Back to Journals » Infection and Drug Resistance » Volume 16
Impact of Irrational Use of Antibiotics Among Patients in the Intensive Care Unit on Clinical Outcomes in Sudan
Authors Abdelkarim OA , Abubakar U , Taha LO, Ashour SA, Abass WY, Osman EM, Muslih MS
Received 17 June 2023
Accepted for publication 26 October 2023
Published 14 November 2023 Volume 2023:16 Pages 7209—7217
DOI https://doi.org/10.2147/IDR.S378645
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Omalhassan Amir Abdelkarim,1 Usman Abubakar,2 Lubna Osman Taha,1 Sondos Ahmed Ashour,1 Wiaam Yousif Abass,1 Eslam Mohamed Osman,1 Mustafa Shith Muslih1
1Department of Pharmacy Practice and Clinical Pharmacy, Faculty of Pharmacy, International University of Africa, Khartoum, Sudan; 2Department of Clinical Pharmacy and Practice, College of Pharmacy, QU Health, Qatar University, Doha, Qatar
Correspondence: Omalhassan Amir Abdelkarim, Department of Pharmacy Practice and Clinical Pharmacy, Faculty of Pharmacy, International University of Africa, Madani St, P.O Box 2469, Al Khartoum, Khartoum, 12223, Sudan, Tel +249912325691, Email [email protected]
Background: Intensive Care Unit (ICU) is a specialized ward where critically ill patients are admitted to provide intensive health care Inappropriate antimicrobial therapy (AMT) and high mortality rates were documented in the ICU. The influence of irrational use of empiric antibiotics on clinical outcomes in ICU patients is not well studied in Sudan.
Aim: This study aims to determine the rational use of antibiotics and its impact on clinical outcomes among ICU patients.
Methods: Using data collection form, a retrospective longitudinal study was conducted among ICU patients at Omdurman Military Hospital, Khartoum State. Patients admitted from January 2019 to December 2019 were included in the study. Patients who stayed in ICU < 48 hr were excluded. Appropriateness of AMT is assessed using culture sensitivity test (CST) and the American Society of Infectious Diseases (IDSA) guideline.
Results: Among 102 patients, 54.9% male, one-third of patients developed nosocomial infections, 80.4% received empiric therapy with broad-spectrum antibiotics. The CST is done in 19%, and 43% patients are prescribed inappropriate AMT. Inappropriate AMT is associated with recurrent infections 38.4% (p=0.028) and high mortality 33.8% (p=0.014). Overall mortality rate 63.7% ICU patients. Elevated mortality in nosocomial 57.8%, decreased with inappropriate AMT in 21.6% patients. Significantly higher mortality rates 90.7% among uncontrolled infections (p< 0.001), 80.6% nosocomial infections (p=0.001), and 76.7% renal compromised (p=0.002).
Conclusion: Empirical AMT reduces the frequency of nosocomial infections, which has an impact on mortality. Inappropriate AMT is significantly associated with uncontrolled infections and lower mortality. Implementing a restrictive infectious control system and effective stewardship programs in hospital ICU wards is recommended.
Keywords: rational use of antibiotics, mortality rate, intensive care unit, clinical outcomes, appropriate antibiotic therapy
Introduction
The intensive care unit (ICU) is a critical care unit for providing care to critically ill patients that involves intensive and specialized medical and nursing monitoring care. Also, it enhances the capacity for multiple methods of physiological organ support to sustain life during life-threatening organ system insufficiency.1 Patients with critical infectious diseases are also managed in the ICU; they can become a significant source of spreading infectious organisms, which may lead to nosocomial infections. Nosocomial infections are associated with increased morbidity, mortality, and health-care costs.2 Nosocomial infections are a serious patient safety concern in the modern healthcare system as it is the fifth most hazardous cause of death among hospitalized patients.3 The mortality rates associated with nosocomial infections can reach 60%, accounting for approximately 40% of total ICU expenditures.4
The World Health Organization (WHO) defines the rational use of drugs as requiring that patients receive medications appropriate to their clinical needs, in doses suitable to their requirements for an adequate period, and at an affordable cost to them and their community.2 Available evidence shows that 20% to 50% of antimicrobial prescriptions in the hospital are inappropriate.2 Reduction in the quality of drug therapy is associated with increased morbidity and mortality, wastage of resources affected in reducing the availability of other vital drugs, increased costs, increased risk of unwanted effects, and the emergence of antimicrobial drug resistance.5 There is also an adverse psychosocial impact of irrational prescribing by perpetuating the notion that every symptom requires medication.6 Extensive and irrational use of antibiotics has also been reported to increase the