Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Impact of Infusion Therapies on Quality of Life in Advanced Parkinson’s Disease
Authors Constantin VA, Szász JA , Dulamea AO, Valkovic P, Kulisevsky J
Received 3 July 2023
Accepted for publication 1 September 2023
Published 14 September 2023 Volume 2023:19 Pages 1959—1972
DOI https://doi.org/10.2147/NDT.S422717
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Viorelia A Constantin,1,* József A Szász,1,2,* Adriana Octaviana Dulamea,3,4 Peter Valkovic,5 Jaime Kulisevsky6
1Second Clinic of Neurology, Târgu Mures County Emergency Clinical Hospital, Târgu Mureș, Romania; 2Department of Neurology, George Emil Palade University of Medicine, Pharmacy, Science and Technology, Târgu Mureș, Romania; 3Neurology Clinic, Fundeni Clinical Institute, Bucharest, Romania; 4University of Medicine and Pharmacy “Carol Davila” Bucharest, Bucharest, Romania; 5Second Department of Neurology, Comenius University Faculty of Medicine and University Hospital Bratislava, Bratislava, Slovakia; 6Movement Disorders Unit at the Neurology Department of Sant Pau Hospital, Barcelona, Spain
*These authors contributed equally to this work
Correspondence: Adriana Octaviana Dulamea, Tel +40212750500, Fax +40212750700, Email [email protected]
Abstract: A high burden of motor and non-motor parkinsonian symptoms is known to have a significant negative impact on the quality of life (QoL) of people with Parkinson’s disease (PD). Effective control of these symptoms with therapies that enable patients to maintain a good QoL is therefore a key treatment goal in PD management. When symptom control can no longer be accomplished with oral or transdermal PD treatment regimens, device-aided therapies (DAT), namely levodopa and apomorphine infusion therapies, and deep brain stimulation, are valuable options to consider. DAT options may also help reduce pill burden and thereby improve compliance with treatment. Since PD therapy relies on symptomatic management, the efficacy and tolerability of any intervention is undoubtedly important, however the impact of different therapies on patient-related outcome measures, in particular health-related QoL, is also a critical consideration for those living with a chronic and disabling condition. This review discusses clinical evidence and ongoing research regarding the QoL benefits of levodopa and apomorphine infusion therapies from studies that have used validated QoL outcome measures. The data suggest that timing of these interventions is important to achieve optimal treatment effects, and that early initiation onto infusion therapies at the point when motor fluctuations emerge, and before patient QoL and functioning have significantly declined, may provide the best long-term outcomes. Healthcare professionals caring for people with PD should therefore discuss all available DAT options with them at an early stage in the course of their disease so they can make informed and timely choices that best suit them, their families and care network.
Keywords: Parkinson’s disease, infusion therapies, motor symptoms, quality of life, patient-reported outcomes
Introduction
Parkinson’s disease (PD) is a progressive condition, and no therapy has yet been identified that can modify the course of the disease or prevent the gradual neurodegeneration.1 Treatment therefore focuses on the management of the recognised motor and non-motor symptoms of PD which can change over time with disease progression.1 For people with PD, a key therapeutic goal is maintaining a good quality of life (QoL). This is especially important when living with a chronic and advancing condition such as PD, so it is critical that symptoms are well managed at all stages.
Oral dopaminergic therapy is the standard initial therapy for patients with PD and its early initiation, even in the absence of marked disability, has been shown to improve QoL.2 Due to the progressive loss of dopaminergic neurons in the brain, after several years the response to oral levodopa changes and becomes less effective3 with the emergence of disabling motor and non-motor complications that impact patients’ QoL.4 This often requires the addition of other adjunctive medications to regain effective symptom control, but eventually even “optimized” oral/transdermal PD treatment regimens may be unable to provide adequate relief of PD motor complications, so an alternative approach is needed. At this point, clinicians may wish to discuss with their patients the option of transitioning to a device-aided therapy (DAT) that provides continuous rather than pulsatile (oral) stimulation, including infusion therapies and deep brain stimulation (DBS).
