Back to Journals » Clinical Ophthalmology » Volume 14
Impact of Marital Status on Survival in Patients with Ocular and Periocular Malignancies: A Retrospective Analysis of 3159 Patients from the SEER Database
Authors Loya A , Ayaz T, Weng CY
Received 10 November 2019
Accepted for publication 24 March 2020
Published 23 April 2020 Volume 2020:14 Pages 1127—1133
DOI https://doi.org/10.2147/OPTH.S238034
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Asad Loya,1 Talha Ayaz,2 Christina Y Weng3
1School of Medicine, Baylor College of Medicine, Houston, TX, USA; 2School of Medicine, University of Texas Medical Branch at Galveston, Galveston, TX, USA; 3Department of Ophthalmology,Cullen Eye Institute, Baylor College of Medicine, Houston, TX, USA
Correspondence: Christina Y Weng
Department of Ophthalmology, Cullen Eye Institute, Baylor College of Medicine, Houston, TX, USA
Tel +1 713 798-6100
Email [email protected]
Background: An ocular or periocular malignancy can profoundly impact patients’ lives as they cope with the challenges of a potentially life-threatening diagnosis and the exhaustive treatment process it entails. An amalgam of biopsychosocial factors can influence prognosis. This study aims to determine whether marital status impacts the long-term survival of patients with these malignancies.
Methods: A retrospective analysis of the Surveillance, Epidemiology, and End Results (SEER) database was performed. Patients with ocular and periocular malignancies diagnosed between 1973 and 2015 were included. The association between survival and marital status was assessed using univariate and multivariate Cox regression. Adjusted covariates included demographic, tumor, and treatment data.
Results: A total of 3159 patients with a mean±SD follow-up period of 6.47± 4.62 (range 0– 17.9) years were studied. At the time of diagnosis, 63.4% (2004/3159) of the cohort were married, 12.9% (409/3159) were single, 16.3% (514/3159) were widowed, and 7.3% (232/3159) were divorced. The mean±SD age of the cohort was 64.4± 15.17 (range 26– 100) years, with histology distributed as 14.6% (462/3159) melanoma, 84.5% (2669/3159) lymphoma, and 0.9% (28/3159) plasmacytoma. Adjusted all-cause mortality risk was higher in single (HR, 1.885, 95% CI 1.535 to 2.314; P< 0.001), widowed (HR, 1.382, 95% CI 1.169 to 1.635; P< 0.001), and divorced (HR, 1.637, 95% CI 1.271 to 2.109; P< 0.001) individuals compared to married individuals. Similarly, adjusted cause-specific mortality risk was higher in single (HR, 1.835, 95% CI 1.332 to 2.528; P< 0.001), widowed (HR, 1.376, 95% CI 1.025 to 1.847; P=0.033), and divorced (HR, 1.873, 95% CI 1.272 to 2.758; P=0.001) individuals compared to married individuals.
Conclusion: Unmarried (single, widowed, and divorced) individuals with ocular or periocular malignancies have unmet social support needs resulting in poorer long-term outcomes. Understanding the prognostic role of such psychosocial factors is necessary to improve the identification of and care for patients with inadequate support.
Keywords: oncology, ocular, cancer, SEER, relationship, database
Introduction
Ocular and periocular malignancies, the most common of which are intraocular melanoma and ocular adnexal lymphoma, are diagnosed in approximately 3500 Americans annually.1–3 These malignancies may result in loss of vision or mortality, and account for an estimated 330–370 deaths per year in the USA.1–3
While intrinsic characteristics of the malignancy can prognosticate mortality, there are other possible factors that influence overall survival rates in these populations. One such factor is marital status, which has been widely studied across many pathologies. With respect to cancer, studies examining non-ocular cancer sites have found improved long-term outcomes in married individuals compared to their non-married counterparts.4–7 It has even been suggested that the effect of marriage may be equivalent to or greater than that of chemotherapy in benefiting cancer survivors.8
In this study, we seek to evaluate the impact of marital status on long-term survival from ocular and periocular malignancies.