risk of toxicity and drug interactions.7 Worldwide, over 50% of antibiotics are prescribed, dispensed, or sold inappropriately, while 50% of patients cannot take them correctly.2 Unfortunately, the situation is alarming; because of inappropriate use, the effective medicines of yesterday become ineffective today. The early initiation of empiric therapy and the selection of optimum antibiotics lead to a better outcome for the patient, prevent complications, and shorten the duration of illness.8 It is crucial for each hospital to have its antibiotic prescribing protocol to ensure appropriate treatment of patients and reduce antibiotic resistance. There are many factors influencing the use of antibiotics in hospitals, for example, the characteristics of the patients, susceptibility to antibiotics, and the hospital restrictions on antibiotic use. In Sudan, there was a significant resistance rate to most antibiotics frequently used in hospitals.9 However, extensive use of both broad- and narrow-spectrum antibiotics in ICU may develop resistant strains of bacteria and other pathogens.10 Antibiotic resistance within the ICU is very high because nosocomial infections require multiple antimicrobial therapies.10 Therefore, this study aims to determine the rational use of antibiotics and its impact on clinical outcomes and mortality rates among ICU patients.
Materials and Methods
Study Design, Settings
A retrospective longitudinal study was conducted among patients admitted to medical ICUs at Omdurman Military Hospital, Khartoum State, between January 2019 and December 2019. Omdurman Military Hospital is a tertiary hospital with more than 500 Beds. The hospital offers medical, surgical, orthopedic, pediatric, obstetrics and gynecology, ear, nose and throat, dental, and psychological services. The two wards of the medical ICU have 20 beds.
Study Population
The study included all patients who fulfilled the inclusion criteria, were admitted into the ICU, and subsequently discharged/died in Omdurman Military Hospital, Sudan. Patients aged ≥ 18 years with ≥48 hours of ICU stay were included in the study.
Study Questionnaire
A data collection form was used; the variables included were compared with previously reported studies. The developed checklist form was subjected to content validity through a focus group discussion of clinical researchers and practitioners to identify any missed variable or clinical consequence. The variables included socio-demographic data, medical history (such as diagnosis, comorbidities, type of infections, performed of CST, suffering from nosocomial infection), laboratory data, medication, and clinical outcomes (death or discharge).
Measurement of Clinical Outcomes
Clinical evaluation for each patient record was performed. The clinical outcomes were determined on the last day regarding “discharge” or “death” after treatment. Renal function was determined using the Cockcroft-Gault equation,11 and guidelines from the Physician Desk Reference and British National Formulary (BNF) were used to determine the dose adjustment and required dose after adjustment.12,13 The need for dose adjustment and required quantity after adjustment were determined using guidelines from the National Institute of Health and Care Excellence.14 The adverse events and drug interactions for every administered drug were determined by Stockley’s drug interaction.15 We defined inappropriate prescriptions as antibiotics for a health event determined not to require antibiotic therapy. The infection treatment was determined by reducing white blood cell (WBC) count and fever. The appropriateness of the antibiotic therapy was determined using the results of a culture sensitivity test (CST) and the American Society of Infectious Diseases (IDSA) guidelines.
Data Analysis
All the data collected through the data collection form were entered into a spreadsheet in Statistical Package for Service Solutions (SPSS version 25) after carefully defining all the variables under study. The analysis was performed in two steps, including a descriptive analysis in which the patient’s clinical situation and various variables were described using frequencies and percentages. Inferential statistics were performed to determine the associations between patient- and therapy-related variables, clinical outcomes, and mortality rate using Pearson’s chi-squared test. A statistically significant was considered when the P-value <0.05
Results
Characteristics of Study Patients
This study involved 102 patients admitted to ICUs between January 2019 and December 2019 and fulfilled the inclusion criteria. The characteristics of the study patients were identified; more than half, 56 (54.9%) of the patients were males, and 67 (66%) were above 55 years old. Moreover, the duration of patient stay in the ICUs ranged between 2 days to a month, and 46 (45%) of the patients admitted to the ICUs for 1 week or less.
