups according to the optimal cutoff value or professional knowledge. When comparing the baseline characteristics between the infected and non-infected groups, the Mann–Whitney U-test was used to analyze the continuous variables, while the chi-square test was used to analyze the categorical variables. Univariate analysis was performed to assess the potential variables associated with NI and In-hospital mortality rate within 28 days of ECMO initiation. NI was modeled as a time-dependent covariate using the Cox regression model to predict the risk factors of death in these patients. Cumulative incidence functions were calculated using the “cmprsk” package in R based on a competing risk model29,31,32. Competing risk regression was used to assess the impact of NI on In-hospital mortality rate and to adjust for the corresponding confounding factors.32 The “etm” package in R software was used to calculate the transition probability of different states (multistate), and the probabilities were illustrated in diagrams.42–44 SPSS software (version 25.0, SPSS, Inc., Chicago, IL, USA) and R V3.6.3 (https://CRAN.R-project.org/) were used to perform all statistical analyses. A bilateral P value of less than 0.05 was considered significant.
Results
Patients’ Clinical Characteristics
In the past 10 years, 503 eligible patients have been enrolled in the study (Figure 1). A total of 213 (42.3%) patients experienced 296 NIs: VAP, 152 (30.2%); BSI, 100 (19.9%); UTI, 4 (0.8%); and other infections (mainly SSIs and SSTIs), 40 (8%). Moreover, 193 (38.4%) patients developed NIs during ICU stay, while 206 (41.0%) patients developed NIs within 28 days after ECMO initiation. A total of 217 (43.1%) patients died during ICU stay, 220 (43.7%) patients died within 28 days after ECMO initiation, and 261 patients (51.9%) died during hospitalization.
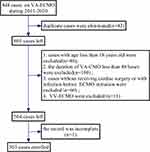 |
Figure 1 Flow chart showing the process of participant selection. |
The frequency distribution of NIs in adult patients who received VA-ECMO after cardiac surgery is shown in Figure 2 The clinical characteristics of these patients are summarized in Supplementary Tables 1 and 2.
The patients’ median age was 61 (51–67) years, and 71.4% of them were men. Compared with the non-infected group, patients with NI had a longer duration of IABP use, mechanical ventilator support, CRRT, and ECMO support. The length of ICU stay in the infected group was significantly longer than that in the non-infected group (12 vs 7, Z = −10.038, P < 0.001). The length of hospital stay was also significantly longer in the infected group than in the non-infected group (24 vs 18.5, Z = −5.764, P < 0.001). Unexpectedly, the 28-day in-hospital crude mortality rate was higher in the non-infected group than in the infected group (Supplementary Tables 1 and 2).
The prevalence rates of NIs during ECMO support and after ECMO removal were 27.8% (140/503) and 20.3% (102/503), respectively. The incidence rates of NI during ECMO support and after ECMO removal were 49‰ (per 1000 ECMO-days) and 25‰ (per 1000 patient-days), respectively (Supplementary Tables 3 and 4).
In total, 275 microorganisms were isolated (Table 1). The most common microorganisms were gram-negative bacteria (194, 70.5%): Acinetobacter baumannii (34.0%), Klebsiella pneumonia (21.6%), and Enterobacterium (20.1%). The gram-positive bacteria count was 48 (17.5%), of which Staphylococcus aureus (85.4%) was the most common pathogen, followed by Enterococcus spp. (8.3%). The fungal count was 33 (12.0%), of which Candida spp. (87.9%) was the most common.
 |
Table 1 Microorganisms of First Nosocomial Infection in Patients on VA-ECMO During ten Years |
Risk Factors of Death in NI Patients on ECMO After Cardiac Surgery
Single-variate Cox regression analysis indicated significant differences in more than 20 variables, such as age group (≥65 years), VIS, SOFA score, NI, and ICU stay (Table 1). When using a Cox regression model with NI as a time-varying covariate, we found 10 independent risk factors for predicting death in patients with NI on ECMO treatment after cardiac surgery (Table 2). Patients with older age (≥65 years), higher VIS (>40), higher SOFA score, higher BMI, cardiopulmonary resuscitation (CPR), and complications such as bleeding, cerebral problems, and time-varying NI had a worse prognosis. The risk of death in patients with NI on ECMO treatment was reduced by 14.8% when the ICU length of stay was extended by 1 more day (hazard ratio [HR] = 0.852, 95% confidence interval [CI] = 0.826–0.880; P < 0.001) (Table 3).