In addition to demonstrating the clinical efficacy of such therapies, a key aspect of modern clinical trials is to include patient-related outcome measures and to evaluate the effect of the intervention on health-related QoL. This descriptive literature review considers factors that impact the QoL of patients with PD as the disease progresses and what symptoms are most troublesome to them. It is based on a PubMed literature review of evidence for the QoL benefits of infusion therapies, focusing in particular on studies that have included QoL as a patient-reported outcome measure.
Impact of Parkinson’s Symptoms and Disease Progression on Quality of Life
Evidence has shown that patients’ QoL is directly related to the PD symptoms they experience,5 particularly when these symptoms are not adequately managed, and impact on their ability to function and carry out their usual daily activities.6,7 Both motor and non-motor symptoms in advanced PD have been shown to adversely affect patients’ QoL.8–10 A “real life” US study of over 700 PD patients was undertaken to evaluate the link between “OFF” episodes and health-related QoL, measured using PDQ-39 and EQ-5D.11 Data were grouped according to whether or not patients experienced “OFF” episodes and by average hours of “OFF” time per day. The results showed that patients who experienced “OFF” episodes had reduced health-related QoL and that the impact on QoL increased in line with increasing average hours of daily “OFF” time, highlighting the need to effectively manage these symptoms.
The usual progression of motor and non-motor symptoms in Parkinson’s is shown in Figure 1. Non-motor symptoms (including sensory dysfunction, sleep disturbance, gut dysfunction and depression) frequently precede motor symptoms and a confirmed clinical diagnosis of PD, often by several years, and have been shown to have detrimental effects on patient QoL.5,12–15 Neuropsychiatric symptoms in particular, including depression, anxiety, apathy, fatigue, and psychotic symptoms, can also have a considerable negative effect on caregivers.16 The onset of motor symptoms, often triggering a diagnosis of PD, further adds to the already impaired QoL17 such that, even in the early stages, patients are already living with a significant QoL burden. The emergence of motor fluctuations despite optimized medical therapy, characterized by increasingly disabling motor symptoms and extended periods of “OFF” often complicated by dyskinesias, signals the progression to advanced PD.18,19 Notably, a 2-year follow-up study of 330 PD patients from 35 centers across Spain found that the development of motor fluctuations was associated with a greater increase in non-motor symptoms burden, measured using the Non-motor Symptoms Scale (NMSS) total score.20
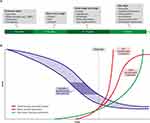 |
Figure 1 A schematic representation of the onset and progression of motor and non-motor symptoms in Parkinson disease (PD). (A) Potential timeline for manifestation of non-motor features of PD. Non-motor symptoms may develop insidiously in the prodromal phase several years before onset of motor features. The duration of this prodromal phase is variable, as is the sequence of the appearance of the non-motor symptoms. Non-motor problems continue to develop throughout the course of the disease, but timing and manifestation may vary between patients. (B) Rates of development and progression of the motor and non-motor features of PD, and the decline in dopaminergic neuronal function. The rate of decline in dopaminergic function in PD may vary between patients (blue shaded section). It is estimated that motor features appear when approximately 50–60% of dopaminergic neurons have been lost (dark blue area). Non-motor features may begin with an earlier pre-motor prodrome, progress more slowly but accumulate greater disability. Abbreviation: RBD, REM sleep behaviour disorder. Notes: Reproduced with permission from Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson's disease. Nat Rev Neurosci. 2017;18(7):435–450; Springer Nature.13 |
For all these reasons, there is a need for timely diagnosis of PD in general and specifically of patients who have reached an advanced stage without diagnosis or appropriate treatment. For these patients, their symptoms may be so disabling that they retain only minimal QoL. Only through early diagnosis will patients achieve a timely referral for suitable therapies and attain the maximum benefit from treatment including preservation of QoL.
