Back to Journals » Journal of Inflammation Research » Volume 16
Impact of Moderate Physical Activity on Inflammatory Markers and Telomere Length in Sedentary and Moderately Active Individuals with Varied Insulin Sensitivity
Authors Almuraikhy S , Sellami M, Al-Amri HS, Domling A, Althani AA, Elrayess MA
Received 27 August 2023
Accepted for publication 7 November 2023
Published 20 November 2023 Volume 2023:16 Pages 5427—5438
DOI https://doi.org/10.2147/JIR.S429899
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Shamma Almuraikhy,1,2,* Maha Sellami,3,* Hadaia Saleh Al-Amri,1,3 Alexander Domling,2 Asmaa A Althani,1,4 Mohamed A Elrayess1,4
1Biomedical Research Center, Qatar University, Doha, Qatar; 2Groningen Research Institute of Pharmacy, Drug Design, Groningen University, Groningen, the Netherlands; 3Physical Education Department (PE), College of Education, Qatar University, Doha, Qatar; 4QU Health, Qatar University, Doha, Qatar
*These authors contributed equally to this work
Correspondence: Mohamed A Elrayess, Email [email protected]
Introduction: Physical activity-associated immune response plays a crucial role in the aging process. This study aimed to determine the impact of short-term moderate physical activity on cytokine levels, oxidative stress markers, and telomere length in lean/overweight young subjects.
Methods: Fasting blood samples were collected from 368 participants at Qatar Biobank. Based on their homeostatic model assessment of insulin resistance (HOMA-IR), participants were categorized as insulin sensitive (IS) or insulin resistant (IR). Subsequently, they were divided into four groups: sedentary IS (n = 90), sedentary IR (n = 90), moderately active IS (n = 94), and moderately active IR (n = 94). Moderate physical activity was defined as walking at least two days per week for more than 150 minutes, as determined by physical activity questionnaires. Serum samples were analyzed for circulating inflammatory cytokines (IL-1β, IL-1RA, IL-6, IL-10, IL-22, MCP-1/CCL2, TNF-α), as well as antioxidant enzyme levels (SOD and catalase). Telomere lengths were measured in the respective DNA samples.
Results: Moderately active IR participants exhibited significantly lower SOD activity, while catalase activity did not show significant differences. Moderately active IS participants had higher IL-6 and IL-10 levels compared to sedentary IS participants, with no significant differences observed in the IR counterparts. Telomere length did not significantly differ between the physically active and sedentary groups.
Conclusion: This study highlights the potential anti-inflammatory and anti-oxidative stress effects of moderate physical activity in individuals with insulin sensitivity and insulin resistance. However, no significant changes in telomere length were observed, suggesting a complex relationship between physical activity and the aging process. Further research is needed to fully understand the underlying mechanisms and optimize the balance between anti-inflammation and anti-oxidation through exercise and lifestyle adjustments.
Keywords: moderate physical activity, antioxidant, interleukins, aging, sedentarily, impaired insulin sensitivity
Introduction
Exercise is recognized as a potent and effective strategy for protecting against inflammation. This beneficial effect can be attributed to a range of factors, including reductions in visceral fat accumulation and the augmented production of anti-inflammatory cytokines during physical activity.1,2 The interplay between exercise and the cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-1 receptor antagonist, TNF receptors and IL-10 is complex and multifaceted.1–4 Indeed variations in cytokine levels can depend on the intensity of the exercise training and the individual’s physical condition.5–7
In addition to the impact on cytokines, exercise also influences the activity of antioxidant enzymes that play crucial roles in managing oxidative stress within the body. Among these enzymes, superoxide dismutase (SOD) and catalase are particularly important. Research has shown lower levels of these antioxidant enzymes in obese patients, indicating impaired antioxidant defenses.8 Regular physical activity, especially of moderate intensity, has been demonstrated to increase the activity of SOD and catalase, potentially enhancing the body’s ability to counteract oxidative stress.9 However, the effects of regular physical activity on antioxidant enzyme activity remain ambiguous, and more in-depth studies are needed.10–15
Another intriguing aspect of the relationship between exercise and health is the influence of physical activity on telomeres, the protective structures located at the ends of chromosomes. Telomeres naturally undergo a process of gradual shortening with aging. However, engaging in regular physical activity has been associated with a reduction in disease risk and a potential preservation of telomere length.16 Studies conducted on US adults have reported significantly longer telomeres in individuals who participate in regular exercise compared to sedentary individuals.17 Furthermore, shortened telomeres have been linked to insulin resistance, which is believed to result from a combination of oxidative stress and chronic inflammation.18–21 The question of whether leukocyte telomere length can serve as a predictive marker for the development of type 2 diabetes remains an area of ongoing research.22,23
Metabolic inflammation is related to the induction of various intracellular stresses, such as mitochondrial oxidative stress,24 and the predominant mechanisms through which a healthy diet and moderate physical exercise could mitigate telomere attrition include decreasing oxidative stress and inflammation.25 Several risk factors, including inflammation and oxidative stress, have been associated with shorter telomere length.26 In light of these considerations, the objective of our study is to comprehensively compare cytokine profiles, oxidative stress markers, and telomere lengths between individuals who are physically active and those who lead sedentary lifestyles. To gain a deeper understanding, we further divide the participants into insulin-resistant (IR) and insulin-sensitive (IS) groups. Our aim is to identify potential differences in oxidative stress status, the impact on telomere length dynamics, and their implications for insulin sensitivity in relation to physical activity. By exploring these relationships, this research aims to further uncover novel therapeutic strategies, refine exercise prescriptions, and develop personalized interventions to harness the benefits of exercise in combating inflammation, oxidative stress, and age-related cellular changes.