 |
Table 2 Single-Variate Cox Regression for Predicting Death of Patients with NI Within 28 Days After ECMO Initiation |
 |
Table 3 Multivariate Cox Regression for Predicting Death of Patients on ECMO After Cardiac Surgery |
Impact of NI on Mortality in Patients on ECMO Treatment After Cardiac Surgery
The transition probability plots of the different states according to the multistate model are shown in Figure 3. The transition probability of NI for states 0–1 (Figure 3A, indicated by the blue line) represents the transition from the non-infection state to the infection state. This indicates that the occurrence of NI was concentrated during the first 5–10 days after ECMO initiation. The incidence of NIs gradually decreased 20 days after the initiation of ECMO. The transition probability from state 0 to state 2 (Figure 3B, indicated by the green line) represents the transition from the non-infection state to the termination state (death in the ICU or discharged alive). The transition probability from state 1 to state 2 (Figure 3C, indicated by the red line) represented the transition from the infection state to the termination state (death in the ICU or being discharged alive). When integrating the three plots, we found that the transition probability was almost unchanged 20 days after ECMO initiation (Figure 3D). Most patients receiving VA-ECMO entered the termination state 20 days after ECMO initiation, died in the ICU, or were discharged from the hospital.
According to the competitive model, the cumulative incidence of death in patients with NIs was higher than that in patients without NI (Z = 5.816, P = 0.0159) (Figure 4). No significant difference was observed in the cumulative incidence function for discharge between the patients with NI and those without NI (Z = 3.313, P = 0.0687) (Figure 5). The competing risk regression analysis further confirmed that the risk of death increased by 50% in patients with NI compared with those without NI (HR = 1.50, 95% CI = 1.14–1.97), after adjusting for age, sex, and SOFA score (Table 4); moreover, the risk of death increased by 2% for every 1-year increase in age (HR = 1.02, 95% CI = 1.00–1.03) and increased by 16% per 1 point increase in SOFA score (HR = 1.16, 95% CI = 1.09–1.22). (Table 4).
 |
Table 4 Results of Competing Risk Regression Analysis for Death and Discharge in Patients on VA ECMO with or Without Nosocomial Infection |
Discussion
To the best of our knowledge, this was the largest single-center retrospective study of adult patients on VA-ECMO treatment. Using the Cox model, we confirmed that time-dependent NI was an independent risk factor for death in patients receiving VA-ECMO treatment after cardiac surgery. For the first time, we confirmed that NI increased the In-hospital mortality rate within 28 days of ECMO initiation, using a competitive risk regression model.
For adult patients receiving VA-ECMO treatment after cardiac surgery, we comprehensively reviewed the epidemiological distribution characteristics of NIs throughout hospitalization, focusing not only on the incidence of NI during ECMO support but also on the incidence of NI after ECMO removal. The prevalence and incidence of NI after ECMO removal reported in our study were 20.3% and 25‰ (per 1000 patient-days), respectively. These values were higher than the results of a retrospective survey on the prevalence and incidence of NI in the ICU in the same period in China.45–48
From the transition probability plot, we found that the peak NI occurrence was close to 10 days after ECMO initiation. To reduce the prevalence of NI, the primary disease should be treated, and the patient should recover as quickly as possible.
When the crude In-hospital mortality rate was compared between patients with NI and those without NI, it was difficult to determine the adverse effects of NI on the In-hospital mortality rate of these patients. NI, discharged alive, and death were the three competing factors.31,49 Concerning NI as a time-dependent intermediate variable, taking discharged alive and death as competing risk factors,31,49 we found that the cumulative incidence of death in patients with NI was higher than that in patients without NI at each time point within 28 days after ECMO initiation. After adjusting for age, sex, and SOFA score, the hazard risk of In-hospital mortality rate increased to approximately 50%, which was consistent with the finding of our previous meta-analysis (mortality increased by 32%). Furthermore, consistent with previous studies,20,22 older age and SOFA score were confirmed to be the independent risk factors for predicting death in adult patients on VA-ECMO treatment in the Cox regression model. The hazard ratio for predicting death in these patients increased by 2% for every 1-year increase in age and by 16% for every 1-point increase in the SOFA score in the competing risk model.
Our predictive model showed that four variables including older age, abnormal white blood cell, mechanical ventilation duration, and ECMO environment in the ICU were independent factors.50 Shortening the mechanical ventilation duration and avoiding ECMO catheterization in ICU as much as possible were the key points to reduce the incidence of NI.