Patients and Methods
This is a retrospective study utilizing the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database,9 a publicly accessible database containing de-identified demographic, case, and follow-up data from cancer patients across the USA. The specific registry accessed was “Incidence – SEER 18 Regs Custom Data (with additional treatment fields), Nov 2017 Sub (1973–2015 varying).” A total of 30,191 cases of ocular and periocular cancer cases were identified between 1973 and 2015 using International Classification of Diseases (ICD) for Oncology third edition topographical codes corresponding to the eye and adnexa (C69.0–69.9) and eyelid (C44.1). Individuals with benign tumors (4693/30,191), non-primary tumors (5370/30,191), age less than or equal to 25 years old (2671/30,191), and unknown/unstaged summary stage (23,600/30,191) were excluded from the study. Of the remaining cohort, individuals with marital status listed as “separated” (22/3581), “unmarried or domestic partner” (4/3581), and “unknown” (396/3581) were excluded because of small numbers or inability to assess.
Extracted data included demographic variables, tumor characteristics, treatment data, and survival status. Demographic variables included in the study were age, sex, race, ethnicity, and marital status. Tumor characteristics included in the study were tumor site, malignancy type, laterality, and summary stage. Summary stage was categorized as local, regional (signifying involvement of regional lymph nodes), or distant (signifying presence of distant metastases). Treatment data included chemotherapy, radiation, and surgery. Extracted survival variables included vital status, cause-specific death classification, and survival months. The primary outcome evaluated was the association between survival and marital status. Marital status was categorized into four types: married (including common law), single (never married), widowed, and divorced.
Asymptotic two-sided Pearson’s chi-squared test was used to compare variables between groups. Survival function curves were generated for adjusted overall survival (OS) and cause-specific survival (CSS). Two different hazard models were created for each OS and CSS that analyzed mortality by marital status: one used univariable Cox regression (no adjustment for other variables) and the other used multivariable Cox regression (adjusted for multiple variables including malignancy type, age, and treatment). Statistical significance was determined using a P-value of less than 0.05. IBM SPSS version 25 was used to conduct the statistical analysis, which was also independently verified by two authors (AL, TA).
According to institutional review board guidelines at the University of Texas Medical Branch at Galveston and Baylor College of Medicine, this study does not meet the definition of “human subject research” as defined by the regulations outlined in 45 CFR 56 and at 21 CFR 5, given that this study involves the use of de-identified data that are publicly available.
Results
Cohort Analysis
A total of 3159 cases were included. At the time of diagnosis, 63.4% (2004/3159) of the cohort were married, 12.9% (409/3159) were single, 16.3% (514/3159) were widowed, and 7.3% (232/3159) were divorced. Of the included cases, 84.5% (2669/3159) were lymphoma (primary vitreoretinal, choroidal, orbital, etc), 14.6% (462/3159) were melanoma, and 0.9% (28/3159) were plasmacytoma. Altogether, 93.1% (2940/3159) of the diagnoses were confirmed by positive histology and 1.6% (50/3159) were confirmed by positive cytology without histology; the remaining diagnoses were confirmed by other or unknown means. Regarding summary stage, 79.9% (2523/3159) of cases were localized, 6.2% (197/3159) were regional, and 13.9% (439/3159) were distant. The most common sites were orbit (NOS), conjunctiva, and eyelid, accounting for 41.0% (1296/3159), 21.7% (685/3159), and 20.6% (652/3159) of all cases, respectively. The mean±SD age of married individuals was 62.3±13.85, of single individuals was 56.85±16.64, of widowed individuals was 79.1±9.87, and of divorced individuals was 63.7±12.44 years. The mean follow-up period was 6.47±4.62 (range 0–17.9) years. Categorical variables distributed by marital status can be found in Table 1.