Progressing from Oral Therapy to a Device-Aided Therapy
Unlike the case for many other neurodegenerative diseases, a range of effective symptomatic therapies are available to Parkinson’s patients. Oral dopamine replacement using its precursor, levodopa, is the current gold standard for PD management and is effective in restoring motor symptom control initially. However, as neurodegeneration progresses, disabling motor complications can emerge which necessitate individual adjustment of the patient’s medication regimen.21 Notably, many studies that have measured QoL as clinical outcome have reported that motor complications may decrease patients’ QoL.22 Ideally, dopaminergic therapy should be personalized for the individual patient’s needs, taking into account their age, symptom profile and severity, and also any co-morbid conditions. Importantly, based on the results of PD MED and Levodopa in Early Parkinson’s Disease (LEAP) studies, historical concerns that early initiation of dopaminergic therapy might promote the emergence of motor complications appear to be unwarranted.23,24 However, for most patients, it is recognised that the efficacy of pulsatile (oral) dosing of medications given in the earlier stages of PD will eventually decline necessitating alternative treatment strategies to ensure effective control of symptoms. As the disease advances, the loss of nigrostriatal terminals within the substantia nigra results in a loss of the capacity to store and progressively release dopamine. At this point, dopamine levels at synapses reflect those in the circulatory system and patients require continuous dopamine administration in order to maintain symptomatic control.25
An additional management challenge in this predominantly elderly population is that of comorbidities and the increased pill burden that may accrue in order to address these issues. In advanced PD, a high pill burden is often associated with poor compliance, a progressively worsening control of motor and non-motor symptoms, and deterioration of QoL.26 Indeed, studies have shown that up to 67% of Parkinson’s patients take <80% of their prescribed PD medications.27 As PD progresses, further medication adjustments become necessary in order to adequately manage symptoms. In addition to oral levodopa therapy (often taken 5–8 times per day or more) the patient may also need several add-on therapies (such as dopamine agonists, monoamine oxidase type B [MAO-B] inhibitors, catechol-O-methyltransferase [COMT] inhibitors), plus therapies to manage non-motor symptoms and comorbid conditions, all of which may result in drug interactions which can have an additional negative impact on the patient’s QoL. In addition, several non-motor symptoms, such as cognitive decline, depression, apathy, or anhedonia, can reduce compliance with medication, particularly where there is a lack of family/carer support or if the person is in institutional care.28 Many patients with PD report problems with sleep, which in turn can have a negative impact on QoL, and so should be managed appropriately. Recently, subcutaneous apomorphine infusion administered at night-time only has been shown to improve insomnia in PD patients.29
Over the years, various effective DATs have become available, transforming the treatment landscape for patients with advanced PD and both relieving the disabling motor and non-motor symptoms, and improving QoL. DAT options include DBS, a surgical approach, and infusion therapies, including subcutaneous apomorphine injection (delivered via a pen injector) as an “as-needed” medication, continuous subcutaneous apomorphine infusion (CSAI), levodopa-carbidopa intestinal gel (LCIG) infusion and, most recently, levodopa-carbidopa-entacapone intestinal gel (LECIG) infusion. Depending on the needs of the individual patient, sometimes DAT options may be switched or even combined if inadequate symptom control is being achieved with a particular DAT.30
Further infusion therapies have been evaluated in Phase 3 clinical trials but have not yet received marketing authorisation: ND0612, a continuous subcutaneous levodopa–carbidopa infusion therapy and ABV-951, a continuous subcutaneous infusion of the levodopa–carbidopa prodrugs, foslevodopa and foscarbidopa. The discussions here will focus on currently available continuous infusion therapies.
The efficacy of these agents in relieving parkinsonian symptoms has been established in controlled clinical trials,31–34 so the question becomes one of optimum timing of therapy and what is best in terms of maintaining patients’ QoL. Should they be reserved for use only once optimised oral medication is no longer effective in controlling motor complications, or should they be considered earlier in the disease evolution, shortly after the emergence of motor fluctuations?
For the latter approach, a change in the way medications are currently deployed for the management of PD would be required. The rationale for earlier introduction of infusion therapies would be to maintain control of motor and non-motor symptoms and to preserve functioning and QoL, rather than reserving their introduction for a later timeline when functional capacity and QoL have already been significantly impacted. As data emerge about the longer-term efficacy of infusion therapies and their efficacy in patients at an earlier stage in their disease evolution, these questions can begin to be explored from an evidence-based perspective.