The advantage of this study is that strict inclusion criteria were used to eliminate many confounding factors. However, this study has several limitations. First, the patients were recruited over a period of 10 years, during which the strategy of medical care and professional skills would have undergone some changes. Second, we only analyzed the first NI in each patient based on the assumption that the same patient with an NI and multiple NIs was at risk of being discharged alive or dead in the ICU. However, this is impractical in clinical practice. Third, we did not assess the impact of different types of NIs on In-hospital mortality rate or discharge individually, because many patients had multiple or mixed NIs during hospitalization. And forth, using culture-proven diagnosis standard might misjudge colonization as infection like most studies did. Moreover, our antibiotic prevention strategy had not been standardized as reported in the literature,51 the impact of changes in antibiotic prophylaxis strategies over the past 10 years on NIs cannot be well explained in this article.
Conclusion
NI is a common complication in adult patients who receive VA-ECMO treatment after cardiac surgery, and the time-dependent NI is an independent risk factor for predicting death in these patients. A competitive risk model analysis showed that NIs certainly increase the risk of death in these patients.
Abbreviations
ECMO, extracorporeal membrane oxygenation; VA-ECMO, venoarterial ECMO; LOS, length of stay; ICU, intensive care unit; SSI, surgical site infection; SSTI, skin and soft tissue infection; IABP, intra-aortic balloon pump; CRRT, continuous renal replacement therapy; VIS, vasoactive inotropic score; SOFA, Sequential Organ Failure Assessment; CPR, cardiopulmonary resuscitation; NI, nosocomial infection; VAP, Ventilator associated pneumonia; BSI, Bloodstream Infection; UTI, urinary tract infection; MDR, multi-drug resistant; LAC, lactate; ALT, alanine aminotransferase; WBC, white blood cell; PLT, platelet count.
Data Sharing Statement
The R code is available from the corresponding author Xiaotong Hou upon reasonable request.
Ethics Approval and Consent to Participate
The study was conducted in accordance with the Declaration of Helsinki. Ethical approval was granted according to the national requirements. This study was approved by the Medical Ethics Committee of Beijing Anzhen Hospital, Capital Medical University. The requirement for informed consent was waived because the study was performed retrospectively and no interventions were applied. The article fully protects the patient’s privacy, and the information related to the patient’s personal identity and the photos, images and words that can identify their personal identity will not be disclosed.
Acknowledgments
We would like to thank senior engineer Yuhao Wu, Electronic Medical Record and Information Center of Anzhen Hospital; we would also like to thank professor Zhuang Tao (Public Health Monitoring and Information Service Center of Chinese Center for Disease Control and Prevention) for providing invaluable statistical support for our study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by the “Sailing” Program of the Key Medical Specialty of Beijing Hospitals Authority in 2021 for Critical Care Medicine (fund number for extracorporeal life support: ZYLX202111). The funding body had no role in the design of the study, collection, analysis, interpretation of data, or manuscript preparation.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Guglin M, Zucker MJ, Bazan VM, et al. Venoarterial ECMO for Adults: JACC Scientific Expert Panel. J Am Coll Cardiol. 2019;73(6):698–716.
2. McCarthy FH, McDermott KM, Kini V, et al. Trends in US extracorporeal membrane oxygenation use and outcomes: 2002-2012. Semin Thorac Cardiovasc Surg. 2015;81–88.
3. Vogel AM, Lew DF, Kao LS, Lally KP. Defining risk for infectious complications on extracorporeal life support. J Pediatr Surg. 2011;46(12):2260–2264.
4. Bizzarro MJ, Conrad SA, Kaufman DA, Rycus P. Infections acquired during extracorporeal membrane oxygenation in neonates, children, and adults. Pediatric Critical Care Med. 2011;12(3):277–281.
5. Kim DW, Yeo HJ, Yoon SH, et al. Impact of bloodstream infections on catheter colonization during extracorporeal membrane oxygenation. J Artificial Organs. 2016;19(2):128–133.
6. Grasselli G, Scaravilli V, Di Bella S, et al. Nosocomial infections during extracorporeal membrane oxygenation: incidence, etiology, and impact on patients’ outcome. Crit Care Med. 2017;45(10):1726–1733.
7. Kutleša M, Santini M, Krajinović V, et al. Nosocomial blood stream infections in patients treated with venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome. Minerva Anestesiol. 2017;83(5):493–501.