Infections and Use of Antibiotics Among Study Patients
Table 1 illustrates that 31 (30.4%) patients developed infections during the study period and were diagnosed with nosocomial disorders. However, 82 (80.4%) patients were being administered empiric treatment with broad-spectrum antibiotics during admission to the ICU. The pattern of nosocomial infection was lower among the patients who initiated empiric antibiotic therapy 25 (24.5%), which is statistically insignificant (p=0.265). The CST samples were drawn from 19 (18.6%) patients, as presented in Figure 1. Moreover, the frequency of antimicrobial drugs prescribed among the study patients is shown in Table 2.
 |
Table 1 Association Between the Development of Nosocomial Infection and Initiation of Empiric Antibiotic Therapy Among Study Patients (n=102) |
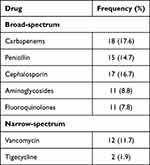 |
Table 2 Frequency of Antimicrobial Drugs Prescribed Among Study Patients |
 |
Figure 1 The frequency of changes in antibiotic therapy after initiation of empiric therapy. Abbreviations: CST, culture sensitivity test; ICU, intensive care units. |
In the current study, 44 (43%) patients were prescribed antibiotics that were not according to the recommendations of IDSA. Of these patients, 23 (52.2%) were discharged after recovering, as presented in Table 3. On the other hand, 58 (57%) patients were prescribed appropriate antibiotics following the IDSA recommendations.
 |
Table 3 Association Between the Clinical Outcomes and the Appropriateness of Antibiotic Therapy Among Study Patients (n=102) |
Association Between Antibiotic Therapy and Clinical Outcomes
Table 3 shows the association between the appropriate use of antibiotic therapy and the clinical outcomes. There was a statistically significant association between the administration of inappropriate antibiotic therapy and recurrent infections among study patients (p-value=0.028) and high mortality among ICU patients (p-value=0.014). The drug–drug interactions 27 (60%) increased among patients who received appropriate antibiotics (p-value<0.001). Furthermore, the mortality rate among patients who received inappropriate antibiotics was 21.6%, as shown in Figure 2.
 |
Figure 2 The mortality rate of various clinical conditions among the study patients (n=102). Abbreviation: CST, Culture Sensitivity Test. |
Mortality Rate Among ICU Patients
The current study also demonstrated an overall mortality rate of 65 (63.7%) among ICU patients. However, mortality rates of 57.8% were observed among patients with nosocomial infections, 46.1% of patients not performing CST, and 42.2% in renal compromised patients. The mortality rates among patients with different clinical conditions are demonstrated in Figure 2.
The current study also documented that more than 50% of the patients who received inappropriate antibiotic therapy were discharged from the ICU (p-value=0.014). However, a higher mortality rate of 59 (90.7%) was documented in patients with uncontrolled infections despite receiving appropriate antibiotic therapy (p-value<0.001). Moreover, 25 (80.6%) and 29 (64.4%) were the mortality rates among the patients with nosocomial infections (p-value=0.001) and patients on ventilators (p-value=0.007), respectively, as presented in Table 4.
 |
Table 4 Associations Between Clinical Variables and the End Outcomes Among ICU Patients |
In our study, 45 (44.1%) patients were on ventilators. However, 22 (48.8%) of them received appropriate antibiotic therapy, and 29 (64.4%) had a high mortality rate. Nevertheless, in ICU patients, most drugs are excreted through the kidneys, and assessing kidney function is critical in ensuring safe and effective therapy administration.16 In this study, 56 (54.9%) were renal-compromised patients who needed special consideration in drug therapy, and out of them, 43 (76.7%) patients died at the end of the study duration, as illustrated in Table 4. Moreover, the frequency of nephrotoxic drugs prescribed among them was documented as 17 (30.4%) proton pump inhibitors (PPIs), 10 (17.9%) penicillin, 9 (16.1%) aminoglycoside, 8 (14.3%) analgesic steroidal drugs, 7 (12.5%) fluoroquinolones, and 5 (8.9%) vancomycin.
Discussion
In the current study, 80.4% of patients were prescribed empiric broad-spectrum antibiotics during ICU admission. However, this rate of patients receiving empiric therapy with broad-spectrum antibiotics is higher than that of a similar study conducted in South Africa that found the rate to be 73.3% and 73.5%, respectively,17,18 a lower pattern was documented in North America, Europe, and Pakistan, at 68% and 28.8%, respectively.19,20 The current study finding was higher than previous studies conducted among ICU patients in Sudan and India, documenting that antibiotics were prescribed to all study patients.21,22 This higher rate of prescribing empirical antibiotics could be explained by the fact that the health-care practitioner was not concordant with standard guidelines in the patient’s therapy.