Materials and Methods
The Data Source and Study Participants
For this study, 368 serum and DNA samples were procured from Qatar Biobank (QBB) participants, accompanied by relevant clinical traits, anthropometric measures, and physical activity questionnaires. Inclusion criteria included young (20–30 years old), lean/overweight (BMI ≥20, <30 Kg/m2), and healthy (no chronic diseases) individuals. Exclusion criteria included those younger than 20 years or over 30 years old, with BMIs less than 20 kg/m2 or greater than 30 kg/m2, or participants with the following chronic diseases: diabetes, glaucoma, macular degeneration, blood clots, cardiovascular disease, bariatric surgery, or cancer. Anthropometric measurements comprised body weight, height (sitting and standing) using the Seca stadiometer, hip and waist circumferences as well as bioimpedance (Tanita). Data also included assessment of muscle strength, grip strength measured in the participants’ right and left hands using a 28 hydraulic hand dynamometer. For blood pressure, using the Omron 705 automated device, two diastolic and systolic blood pressure measurements were obtained, and if these differed by 5 mmHg or more, a third measurement was made. Hematology and blood biochemistry were analyzed by the laboratories of the Hamad Medical Centre Laboratory, Qatar.27 Ethical approvals for this study were obtained from both the Institutional Review Board of Qatar Biobank (QF-QBB-RES-ACC-00066) and Qatar University (QU-IRB 1716-E/22). All participants provided informed consent. Based on their Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) index, participants were categorized as insulin sensitive (IS) if HOMA-IR was ≤1.85, and insulin resistant (IR) if HOMA-IR was ≥1.85.28 To classify individual into two groups (Sedentary and active), we have used results of physical activity questionnaire (self-reported activity) collected and performed by Qatar Biobank, in addition to results of fitness test such as Handgrip test together with the aerobic performance test duration (which was not reported to the current data). The two strategies were used to obtain accurate result regarding the fitness level of the individual, as the self-report questionnaire may not be sufficient to determine the level of physical activity. According to scientific literature, CDC, ACSM and WHO suggest that a physically active individual is the one who is engaged into physical activity for at least 150 minutes of moderate-intensity aerobic activity, 75 minutes of vigorous-intensity aerobic activity, or an equivalent combination each week, along with muscle-strengthening activities on two or more days per week.29–32
The participants, averaging 25 years of age, included males (n = 189) and females (n = 179) with an average BMI of 25.05. They were categorized into four groups as follows:
Insulin Sensitive Sedentary (ISS, n = 90): 44% male, 46% female, Insulin Sensitive Active (ISA, n = 94): 49% male, 45% female, Insulin Resistant Sedentary (IRS, n = 90): 44% male, 46% female and Insulin Resistant Active (IRA, n = 94): 52% male, 42% female.
Cytokines Profiling
The ProcartaPlex™ Human Mix & Match cytokine multiplex kit (MAN0024966, Invitrogen) was used to simultaneously profile cytokines, including IL-1 beta, IL-1RA, IL-6, IL-10, IL-22, MCP-1/CCL2, and TNF-alpha using LUMINEX 200 in the sera of 368 samples, according to manufacturer’s instructions. Separate standard curves were used to validate the assay for the detection and quantification of cytokines according to manufacturer’s instructions using Xponent software.
Antioxidant Enzymes Activity Measurements
Activities of superoxide dismutase and catalase were determined in the sera of 368 samples using the colorimetric activity assays (EIACATC and EIASODC, respectively), according to manufacturer’s instructions (ThermoFisher Scientific, Frederick, MD, USA). Absorbance was then read using cytation5 (BioTek, imaging reader, USA).