8. Bouglé A, Bombled C, Margetis D, et al. Ventilator-associated pneumonia in patients assisted by veno-arterial extracorporeal membrane oxygenation support: epidemiology and risk factors of treatment failure. PLoS One. 2018;13(4):e0194976.
9. Wang J, Huang J, Hu W, Cai X, Hu W, Zhu Y. Risk factors and prognosis of nosocomial pneumonia in patients undergoing extracorporeal membrane oxygenation: a retrospective study. J Int Med Res. 2020;48(10):300060520964701.
10. Wang J, Wang L, Jia M, Du Z, Hou X. Extracorporeal Membrane Oxygenation-Related Nosocomial Infection after Cardiac Surgery in Adult Patients. Braz J Cardiovasc Surg. 2021;36(6):743–751.
11. Burket JS, Bartlett RH, Vander Hyde K, Chenoweth CE. Nosocomial infections in adult patients undergoing extracorporeal membrane oxygenation. Clinical Infectious Diseases. 1999;28(4):828–833.
12. Hsu MS, Chiu KM, Huang YT, Kao KL, Chu SH, Liao CH. Risk factors for nosocomial infection during extracorporeal membrane oxygenation. J Hospital Infection. 2009;73(3):210–216.
13. Sun HY, Ko WJ, Tsai PR, et al. Infections occurring during extracorporeal membrane oxygenation use in adult patients. J Thoracic Cardiovascular Surgery. 2010;140(5):1125–1132.
14. Schmidt M, Bréchot N, Hariri S, et al. Nosocomial infections in adult cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation. Clin Infect Dis. 2012;55(12):1633–1641.
15. Pieri M, Agracheva N, Fumagalli L, et al. Infections occurring in adult patients receiving mechanical circulatory support: the two-year experience of an Italian national referral tertiary care center. Medicina Intensiva. 2013;37(7):468–475.
16. Aubron C, Cheng AC, Pilcher D, et al. Infections acquired by adults who receive extracorporeal membrane oxygenation: risk factors and outcome. Infect Control Hosp Epidemiol. 2013;34(1):24–30.
17. Kim GS, Lee KS, Park CK, et al. Nosocomial Infection in Adult Patients Undergoing Veno-Arterial Extracorporeal Membrane Oxygenation. J Korean Med Sci. 2017;32(4):593–598.
18. Juthani BK, Macfarlan J, Wu J, Misselbeck TS. Incidence of nosocomial infections in adult patients undergoing extracorporeal membrane oxygenation. Heart and Lung. 2018;47(6):626–630.
19. Allou N, Lo Pinto H, Persichini R, et al. Cannula-related infection in patients supported by peripheral ECMO: clinical and microbiological characteristics. ASAIO J. 2019;65(2):180–186.
20. Silvetti S, Ranucci M, Pistuddi V, et al. Bloodstream infections during post-cardiotomy extracorporeal membrane oxygenation: incidence, risk factors, and outcomes. Int J Artificial Organs. 2019;42(6):299–306.
21. Menaker J, Galvagno S, Rabinowitz R, et al. Epidemiology of blood stream infection in adult extracorporeal membrane oxygenation patients: a cohort study. Heart and Lung. 2019;48(3):236–239.
22. Wang JR, Huang JY, Hu W, Cai XY, Hu WH, Zhu Y. Bloodstream infections in patients undergoing extracorporeal membrane oxygenation. Pakistan J Med Sci. 2020;36(6):1171–1176.
23. Ko RE, Huh K, Kim DH, et al. Nosocomial infections in in-hospital cardiac arrest patients who undergo extracorporeal cardiopulmonary resuscitation. PLoS One. 2020;15(12):e0243838.
24. Selçuk Ü, Sargın M, Baştopçu M, et al. Microbiological Spectrum of Nosocomial ECMO Infections in a Tertiary Care Center. Br j Cardiovascular Surgery. 2021;36(3):338–345.
25. Huang W, Ye H, Cheng Z, et al. Outcomes from the Use of Perioperative Extracorporeal Membrane Oxygenation in Patients Undergoing Thoracic Surgery: an 8-Year Single-Center Experience. Med Sci Monit. 2021;27:e931842.
26. Giraldi G, Montesano M, Sandorfi F, Iachini M, Orsi GB. Excess length of hospital stay due to healthcare acquired infections: methodologies evaluation. Ann Ig. 2019;31(5):507–516.
27. Wolkewitz M, Schumacher M, Rücker G, Harbarth S, Beyersmann J. Estimands to quantify prolonged hospital stay associated with nosocomial infections. BMC Med Res Methodol. 2019;19(1):111.