Our study also showed that appropriate antibiotic therapy was prescribed in 57% of patients. This finding is higher than the previous study, where 14% of patients were appropriately prescribed antibiotics and discharged after recovery.19 However, mortality among patients who received inappropriate antibiotics was higher, 96.5%, than in our study, 47.7%. In contrast, our study result was lower than another study, finding that empiric antibiotic treatment was appropriate in 77.5%.23 Moreover, a previous study conducted in Khartoum documented inappropriate empiric choice in 44.9%, and broad antibiotics were used among 40.6% of study patients.24 This discrepancy could be explained by the hospitals’ lack of established protocols or infectious control committees. Consequently, standard treatment guidelines for empirical antibiotic therapy should be developed for Sudanese patients based on the local antibiotic resistance susceptibility pattern. However, all ICU patients are at high risk for developing infections due to intense pathological and critical conditions. Therefore, all measures should be taken to prevent infections. Nevertheless, antibiotics could be appropriately used with an antibiotic stewardship program and drug utilization evaluation (DUE) to maximize the therapeutic response and reduce unintended side effects.25
However, in the absence of an antibiotic control or stewardship program in the hospital and none of the prescribed therapies reviewed by clinical pharmacists specialized in infectious diseases, inappropriate therapy was prescribed in 43% of patients, which is very low compared to previous data.26 Inconsistent with an earlier study, inappropriate empiric antimicrobial therapy was associated with serious interactions and unwanted adverse reactions.20 However, inappropriate use of antibiotics among hospitalized patients was not only reported in developing countries 97% and 58.5% in Indonesia and Sudan, respectively,27,28 but also documented in the developed county as in the United States among 44–97% of hospitalized patients.29 Nevertheless, the irrational use of antibiotics leads to an escalation in the morbidity and mortality rate in the community, health-care costs, and the development of antibiotic resistance.30,31 The mortality rate among inappropriate antibiotic therapy ICU patients in this study was 21.6%. This result differs from another study that showed mortality of inappropriately treated 88% of patients.32 The use of standardization and guidelines for antibiotic therapy may explain this discrepancy in mortality among ICU patients.
In the current study, the spread of nosocomial infections was documented among one-third of study patients; this finding is consistent with the result of a previous retrospective study.19 This might be related to poor hygienic conditions; proper sterilization and disinfection could not be attained at all levels due to high patient influx.33,34 However, in our study, a higher percentage, 46.1%, of the patients who received empiric therapy did not develop nosocomial infection during ICU admission. Similarly, previous studies demonstrated a reduction of nosocomial infections among ICU patients associated with empiric antimicrobial therapy.19,35 The explanation of this finding might be due to early effective treatment of suspected infections by empirical therapy. Therefore, using antibiotics judiciously and following established guidelines can prevent the emergency of antibiotic resistance and ensure optimal patient outcomes.
Furthermore, this study also found that the CST was done in 19% of patients, while a prospective study performed in 43 Italian ICU patients found that CST followed the onset of empiric antibiotic treatment in 93% of the cases.16 The test was done for 46.7% of the patients in a study conducted in India.21 Also, our findings disagree with a previous study in an ICU in Sudan, which showed that CST was not done for all patients.22 This discrepancy could be explained by the socio-economic situation in developing countries, which leads to insufficient health services and a low rate of CST use. However, excessive and inappropriate use of empiric antibiotics is a major cause of antibiotic resistance, especially if the antibiotics were administered for a long time without a CST to identify the type of organism.