Measurement of DNA Telomere Length
PureLink® Genomic DNA Kits (Invitrogen, Life Technologies, Carlsbad, CA, USA) were used for isolation of genomic DNA from the clotted blood at the bottom of the serum tubes from 368 samples described in Table 1, according to manufacturer’s instructions. The concentration and the quality of DNA were measured using the Nanodrop. Absolute Human Telomere Length Quantification qPCR Assay Kit (ScienCell, Carlsbad, CA, USA) was used to measure the average telomere length in extracted DNA samples according to manufacturer’s instructions. The kit includes a telomere primer set that amplifies telomere sequences, a single copy reference region for data normalization and a reference genomic DNA sample with known telomere length as a reference for calculating the telomere length of target samples. Briefly, two qPCR reactions were prepared for each genomic DNA sample: one with telomere (TL) and one with single copy reference (SCR) primer stock solutions. qPCR reactions were prepared by adding genomic DNA template (5 ng/µL) to primer stock solution (TL or SCR) and GoldNStart TaqGreen qPCR master mix. qPCR was run using an initial denaturation of 95 °C for 10 min, followed by 32 cycles of denaturation at 95 °C for 20s, annealing at 52 °C for 20s and extension at 72 °C for 45s using StepOne™ Real-Time PCR System (ThermoFisher). For quantification of TL, ∆Cq (TL) was quantified by assessing the cycle number difference of TL between two genomic DNA samples (sample of interest and the reference genomic DNA sample with known telomere length). For SCR, ∆Cq (SCR) was assessed by quantifying the cycle number difference of SCR between two genomic DNA samples (sample of interest and the reference genomic DNA sample with known telomere length). ∆∆Cq was calculated as ∆Cq (TL)−∆Cq (SCR). Fold change was assessed as 2-∆∆Cq.33
 |
Table 1 General Characteristics of Study Participants |
Statistical Analysis
All analyses were performed using R Studio (v 4.0.3) software (R Foundation for statistical computing, Vienna, Austria). Nominal variables are displayed as numbers with percentages and the differences were determined using Chi-square test. Numeric (continuous) data are presented as mean (standard deviation) and were compared using Student’s t-test/Mann–Whitney U-test and 1-way ANOVA. Shapiro Wilk test was used to examine the normality of distribution and skewed data were log-transformed. Linear regression analysis evaluating the association between ISA vs ISS, IRA vs IRS, and active vs sedentary participants with correction for confounders’ age, BMI, gender, and fasting time. Means difference of each analysts between active and sedentary at each level of HOMA-IR was calculated using R Emmeans package while correcting for confounders: age, BMI, gender, and fasting time. Nominal p-values were corrected for multiple testing using the false discovery rate (FDR) method. The analysis incorporated the interaction between the HOMA-IR category and physical activity groups; the estimate represents the offset in the intercept from the sedentary baseline to active individuals.
Results
General Characteristics of Study Population
Overall, 368 lean/overweight (BMI ≥20, <30 Kg/m2), young (20–30 years old) individuals were included in this study. The participants were grouped into ISA, ISS, IRA and IRS based on their HOMA-IR and physical activity status. Significant differences were observed among the four groups. BMI showed a significant difference between the ISS and IRS groups (p-value: 0.008), with higher BMI observed in the IRS group. Moreover, average systolic blood pressure displayed a significant difference between the ISS and IRS groups (p value ≤ 0.001), with higher values in the IRS group. Average diastolic blood pressure also exhibited a significant difference between the ISS and IRS groups (p-value = 0.027), again with higher values in the IRS group. These findings suggest potential variations in cardiovascular health and body composition between the two groups. Regarding measured cytokines, IL-6 levels showed a significant difference between the ISS and ISA groups (p-value: 0.007), with higher levels found in the ISA group. Similarly, IL-10 levels exhibited a non-significant trend of difference between the ISS and ISA groups (p-value: 0.079), with higher levels in the ISA group as well. However, no significant differences were found in IL-22, MCP-1/CCL2, and TNF-α levels among the four groups. Furthermore, white blood cell count demonstrated a significant difference between the ISS and IRS groups (p value = 0.003), with higher counts in the IRS group. Similarly, triglyceride levels showed a significant difference between the ISS and IRS groups (p-value = ≤0.001), with higher levels observed in the IRS group. Other factors, such as waist size and hip size, also displayed significant differences among the groups. Basic subject’s characteristics are summarized in Table 1.