28. Wolkewitz M, von Cube M, Schumacher M. Multistate Modeling to Analyze Nosocomial Infection Data: an Introduction and Demonstration. Infect Control Hosp Epidemiol. 2017;38(8):953–959.
29. Cortese G, Gerds TA, Andersen PK. Comparing predictions among competing risks models with time-dependent covariates. Stat Med. 2013;32(18):3089–3101.
30. Beyersmann J, Schumacher M, Allignol A. Multistate models and their connection to competing risks. In: Beyersmann J, Allignol A, Schumacher M, editors. Competing Risks and Multistate Models with R. New York: Springer New York; 2012:169–175.
31. Barnett A, Graves N. Competing risks models and time-dependent covariates. Crit Care. 2008;12(2):134.
32. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509.
33. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16(3):128–140.
34. Quintana MT, Mazzeffi M, Galvagno SM, et al. A Retrospective Study of Infection in Patients Requiring Extracorporeal Membrane Oxygenation Support. Ann Thorac Surg. 2021;112(4):1168–1175.
35. Yun JH, Hong SB, Jung SH, et al. Epidemiology and Clinical Characteristics of Bloodstream Infection in Patients Under Extracorporeal Membranous Oxygenation. J Intensive Care Med. 2020;36(6):1053–1060.
36. Li ZJ, Zhang DF, Zhang WH. Analysis of Nosocomial Infection and Risk Factors in Patients with ECMO Treatment. Infect Drug Resist. 2021;14:2403–2410.
37. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332.
38. Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 2010;11(2):234–238.
39. Hyun J, Kim AR, Lee SE, et al. Vasoactive-Inotropic Score as a Determinant of Timely Initiation of Venoarterial Extracorporeal Membrane Oxygenation in Patients With Cardiogenic Shock. Circ J. 2022;86(4):687–694.
40. Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710.
41. Laimoud M, Alanazi M. The Validity of SOFA Score to Predict Mortality in Adult Patients with Cardiogenic Shock on Venoarterial Extracorporeal Membrane Oxygenation. Crit Care Res Pract. 2020;2020:3129864.
42. Guo HL, Zhao GJ, Ling XW, Xu JJ, Lu CJ, Liu ZJ. Using competing risk and multistate model to estimate the impact of nosocomial infection on length of stay and mortality in burn patients in Southeast China. BMJ Open. 2019;8(11):e020527.
43. Allignol A, Schumacher M, Beyersmann J. Estimating summary functionals in multistate models with an application to hospital infection data. Comput Stat. 2011;26(2):181–197.
44. Wangler M, Beyersmann J, Schumacher M. changeLOS: an R-package for change in length of hospital stay based on the Aalen-Johansen estimator. Newsletter R Project. 2006;6:31.
45. Zhao X, Wang L, Wei N, et al. Epidemiological and clinical characteristics of healthcare-associated infection in elderly patients in a large Chinese tertiary hospital: a 3-year surveillance study. BMC Infect Dis. 2020;20(1):121.
46. He H, Ma X, Su L, et al. Effects of a national quality improvement program on ICUs in China: a controlled pre-post cohort study in 586 hospitals. Crit Care. 2020;24(1):73.
47. Zhang Y, Du M, Johnston JM, et al. Incidence of healthcare-associated infections in a tertiary hospital in Beijing, China: results from a real-time surveillance system. Antimicrob Resist Infect Control. 2019;8:145.
48. Huoi C, Vanhems P, Nicolle MC, Michallet M, Bénet T. Incidence of hospital-acquired pneumonia, bacteraemia and urinary tract infections in patients with haematological malignancies, 2004-2010: a surveillance-based study. PLoS One. 2013;8(3):e58121.
49. Wolkewitz M, Vonberg RP, Grundmann H, et al. Risk factors for the development of nosocomial pneumonia and mortality on intensive care units: application of competing risks models. Crit Care. 2008;12(2):R44.
50. Li X, Wang L, Li C, et al. A nomogram to predict nosocomial infection in patients on venoarterial extracorporeal membrane oxygenation after cardiac surgery. Perfusion. 2022;2676591221130484.
51. Shah A, Sampathkumar P, Stevens RW, et al. Reducing Broad-Spectrum Antimicrobial Use in Extracorporeal Membrane Oxygenation: reduce AMMO Study. Clin Infect Dis. 2021;73(4):e988–e996.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