In the present study, an overall mortality rate of 63.7% was found among patients in ICU. However, a lower mortality rate was documented in previous studies in Sudan, Northland, India, and Turkey.22,36–39 Higher result was observed in previous studies.19,40,41 This discrepancy confesses to variations between countries in implementing great systems for infectious control and antibiotics stewardship programs and concord with standard guidelines. Consequently, higher mortality rate among all ICU patients was found in patients with nosocomial infections, 57.8%. These results were lower than those in Spain and Turkey at 69%.40,42 This variation could be related to the higher impact of nosocomial infections contracted in critical care, which are ventilator-associated pneumonia, central line-associated bloodstream infection, and urinary catheter-related urinary tract infection. Furthermore, this study displays that approximately 81% of the mortality among patients experienced nosocomial infections. Lower mortality, 28.1%, was documented in an Indian study.38 The difference is probably related to good hygiene practices and ventilatory care. Consequently, this study found a lower mortality rate in patients who had not initiated empiric antibiotic therapy, 5.6%. This finding is probably supported by the fact that inadequate initial empiric antimicrobial treatment was not associated with higher mortality in ICU patients in Canada.43
The present study found a high mortality rate of 76.7% among renal-compromised patients, which is lower than Pakistan’s study result of 85.4%.19 Our study results are higher than a previously reported study in seven adult ICUs of the tertiary medical center in 57%.44 High mortality among renal compromised patients is usually due to use of corticosteroids and other nephrotoxic agents without adequate dose monitoring, which leads to decreased glomerular filtration rate in ICU patients. This variation in mortality could be due to disparity in CLOSE monitoring for the renal profile of ICU patients. However, inappropriate use of antimicrobials and noncompliance with infection control precautions are the main risk factors for antimicrobial resistance. Antimicrobial resistance has become a global concern and particularly a life-threatening issue in Sudan. Therefore, implementing infection control with specialized health-care providers, including clinical pharmacists, hospital protocol, and a strong stewardship program will minimize the risk of infections and resistant strains in health-care settings, particularly in ICUs, and improve patient quality of life.
Limitation
The present study provides insight into the rational and inappropriate use of antibiotics and its impact on ICU patients’ clinical outcomes. To the best of our knowledge, this is the first study that evaluates the rational and inappropriate use of antibiotics and mortality rates among ICU patients in Sudan. The findings reveal the requirement for more infection control and reasonable use of antimicrobial therapy to improve clinical outcomes and mortality rate. Despite these, some limitations should be considered with caution. Firstly, the research was conducted as a retrospective study. Therefore, some information on study patients may be missed. Secondly, national guidelines and hospital protocols for antimicrobial therapy need to be well established. Therefore, the use of IDSA guidelines is probably not comparable. The emphasized limitations of this study should be abridged in future studies.
Conclusion
Irrational and inappropriate antibiotic therapy among ICU patients is a leading cause of antimicrobial resistance and nosocomial infection. Inappropriate antibiotic therapy is significantly associated with uncontrolled infections and nosocomial infections. The highest mortality rate was observed among study patients. Nosocomial infection, uncontrolled infection, ventilator, and renal compromised patients significantly influenced mortality. Intensive care units must maintain regional antimicrobial resistance surveillance and provide the necessary antibiogram data in Sudan. Local protocols, improving awareness, training, and continuous education for health-care providers, and optimizing antimicrobial medicines should be top priorities in hospital ICUs. Medication review with close monitoring of ICU patients under a competent intensive care specialized clinical pharmacist’s supervision can help reduce various factors influencing the mortality rate and other clinical outcomes. Health-care policymakers must implement infection control systems and effective antimicrobial stewardship programs to improve health-care outcomes and patients’ quality of life.
Abbreviations
AMT, Antimicrobial Therapy; BNF, British National Formulary; CST, Culture Sensitivity Test; ICU, Intensive Care Unit; IDSA, American Society of Infectious Diseases; NICE, National Institute of Health and Care Excellence; PPIs, Proton Pump Inhibitors; SPSS, Statistical Package for Service Solutions; WBC, White Blood Cell; WHO, The World Health Organization.
Data Sharing Statement
The datasets analyzed and used during this study are available from the corresponding author upon reasonable request.
Ethics Approval
Ethical approval was taken from the Research Ethical Committee, Faculty of Pharmacy, International University of Africa. Permission to access patient records was obtained from the administration of Omdurman Military Hospital to ensure confidentiality.
Acknowledgments
The authors thank the Omdurman Military Hospital Administration for permission to conduct this study.
Author Contributions
All authors made a noteworthy contribution to the work reported, whether that is in the study design, conception, execution, data analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; provided final approval of the manuscript to be published; have agreed on the journal to which the paper has been submitted; and agree to be responsible for all aspects of the work.
Disclosure
All authors have no competing interests, and they declare for that.
References
1. Marshall JC, Bosco L, Adhikari NK, et al. What is an intensive care unit? A report of the World Federation of Societies of Intensive and Critical Care Medicine task force. J.Crit.Care. 2017;37:270–276. doi:10.1016/j.jcrc.2016.07.015
2. Shaydullina LY, Ziganshina LE. Clinical Pharmacology- one of the World Health Organization’s strategies in promoting rational use of medicines. KMJ. 2012;93(6):916–920.