Differences in the Levels of Cytokines, Oxidative Stress Markers and Telomere Length Between ISA Vs ISS and IRA Vs IRS
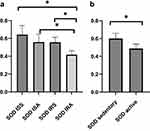
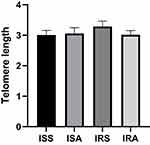
A linear model was used to evaluate the differences in cytokines, oxidative stress markers and telomere length between ISS vs ISA and IRS vs IRA individuals after correcting for confounding factors, including gender, age, BMI and fasting time. Among the detected cytokines, IL-6 and IL-10 showed significantly higher levels in ISA compared to ISS (p = 0.023, 0.027, respectively) as shown in Table 2 and Figure 1. On the other hand, our results showed significantly lower level of SOD in IRA group compared to IRS (p value = 0.005), as presented in Table 3 and Figure 2. There were no significant differences in telomere lengths among ISS, ISA, IRS and IRA individuals (Figure 3).
 |
Table 2 Linear Regression Analysis Evaluating the Differences in Cytokines, Oxidative Stress Markers and Telomere Length (TL) Between ISA Vs ISS After Correcting for Potential Confounders |
 |
Table 3 Linear Regression Analysis Evaluating the Differences in Cytokines, Oxidative Stress Markers and Telomere Length (TL) Between IRA Vs IRS After Correcting for Potential Confounders |
Spearman Correlation Between Cytokines, SOD, Catalase, Telomere Length and Triglycerides
Correlation analysis was conducted to explore the relationships between different cytokines, such as IL-6, IL-10, and MCP-1, as well as oxidative stress markers like SOD and catalase, telomere length, and clinical traits within the entire cohort. The results revealed several significant correlations summarized in Figure 4. IL-6 showed positive correlations with TNF-α and SOD, IL-10 exhibited a positive correlation with IL-1β, MCP-1 displayed a positive correlation with IL-1RA, SOD correlated positively with HDL-cholesterol and IL-6. Conversely, IL-6 demonstrated a negative correlation with MCP-1, IL-10 showed a negative correlation with HDL-cholesterol, MCP-1 exhibited negative correlations with IL-6 and catalase, and SOD displayed negative correlations with triglycerides, catalase, and C-peptide.
Discussion
In this study, the ISA group displayed elevated circulating levels of IL-6 and IL-10 compared to the ISS group. However, no significant difference was observed between the IR active and sedentary groups, likely because IR individuals had higher levels of IL-6. The role of IL-6 in insulin resistance has been a subject of debate for many years. However, some previous studies suggest that IL-6 does not promote insulin resistance and, in certain cases, may even have beneficial effects on this disorder.34 PA triggers the release of higher amounts of specific cytokines, primarily IL-6.35 IL-6 plays a role in regulating energy expenditure, likely through its influence on brown adipose tissue via central nervous system effects. Our results demonstrated significant positive correlations between IL-6 and TNF-α levels. Both IL-6 and TNF-α were found to be elevated in obese and insulin-resistant patients. However, TNF-α exhibited inconsistent levels following prolonged exercise.36 Skeletal muscles release higher quantities of IL-6 during workouts, and this release is dependent on the type, intensity, and duration of the activity.37,38 Consequently, anti-inflammatory cytokines such as IL-10 are released in response to acute inflammation.39 IL-6 has been extensively studied in the scientific community as the key cytokine in exercise physiology, showcasing its pivotal role. In response to exercise, IL-6 levels exhibit a significant exponential increase (up to 100-fold), which subsequently decline rapidly in the post-exercise period. Despite its well-known pro-inflammatory function, IL-6 also exerts anti-inflammatory effects during exercise. One proposed mechanism for these effects involves IL-6 inducing the production of anti-inflammatory cytokines, specifically IL-10 and IL-1R.40
Our emerging data has also showed higher IL-10 levels in the active insulin-sensitive groups. ISA individuals showed a four-fold higher levels of IL-10 compared to their sedentary counterparts. Studies have shown that IL-10 appears during the recovery period after PA.36 It is evident that certain cytokines promote muscle cell function during physical activity, thereby enhancing exercise performance.38 IL-10 is produced by activated macrophages and Th2 cells. It inhibits the production of IFN-γ by Th1 cells, shifting immune responses toward a Th2 type. IL-10 also suppresses cytokine production by activated macrophages and the expression of class II MHC and co-stimulatory molecules by these macrophages, thereby reducing immune responses.