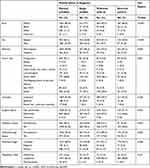 |
Table 1 Demographic, Tumor, and Treatment Characteristics of Subjects with Ocular and Periocular Tumors |
Marital Status Survival Analysis
When adjusting for demographic (including age), tumor, and treatment characteristics, all-cause mortality risk was higher in single (HR, 1.885, 95% CI 1.535 to 2.314; P<0.001), widowed (HR, 1.382, 95% CI 1.169 to 1.635; P<0.001), and divorced (HR, 1.637, 95% CI 1.271 to 2.109; P<0.001) individuals compared to married individuals. Similarly, adjusted cause-specific mortality risk was higher in single (HR, 1.835, 95% CI 1.332 to 2.528; P<0.001), widowed (HR, 1.376, 95% CI 1.025 to 1.847; P=0.033), and divorced (HR, 1.873, 95% CI 1.272 to 2.758; P=0.001) individuals compared to their married counterparts (Figure 1). Unadjusted and adjusted hazard ratios are displayed in Table 2.
 |
Table 2 Univariable and Multivariable Cox Regression |
Adjusted all-cause mortality also significantly varied by age at diagnosis (P<0.001), sex (P<0.001), tumor site (P<0.001), radiation treatment (P<0.001), chemotherapy treatment (P=0.001), and summary stage (P<0.001) (Supplemental Table 1). Adjusted cause-specific survival significantly varied by age at diagnosis (P<0.001), sex (P=0.007), tumor site (P<0.001), radiation treatment (P=0.008), chemotherapy treatment (P<0.001), summary stage (P<0.001), and laterality (P=0.012) (Supplemental Table 2).
Discussion
Using a national cancer database, we performed a retrospective analysis of 3159 cases of ocular and periocular tumors to evaluate the impact of marital status on survivorship. Although the role of marital status on survival has been studied extensively in the context of other cancers, to the authors’ knowledge, this is the first study analyzing its relevance to survival from eye-associated cancers. When adjusting for demographic, tumor, and treatment variables, both all-cause and cause-specific mortality risk were significantly higher for single, widowed, and divorced individuals compared to married individuals. In those diagnosed with ocular and periocular malignancies, at any given time, single individuals have a 1.89 times increased risk of all-cause death; widowed individuals have a 1.38 times increased risk of death; and divorced individuals have a 1.64 times increased risk of death from any cause compared to those who are married. Similarly, with regard to malignancy-related deaths alone, single individuals have a 1.84 times increased risk of death; widowed individuals have a 1.38 times increased risk of death; and divorced individuals have a 1.87 times increased risk of death compared to married counterparts.
There are several possible explanations for our observation. Receiving a cancer diagnosis is an extremely stressful and life-changing experience for a patient. During this time, psychosocial support is of critical importance in coping; for many individuals, this support may be offered through a spousal relationship. The inherent social support associated with marriage may contribute to better mental health and psychological status, which may improve a patient’s outlook and, therefore, ability to face cancer and its hardships. Goldzweig et al showed that married individuals had decreased levels of distress, depression, and anxiety following a cancer diagnosis.15 Our finding that married individuals experienced improved OS and CSS compared to non-married individuals may be due to the reduction of such negative emotions and their associated complications. The support of a marital partner and presence of a cohesive family have also been shown to increase adherence to treatment regimens, while the presence of conflict within family has been shown to increase non-adherence.16 Since oncologic diagnoses often require multiple visits for evaluation and treatment, and adherence to treatment is critical for survival, it seems possible that our findings may be driven by patient compliance related to marital status. Finally, socioeconomic status (SES) may directly or indirectly affect ocular cancer survival. While there were no time-specific socioeconomic data available in this database, it is possible that married individuals may be more financially stable than their non-married counterparts and that our findings are actually reflective of SES rather than marital status. Financial stability could affect multiple facets of care, including compliance, treatment choices, ability to afford medications, and even baseline health, and thus it is feasible that it could significantly affect survival. Further study is needed in this area.