3. Mauldin PD, Salgado CD, Hansen IS, Durup DT, Bosso JA. Attributable hospital cost and length of stay associated with healthcare-associated infections caused by antibiotic-resistant gram-negative bacteria. Antimicrob Agents and Chemother. 2010;54(1):109–115. doi:10.1128/AAC.01041-09
4. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated care costs. Crit Care Med. 2001;29(7):1303–1310. doi:10.1097/00003246-200107000-00002
5. Srinivasan S. A network for the rational and ethical use of drugs. Indian J Med Ethics. 2004;(1). doi:10.20529/IJME.2004.005
6. Chaturvedi VP, Mathur AG, Anand AC. Rational drug use common as common sense? Med J Armed Forces India. 2012;68(3):206–208. doi:10.1016/j.mjafi.2012.04.002
7. Littmann J, Rid A, Buyx A. Tackling antimicrobial resistance: ethical framework for rational antibiotic use. Eur J Public Health. 2017;28(2):359–363. doi:10.1093/eurpub/ckx165
8. Depuydt P, De Waele JJ. Optimal and responsible use of antibiotics. Curr.Opin.Crit.Care. 2019;25(5):458–464. doi:10.1097/MCC.0000000000000645
9. Abdellatif AO, Ali IN, Mohamed MA, Ibrahim MA, Mohamed WA. Evaluation of the Antibiotic Resistance Pattern at the Medical Services Administration Hospital in Khartoum, Sudan, 2021. J Res Appl Sci Biotechnol. 2022;1(4):50–56. doi:10.55544/jrasb.1.4.6
10. Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infectious Dis. 2006;42 Suppl 2:S82–89. doi:10.1086/499406
11. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi:10.1159/000180580
12. Staff PDR. Physicians’ Desk Reference. New York: Thomson Healthcare; 2011.
13. Society RP MedicinesComplete [homepage]; 2019. Available from: https://about.medicinescomplete.com/.
14. The National Institute for Health and Care Excellence (NICE) Antimicrobial Prescribing; 2019. Available from: https://www.nice.org.uk/search?antimicrobialprescribing.
15. Preston CL. Stockley’s Drug interaction.In: Royal Pharmaceutical Society. London: Pharmapress; 2015.
16. Malacarne P, Rossi C, Bertolini G. Antibiotic usage in intensive care units: a pharmaco-epidemiological multicentre study. J Antimicrob Chemother. 2004;54(1):221–224. doi:10.1093/jac/dkh299
17. Aarts M-AW, Brun-Buisson C, Cook DJ, et al. Antibiotic management of suspected nosocomial ICU-acquired infection: does prolonged empiric therapy improve outcome? Intensive Care Med. 2007;33(8):1369–1378. doi:10.1007/s00134-007-0723-y
18. Paruk F, Richards G, Scribante J, Bhagwanjee S, Mer M, Perrie H. Antibiotic prescription practices and their relationship to outcome in South Africa: findings of the prevalence of infection in South African intensive care units (PISA) study. S Afr Med J. 2012;102(7):613–616. doi:10.7196/SAMJ.5833
19. Ali M, Naureen H, Tariq MH, et al. Rational use of antibiotics in an intensive care unit: a retrospective study of the impact on clinical outcomes and mortality rate. Infect Drug Resist. 2019;12:493–499. doi:10.2147/IDR.S187836
20. Chunnilall D, Peer A, Naidoo I, Essack S. An evaluation of antibiotic prescribing patterns in adult intensive care units in a private hospital in KwaZulu-Natal. S Afr J Infect Dis. 2015;30(1):17–22.
21. Kumari A, Goyal J, Chandalia S, Rustage K, Attri R, Goyal B. Evaluation of rational use of antibiotics in intensive care unit of a tertiary care hospital in north India. Med Int J Pharmacology. 2019;11(2):21–24.
22. Sanhoury OM, Eldalo AS. Evaluation of Meropenem Utilization in Intensive Care Unit in Sudan. Int J Clin Pharmacol Pharmacother. 2016;1(106). doi:10.15344/2456-3501/2016/106
23. Vallés J, Pobo A, García-Esquirol O, Mariscal D, Real J, Fernández R. Excess ICU mortality attributable to ventilator-associated pneumonia: the role of early vs late onset. Intensive Care Med. 2007;33(8):1363–1368. doi:10.1007/s00134-007-0721-0
24. Kheder SI. Physicians’ knowledge and perception of antimicrobial resistance: a survey in Khartoum state hospital settings. British Journal of Pharmaceutical Research. 2013;3(3):347–362. doi:10.9734/BJPR/2013/2117
25. Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infectious Dis. 2007;44(2):159–177. doi:10.1086/510393
26. Baqi S, Damani N, Shah SA, Khanani R. Infection Control at a Government Hospital in Pakistan. Int J Infect Control. 2009;5.