It is plausible that IL-6 and IL-10 work synergistically to regulate immune responses and inhibit the development of severe inflammatory reactions. McGeachy et al41 demonstrated that IL-10 is upregulated in the presence of IL-6, which aligns with our findings. Fluctuations in IL-10 in response to PA were associated with increased insulin sensitivity and protection of skeletal muscle against the detrimental effects of inflammatory responses due to macrophage infiltration. Moreover, our study reported a positive correlation between IL-10 and IL-1β, as well as a negative correlation with HDL cholesterol. Notably, Kim et al42 demonstrated that in non-diabetic obese patients, a decrease in serum total cholesterol levels was accompanied by an increase in serum IL-10 concentration. Although IL-1β remained unchanged in both insulin-sensitive and insulin-resistant groups, whether active or sedentary, some cytokines such as IL-6 and IL-10 seem to be influenced by the level of PA. As previously mentioned, the intensity and duration of PA may play a significant role in the disparities of results. For instance, Stewart et al43 found that a 12-week aerobic exercise training did not alter plasma IL-6 and IL-1β levels when comparing young active groups (18–35 years) to older groups, suggesting that short-term training (months) might not be sufficient to induce changes in proinflammatory cytokines. Furthermore, there are more similarities than differences between the immune function of resting athletes and non-athletes, according to Nieman et al.44,45
Catalase, glutathione peroxidase, and SOD are important antioxidant enzymes. Our data has revealed that active individuals had lower SOD than sedentary counterparts regardless of their insulin sensitivity status. Despite the numerous health benefits of regular muscular activity, exercise causes a rise in the free radicals and other types of reactive oxygen species (ROS). Regular training, especially aerobic training, strengthens enzymatic antioxidant defenses.46 However, previous studies have also suggested that exercise or PA can decrease oxidative stress status.47 According to a recent meta analysis, Xu et al48 observed that higher SOD outcome could not reveal the antioxidant balance as it is a “complex system” that may depends on several factors. Findings from the meta-analysis suggested that results are clearly contradictory and most likely depend on the sampling period as well as the length and training intensity, which have varied widely between researches. Some of these studies demonstrated that post training SOD would reduce the lactic acid generated by endurance exercise.49 This has been confirmed by another study, which indicates that elevated SOD improves fatigue and muscle recovery.50 These impacts are again explained by the ability of SOD to fight against oxidative stress. Indeed, intense or prolonged physical exercise is a generator of free radicals. According to a recent research, endurance exercise increases extracellular SOD (EcSOD) levels in various peripheral organs by encouraging EcSOD expression in skeletal muscle.51 However, according to Ooawakara et al,52 three month of endurance training may only increase the EC-SOD reserves in intracellular level. Our data also revealed higher SOD in ISA compared to IRA counterparts. According to Tinahone et al,8 the patients with elevated IR had lower plasma SOD activity, which is in line with our findings. This result may provide evidence of relation of lower concentrations of superoxide anions and IR, despite physical activity status.
Physical activity status exhibited no change in telomere length in all groups. Our previous study showed that high-intensity elite athletes had elevated levels of IL-6 and IL-10 and longer telomeres compared to low and moderate intensity counterparts.53 Endurance exercise (eg running, brisk walking, or swimming) and a healthy lifestyle in general have been associated with longer TL and may therefore delay cellular aging. Beneficial effects of PA on cell regeneration and senescence have already been observed. Long-term endurance training is associated with higher telomerase activity and reduced telomere wear in young and middle-aged endurance athletes compared to inactive individuals.54,55 The results of that study show that only endurance activities can improve telomerase activity and the length of leukocyte telomeres in a punctual and chronic way (26 weeks of training), thus confirming the interest of practicing regularly and/or longer duration moderate training to allow higher telomerase activity and longer TL. Such phenomenon is also observed in twins where one would be active and the other inactive. As reported that higher intense PA would result in longer TL in twin comparing to their sibling.56 The absence of a significant difference in telomere length between sedentary and physically active individuals in our study may be attributed to several factors. These may include the specific intensity and duration of physical activity, variations in telomerase activity, and the influence of other biological and environmental factors that were not accounted for in our analysis. Further research is needed to explore the complex relationship between physical activity, telomere length, and the mechanisms involved in cellular aging.
While the study provided valuable insights, its cross-sectional design and reliance on self-reported physical activity data limited the ability to establish causality or determine long-term effects. Another limitation is relying on HOMA-IR crude cut-off as a reference for insulin resistance. Different results might have arisen if we had defined an IR group based on HbA1c levels. Furthermore, the findings suggested distinct effects of moderate activity in insulin-sensitive and insulin-resistant individuals, but no significant changes in telomere length were observed, indicating a lack of direct correlation between moderate physical activity and the aging process in this specific population. While telomere length is often considered a measure of aging, it is important to acknowledge that aging is influenced by numerous factors, and physical activity exerts its impact on aging through various mechanisms beyond just telomere length. Further research with longitudinal designs, objective physical activity measures, and diverse populations is necessary to validate and expand upon these findings.