Aside from a lack of SES data, there were other limitations in this study. Variables such as stage and grade of cancer were not available for all database patients; since these are important predictors of survival, our analysis was limited to a much smaller number of included cases. As a result, other common ocular and periocular neoplasms, such as squamous cell carcinoma, angiosarcoma, and sebaceous cell carcinoma, were excluded from our analysis. In addition, localized cutaneous (eyelid) basal cell and squamous cell carcinomas are not reported within SEER, limiting our ability to account for these within our study. Furthermore, our analysis was confined to the variables within the SEER database, which limited our ability to account for other active medical problems, past medical history, detailed cancer treatment data, or changes in marital status post-database entry, among other factors. Nonetheless, we were able to minimize confounding effects by adjusting for treatment data within two discrete categories (yes, no/unknown). Lastly, as case data were identified through ICD codes, it is possible that inaccurate coding affected our results.
Although there are no published studies specifically evaluating how marital status relates to ocular tumor survival, research has suggested that marital status can impact the advancement of ocular disease and visual impairment.17–19 Studies evaluating marital status in the context of non-ocular cancers demonstrate findings similar to ours, with improved outcomes in married individuals compared to non-married individuals.4–7 One study of non-small cell lung cancer patients found improved overall and cause-specific survival in married patients compared to non-married patients.4 Another analysis evaluating prostate cancer patients found that married men were diagnosed at lower Gleason scores, were more likely to undergo surgical intervention, and had higher 5-year survival rates compared to single, divorced/separated, and widowed men.5 One large population-based study including all cancers found that unmarried individuals were significantly more likely to receive a diagnosis at an advanced stage, had an increased likelihood of not receiving treatment, and displayed decreased survival compared to their married counterparts.6
Other factors were also independently associated with significant differences in mortality risk. As demonstrated in prior literature, increased age at diagnosis,10 bilateral tumors,11,12 and higher summary stage10 were associated with increased mortality. Similar to previous studies, mortality risk also significantly differed by the malignancy’s site of origin within the eye and orbit.13 Differences found with regard to chemotherapy and radiation treatment, although important statistically in adjustment, have unclear clinical significance as subgroups were classified as “yes” or “no/unknown”. Individuals with unknown status may have received treatment confounding this finding, a known limitation of the SEER dataset. Previous studies, however, have also documented reduced mortality with radiotherapy use.10,14 As types and modalities of chemotherapy administration continue to evolve, further investigation is necessary for its role in ocular and periocular cancer. Interestingly, males had improved survival compared to their female counterparts. In contrast, Mahendraraj et al, in their study examining survival trends in uveal melanoma, reported poorer survival in males.10 Additional investigation is necessary to confirm and further clarify the mechanism for survival disparities by sex.
In light of our findings, it may be important to address a patient’s marital status when determining treatment plans and risk stratification. While further analysis is needed to determine which aspects of marital status have the greatest impact on survival, offering non-married patients additional social support may prove to be a valuable component in their overall care plan.
Conclusion
Our study findings suggest that in patients with ocular and periocular malignancies, non-married (single, widowed, and divorced) individuals experience poorer all-cause and cause-specific survival compared to married individuals. An improved understanding of prognostically significant psychosocial factors, such as presence of spousal support, is valuable at a clinical level. Clinicians can potentially apply this information to real-world practice by conducting a more comprehensive risk assessment for individual patients to identify patients who may have inadequate social support and provide them with additional resources. If there is indeed a psychosocial advantage provided by marriage, then it may be beneficial to ensure that non-married individuals receive alternative types of support in order to optimize survival outcomes.
Funding
No funding was acquired for this study.