27. Abdulah R. Antibiotic abuse in developing countries. Pharm Regul Aff. 2012;1(2):1000–1106. doi:10.4172/2167-7689.1000e106
28. Elfaki A. Assessment of antibiotics prescription in hospitalized patients at El Obeid Hospital, Sudan. Sudan J Med Sci. 2009;4(3):56.
29. Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. Morbidity Mortality Weekly Rep. 2014;63(9):194–200.
30. French GL. Clinical impact and relevance of antibiotic resistance. Adv Drug Deliv Rev. 2005;57(10):1514–1527. doi:10.1016/j.addr.2005.04.005
31. Monroe S, Polk R. Antimicrobial use and bacterial resistance. Curr Opin Microbiol. 2000;3(5):496–501. doi:10.1016/S1369-5274(00)00129-6
32. Yamaga S, Shime N. Association between appropriate empiric antimicrobial therapy and mortality from bloodstream infections in the intensive care unit. J Infect Chemother. 2018;24(4):267–271. doi:10.1016/j.jiac.2017.11.011
33. Akhtar N. Hospital-acquired infections in a medical intensive care unit. J Coll of Physicians Sur Pak. 2010;20(6):386–390.
34. Ikram A, Hussain Shah SI, Naseem S, et al. Status of hospital infection control measures at seven major tertiary care hospitals of northern Punjab. J Coll Physicians Surg Pak. 2010;20(4):266–270.
35. Kollef MH. Appropriate empirical antibacterial therapy for nosocomial infections: getting it right the first time. Drugs. 2003;63(20):2157–2168. doi:10.2165/00003495-200363200-00001
36. Bergmans DC, Bonten MJ, Gaillard CA, et al. Indications for antibiotic use in ICU patients: a one-year prospective surveillance. J Antimicrob Chemother. 1997;39(4):527–535. doi:10.1093/jac/39.4.527
37. Colpan A, Akinci E, Erbay A, Balaban N, Bodur H. Evaluation of risk factors for mortality in intensive care units: a prospective study from a referral hospital in Turkey. Am J Infect Control. 2005;33(1):42–47. doi:10.1016/j.ajic.2004.09.005
38. Pradhan NP, Bhat S, Ghadage D. Nosocomial infections in the medical ICU: a retrospective study highlighting their prevalence, microbiological profile and impact on ICU stay and mortality. J Assoc Physicians India. 2014;62(10):18–21.
39. Rosenthal VD, Guzman S, Orellano PW. Nosocomial infections in medical-surgical intensive care units in Argentina: attributable mortality and length of stay. Am J Infect Control. 2003;31(5):291–295. doi:10.1067/mic.2003.1
40. Bueno-Cavanillas A, Delgado-Rodríguez M, López-Luque A, Schaffino-Cano S, Gálvez-Vargas R. Influence of nosocomial infection on mortality rate in an intensive care unit. Crit Care Med. 1994;22(1):55–60. doi:10.1097/00003246-199401000-00013
41. Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1994;271(20):1598–1601. doi:10.1001/jama.1994.03510440058033
42. Cevik MA, Yilmaz GR, Erdinc FS, Ucler S, Tulek NE. Relationship between nosocomial infection and mortality in a neurology intensive care unit in Turkey. J Hosp Infect. 2005;59(4):324–330. doi:10.1016/j.jhin.2004.10.012
43. Savage RD, Fowler RA, Rishu AH, et al. The Effect of Inadequate Initial Empiric Antimicrobial Treatment on Mortality in Critically Ill Patients with Bloodstream Infections: a Multi-Centre Retrospective Cohort Study. PLoS One. 2016;11(5):e0154944. doi:10.1371/journal.pone.0154944
44. Mandelbaum T, Scott DJ, Lee J, et al. Outcome of critically ill patients with acute kidney injury using the AKIN criteria. Crit Care Med. 2011;39(12):2659. doi:10.1097/CCM.0b013e3182281f1b
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