Conclusion
This study provides valuable insights into the selective impact of physical activity on specific cytokines, particularly IL-6 and IL-10, in insulin-sensitive individuals. It also suggests that the mode and duration of physical activity influence other cytokines, such as IL-1beta, IL-1RA, IL-22, MCP-1/CCL2, and TNF-α. Importantly, the exercise-induced elevation in IL-6 does not lead to inflammatory exacerbations and is associated with beneficial effects. This presents exciting opportunities for utilizing exercise and its cytokine profile in the treatment of various conditions, necessitating further research in sports medicine and immunobiology. Moreover, the study highlights higher IL-6 and IL-10 levels in physically active insulin-sensitive individuals, indicating potential anti-inflammatory roles in glucose metabolism and insulin resistance. However, no significant changes in telomere length were observed between active and sedentary groups, suggesting that physical activity alone may not be sufficient to ensure longer telomeres. Factors such as exercise duration, intensity, recovery time, and diet may influence telomere dynamics. Future longitudinal studies are necessary to uncover the complex relationships between antioxidant enzymes, other molecules, and their biological functions. Additionally, optimizing the balance between oxidation and antioxidation through regular exercise and lifestyle adjustments warrants further investigation.
Data Sharing Statement
Data are available from the corresponding author upon reasonable request.
Institutional Review Board Statement
This study is performed in line with the World Medical Association Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. All protocols were approved by the Institutional Review Boards of the Qatar Biobank (QF-QBB-RES-ACC-00066) and Qatar University (QU-IRB 1716-E/22) and has received Expedited Review according to Qatar Ministry of Public Health (MoPH) regulations.
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Acknowledgments
The researchers would like to thank Qatar Biobank for providing samples, Physical education department of College of Education and biomedical center of Qatar University for participating in the study design and biochemical assay.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by Qatar University IRCC grant number IRCC-2022-467 (MAE, MS).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Walsh NP, Gleeson M, Shephard RJ, et al. Position statement. Part one: immune function and exercise. Exerc Immunol Rev. 2011;17:6–63.
2. Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev. 2000;80(3):1055–1081. doi:10.1152/physrev.2000.80.3.1055
3. Bruunsgaard H, Skinhoj P, Qvist J, Pedersen BK. Elderly humans show prolonged in vivo inflammatory activity during pneumococcal infections. J Infect Dis. 1999;180(2):551–554. doi:10.1086/314873
4. Croisier JL, Camus G, Venneman I, et al. Effects of training on exercise-induced muscle damage and interleukin 6 production. Muscle Nerve. 1999;22(2):208–212. doi:10.1002/(SICI)1097-4598(199902)22:2<208::AID-MUS8>3.0.CO;2-B
5. Wang S, Zhou H, Zhao C, He H. Effect of Exercise Training on Body Composition and Inflammatory Cytokine Levels in Overweight and Obese Individuals: a Systematic Review and Network Meta-Analysis. Front Immunol. 2022;13:921085. doi:10.3389/fimmu.2022.921085
6. Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol. 1999;515(Pt 1):287–291. doi:10.1111/j.1469-7793.1999.287ad.x
7. Prokopchuk O, Liu Y, Wang L, Wirth K, Schmidtbleicher D, Steinacker JM. Skeletal muscle IL-4, IL-4Ralpha, IL-13 and IL-13Ralpha1 expression and response to strength training. Exerc Immunol Rev. 2007;13:67–75.
8. Tinahones FJ, Murri-Pierri M, Garrido-Sanchez L, et al. Oxidative stress in severely obese persons is greater in those with insulin resistance. Obesity. 2009;17(2):240–246. doi:10.1038/oby.2008.536
9. Hoseini R, Rahim HA, Ahmed JK. Concurrent alteration in inflammatory biomarker gene expression and oxidative stress: how aerobic training and vitamin D improve T2DM. BMC Complement Med Ther. 2022;22(1):165. doi:10.1186/s12906-022-03645-7
10. Nishida Y, Higaki Y, Taguchi N. Objectively measured physical activity and inflammatory cytokine levels in middle-aged Japanese people. Prev Med. 2014;64:81–87. doi:10.1016/j.ypmed.2014.04.004
11. Tonkonogi M, Walsh B, Svensson M, Sahlin K. Mitochondrial function and antioxidative defence in human muscle: effects of endurance training and oxidative stress. J Physiol. 2000;528(Pt 2):379–388. doi:10.1111/j.1469-7793.2000.00379.x
12. Miyazaki H, Oh-ishi S, Ookawara T, et al. Strenuous endurance training in humans reduces oxidative stress following exhausting exercise. Eur J Appl Physiol. 2001;84(1–2):1–6. doi:10.1007/s004210000342
13. Leeuwenburgh C, Hollander J, Leichtweis S, et al. Adaptations of glutathione antioxidant system to endurance training are tissue and muscle fiber specific. Am J Physiol. 1997;272(1 Pt 2):R363–9. doi:10.1152/ajpregu.1997.272.1.R363
14. Tiidus PM, Pushkarenko J, Houston ME. Lack of antioxidant adaptation to short-term aerobic training in human muscle. Am J Physiol. 1996;271(4 Pt 2):R832–6. doi:10.1152/ajpregu.1996.271.4.R832
15. Dimauro I, Grazioli E, Lisi V, et al. Systemic response of antioxidants, heat shock proteins, and inflammatory biomarkers to short-lasting exercise training in healthy male subjects. Oxidative Medicine and Cellular Longevity. 2021;2021:1–15. doi:10.1155/2021/1938492
16. Arsenis NC, You T, Ogawa EF, Tinsley GM, Zuo L. Physical activity and telomere length: impact of aging and potential mechanisms of action. Oncotarget. 2017;8(27):45008–45019. doi:10.18632/oncotarget.16726
17. Tucker LA. Physical activity and telomere length in U.S. men and women: an NHANES investigation. Prev Med. 2017;100:145–151. doi:10.1016/j.ypmed.2017.04.027
18. von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27(7):339–344. doi:10.1016/S0968-0004(02)02110-2
19. Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans. 2007;35(5):1147–1150. doi:10.1042/BST0351147
20. Navarro-Mateu F, Rubio-Aparicio M, Cayuela P, et al. The association of telomere length with substance use disorders: systematic review and meta-analysis protocol. Syst Rev. 2019;8:298. doi:10.1186/s13643-019-1199-x
21. Cheng F, Luk AO, Shi M, et al. Shortened leukocyte telomere length is associated with glycemic progression in type 2 diabetes: a prospective and Mendelian randomization analysis. Diabetes Care. 2022;45:701–709.