Disclosure
Dr. Weng is a consultant for and reports personal fees from Alcon, Inc., Allergan, Inc., and Alimera Sciences, Inc. The authors report no other conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi:10.3322/caac.21387
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
4. Wu Y, Ai Z, Xu G. Marital status and survival in patients with non-small cell lung cancer: an analysis of 70006 patients in the SEER database. Oncotarget. 2017;8(61):103518–103534. doi:10.18632/oncotarget.21568
5. Liu Y, Xia Q, Xia J, et al. The impact of marriage on the overall survival of prostate cancer patients: a Surveillance, Epidemiology, and End Results (SEER) analysis. Can Urol Assoc J. 2018;13:5. doi:10.5489/cuaj.5413
6. Goodwin JS, Hunt WC, Key CR, Samet JM. The effect of marital status on stage, treatment, and survival of cancer patients. JAMA. 1987;258(21):3125–3130
7. Aizer AA, Chen M-H, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869–3876. doi:10.1200/JCO.2013.49.6489
8. Kissane DW. Marriage is as protective as chemotherapy in cancer care. J Clin Oncol. 2013;31(31):3852–3853. doi:10.1200/JCO.2013.51.5080
9. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER 18 Regs Custom Data (with additional treatment fields), Nov 2017 Sub (1973-2015 varying) - Linked To County Attributes - Total U.S., 1969-2016 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2018, based on the November 2017 submission. Available from: www.seer.cancer.gov.
10. Mahendraraj K, Lau CS, Lee I, Chamberlain RS. Trends in incidence, survival, and management of uveal melanoma: a population-based study of 7516 patients from the Surveillance, Epidemiology, and End Results database (1973–2012). Clin Ophthalmol. 2016;10:2113–2119. doi:10.2147/OPTH.S113623
11. Andreoli MT, Chau FY, Shapiro MJ, Leiderman YI. Epidemiological trends in 1452 cases of retinoblastoma from the Surveillance, Epidemiology, and End Results (SEER) registry. Can J Ophthalmol. 2017;52(6):592–598. doi:10.1016/j.jcjo.2017.05.012
12. Jenkins C, Rose G, Bunce C, et al. Clinical features associated with survival of patients with lymphoma of the ocular adnexa. Eye. 2003;17(7):809–820. doi:10.1038/sj.eye.6700379
13. Mahendraraj K, Shrestha S, Lau CS, Chamberlain RS. Ocular melanoma-when you have seen one, you have not seen them all: a clinical outcome study from the Surveillance, Epidemiology and End Results (SEER) database (1973–2012). Clin Ophthalmol. 2017;11:153. doi:10.2147/OPTH.S120530
14. Olszewski AJ, Desai A. Radiation therapy administration and survival in Stage I/II extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Int J Radiat Oncol. 2014;88(3):642–649. doi:10.1016/j.ijrobp.2013.11.225
15. Goldzweig G, Andritsch E, Hubert A, et al. Psychological distress among male patients and male spouses: what do oncologists need to know? Ann Oncol. 2010;21(4):877–883. doi:10.1093/annonc/mdp398
16. DiMatteo MR. Social support and patient adherence to medical treatment: a meta-analysis. Heal Psychol. 2004;23(2):207–218. doi:10.1037/0278-6133.23.2.207
17. Riva I, Legramandi L, Katsanos A, et al. Influence of sociodemographic factors on disease characteristics and vision-related quality of life in primary open-angle glaucoma patients. J Glaucoma. 2018;27(9):776–784. doi:10.1097/IJG.0000000000000989
18. Anastasopoulos E, Haidich AB, Coleman AL, et al. Risk factors for age-related macular degeneration in a Greek population: the thessaloniki eye study. Ophthalmic Epidemiol. 2018;25(5–6):457–469. doi:10.1080/09286586.2018.1512634
19. Gan S, Zhou X, Yan J, et al. The prevalence and risk factors of visual impairment among rural residents aged 50 years and above in Yugan county, China. Ophthalmic Epidemiol. 2018;25(5–6):331–337. doi:10.1080/09286586.2018.1476557
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