22. Zhao J, Zhu Y, Lin J, et al. Short leukocyte telomere length predicts risk of diabetes in American Indians: the strong heart family study. Diabetes. 2014;63(1):354–362. doi:10.2337/db13-0744
23. You NC, Chen BH, Song Y, et al. A prospective study of leukocyte telomere length and risk of type 2 diabetes in postmenopausal women. Diabetes. 2012;61(11):2998–3004. doi:10.2337/db12-0241
24. Muriach M, Flores-Bellver M, Romero FJ, Barcia JM. Diabetes and the brain: oxidative stress, inflammation, and autophagy. Oxid Med Cell Longev. 2014;2014:102158. doi:10.1155/2014/102158
25. Ibarra MJN, Hernández J, Juvera GCJNH. Diet, physical activity and telomere length in adults. Nutricion hospitalaria. 2019;36(6):1403–1417. doi:10.20960/nh.02673
26. Kalea AZ, Drosatos K. Nutriepigenetics and cardiovascular disease. Diabetes Care. 2018;21:252.
27. Al Kuwari H, Al Thani A, Al Marri A, et al. The Qatar Biobank: background and methods. BMC Public Health. 2015;15:1–9.
28. Gayoso-Diz P, Otero-Gonzalez A, Rodriguez-Alvarez MX, et al. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: effect of gender and age: EPIRCE cross-sectional study. BMC Endocr Disord. 2013;13(1):47. doi:10.1186/1472-6823-13-47
29. Chaabna K, Mamtani R, Abraham A, Maisonneuve P, Lowenfels AB, Cheema S. Physical Activity and Its Barriers and Facilitators among University Students in Qatar: a Cross-Sectional Study. Int J Environ Res Public Health. 2022;19(12):7369. doi:10.3390/ijerph19127369
30. Sayegh S, Van Der Walt M, Al-Kuwari MG. One-year assessment of physical activity level in adult Qatari females: a pedometer-based longitudinal study. Int J Womens Health. 2016;8:287–293. doi:10.2147/IJWH.S99943
31. Griffin A, Roselli T, Clemens SL. Trends in Total Physical Activity Time, Walking, and Vigorous Physical Activity Time in Queensland Adults From 2004-2018. J Phys Act Health. 2020;17(6):592–602. doi:10.1123/jpah.2019-0282
32. Majed L, Sayegh S, Chrismas BCR. Reference Walking Speeds for Healthy Young Adults in Qatar: moderating Effect of Obesity and Physical Activity. SAGE Open. 2022;12(1):21582440221079919. doi:10.1177/21582440221079919
33. Sellami M, Al-Muraikhy S, Al-Jaber H, et al. Age and sport intensity-dependent changes in cytokines and telomere length in elite athletes. Antioxidants. 2021;10:1035.
34. Carey AL, Febbraio MA. Interleukin-6 and insulin sensitivity: friend or foe? Diabetologia. 2004;47(7):1135–1142. doi:10.1007/s00125-004-1447-y
35. Wedell-Neergaard AS, Lang Lehrskov L, Christensen RH, et al. Exercise-Induced Changes in Visceral Adipose Tissue Mass Are Regulated by IL-6 Signaling: a Randomized Controlled Trial. Cell Metab. 2019;29(4):844–855 e3. doi:10.1016/j.cmet.2018.12.007
36. Pedersen BK, Bruunsgaard H, Ostrowski K, et al. Cytokines in aging and exercise. Int J Sports Med. 2000;21 Suppl 1:S4–9. doi:10.1055/s-2000-1444
37. Pedersen BK, Steensberg A, Fischer C, et al. Searching for the exercise factor: is IL-6 a candidate? J Muscle Res Cell Motil. 2003;24(2/3):113–119. doi:10.1023/A:1026070911202
38. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8):457–465. doi:10.1038/nrendo.2012.49
39. Hiscock N, Fischer CP, Sacchetti M, van Hall G, Febbraio MA, Pedersen BK. Recombinant human interleukin-6 infusion during low-intensity exercise does not enhance whole body lipolysis or fat oxidation in humans. Am J Physiol Endocrinol Metab. 2005;289(1):E2–7. doi:10.1152/ajpendo.00274.2004
40. Docherty S, Harley R, McAuley JJ, et al. The effect of exercise on cytokines: implications for musculoskeletal health: a narrative review. BMC Sports Sci Med Rehabil. 2022;14:5. doi:10.1186/s13102-022-00397-2
41. McGeachy MJ, Bak-Jensen KS, Chen Y, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8(12):1390–1397. doi:10.1038/ni1539
42. Kim SJ, Lim J, Nam GE, Park HS. Correlation between Serum Lipid Parameters and Interleukin-10 Concentration in Obese Individuals. J Obes Metab Syndr. 2021;30(2):173–177. doi:10.7570/jomes20122
43. Stewart LK, Flynn MG, Campbell WW, et al. The influence of exercise training on inflammatory cytokines and C-reactive protein. Med Sci Sports Exerc. 2007;39(10):1714–1719. doi:10.1249/mss.0b013e31811ece1c
44. Nieman DC, Pedersen BK. Exercise and immune function. Recent Developments Sports Med. 1999;27:73–80.
45. Pedersen BK, Toft AD. Effects of exercise on lymphocytes and cytokines. Br J Sports Med. 2000;34:246–251. doi:10.1136/bjsm.34.4.246
46. Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88(4):1243–1276. doi:10.1152/physrev.00031.2007
47. de Sousa CV, Sales MM, Rosa TS, Lewis JE, de Andrade RV, Simoes HG. The Antioxidant Effect of Exercise: a Systematic Review and Meta-Analysis. Sports Med. 2017;47(2):277–293. doi:10.1007/s40279-016-0566-1
48. Xu Y, Liang M, Ugbolue UC, Fekete G, Gu Y. Effect of Physical Exercise Under Different Intensity and Antioxidative Supplementation for Plasma Superoxide Dismutase in Healthy Adults: systematic Review and Network Meta-Analysis. Front Physiol. 2022;13:707176. doi:10.3389/fphys.2022.707176
49. Skarpanska-Stejnborn A, Pilaczynska-Szczesniak L, Basta P, Deskur-Smielecka E, Woitas-Slubowska D, Adach Z. Effects of oral supplementation with plant superoxide dismutase extract on selected redox parameters and an inflammatory marker in a 2000-m rowing-ergometer test. Int J Sport Nutr Exerc Metab. 2011;21(2):124–134. doi:10.1123/ijsnem.21.2.124
50. Carillon J, Notin C, Schmitt K, Simoneau G, Lacan D. Dietary supplementation with a superoxide dismutase-melon concentrate reduces stress, physical and mental fatigue in healthy people: a randomised, double-blind, placebo-controlled trial. Nutrients. 2014;6(6):2348–2359. doi:10.3390/nu6062348
51. Yan Z, Spaulding HR. Extracellular superoxide dismutase, a molecular transducer of health benefits of exercise. Redox Biol. 2020;32:101508. doi:10.1016/j.redox.2020.101508
52. Ookawara T, Haga S, Ha S, et al. Effects of endurance training on three superoxide dismutase isoenzymes in human plasma. Free Radic Res. 2003;37(7):713–719. doi:10.1080/1071576031000102132
53. Sellami M, Al-Muraikhy S, Al-Jaber H, et al. Age and Sport Intensity-Dependent Changes in Cytokines and Telomere Length in Elite Athletes. Antioxidants. 2021;10:548.
54. Werner CM, Hecksteden A, Zundler J, et al. Differential effects of endurance, interval, and resistance training on telomerase activity and telomere length in a randomized, controlled study. Eur Heart J. 2019;40(1):34–46. doi:10.1093/eurheartj/ehy585
55. Borghini A, Giardini G, Tonacci A, et al. Chronic and acute effects of endurance training on telomere length. Mutagenesis. 2015;30(5):711–716. doi:10.1093/mutage/gev038
56. Mundstock E, Zatti H, Louzada FM, et al. Effects of physical activity in telomere length: systematic review and meta-analysis. Ageing Res Rev. 2015;22:72–80. doi:10.1016/j.arr.2015.02.004
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.