Factors Influencing Optimal Outcomes with Infusion Therapy
In recent years, a growing number of retrospective evaluations and post hoc analyses have provided insights into optimal use of infusion therapy in advanced PD. One study has suggested that older PD patients may be less well suited to LCIG therapy, since younger age when undergoing insertion of the percutaneous endoscopic gastro-jejunostomy (PEG-J) system was found to be one of the positive predictive factors for long-term efficacy.35 Further, the risk of patients discontinuing LCIG treatment was found to be greater in patients aged 70 years or over.36 Longer disease duration at the time of LCIG treatment initiation has been associated with reduced efficacy in controlling motor complications (namely the duration of OFF time reduction and increase in ON time without troublesome dyskinesia), and a greater incidence of severe adverse events. It has been suggested that LCIG infusion may have greater benefits in patients with less advanced disease and whose motor complications prior to PEG-J insertion are less severe.37
Similar conclusions derived from a ten-year comprehensive retrospective analysis of LCIG treatment: a more advanced stage of PD and cognitive impairment at the time of LCIG initiation were predictive factors for therapy discontinuation.38 These results highlight that “delayed” LCIG initiation may result in loss of some of the benefits of prompt and timely intervention.39
The recent BALANCE study compared the long-term effect of LCIG therapy with standard-of-care treatment on health-related QoL and found that in routine clinical practice, initiation of LCIG treatment was often delayed, and only commenced well beyond the usual clinical criteria for this therapy. The authors suggested that this approach is likely to result in reduced clinical benefits for PD patients.40
Infusion Therapies and Quality of Life in PD
Evaluation of the QoL of PD patients as part of clinical studies and investigation of the relationship between QoL and motor and non-motor PD symptoms, is critical in order to aid clinicians in making informed decisions about treatment and patients’ optimal wellbeing. A systematic review undertaken to assess the tools used to assess QoL in clinical research identified 42 different instruments used across 116 different studies.41 The most commonly used tools were the disease-specific 39-items and the short-form 8-items Parkinson’s Disease Questionnaire (PDQ-39 and PDQ-8, respectively). A range of non-disease-specific tools to assess health quality are also used, including EQ-5D (a 5-dimension instrument developed by the EuroQoL Group), EQ-VAS (a visual analogue scale version), SEIQoL-Q (Schedule for the Evaluation of Individual Quality of Life) and PWI-A (Personal Wellbeing Index–Adult). The findings of this review highlight the need for common, validated outcome measures to be used in PD clinical research that will enable comparison across studies.
Published clinical trials of infusion therapies in advanced PD that include QoL outcome measures, and ongoing studies in this setting, are summarized below and in Table 1 and Table 2, respectively.
 |
Table 1 Published Clinical Trials of Infusion Therapies in Advanced PD That Include QoL Outcomes |
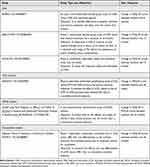 |
Table 2 Ongoing Studies of Infusion Therapies in Advanced PD That Include QoL Outcomes |
Continuous Subcutaneous Apomorphine Infusion (CSAI)
Published Studies
Improvements in QoL in patients with advanced PD treated with CSAI have been reported in several prospective and observational, “real-world” studies.
The OPTIPUMP study was prospective, open-label, observational cohort study performed in a “real-life” clinical setting in France and enrolled 142 patients with PD whose symptoms were not managed effectively with oral treatments.42 QoL scores at initiation of treatment and at follow-up after 6 months were assessed using the PDQ-39 instrument. The authors reported significant improvements in health-related QoL after 6 months of CSAI treatment (p < 0.001).
A retrospective study was undertaken at the same French center to evaluate the effect of CSAI on motor fluctuations, cognition, psychiatric domains, and QoL in 22 PD patients who did not have cognitive impairment. Scores were assessed before and 6 months after the commencing add-on CSAI therapy.44 The results showed that CSAI improved motor symptoms and QoL and there were no negative effects on cognitive or psychiatric domains.
The TOLEDO study was a randomized, double-blind, multicenter trial comparing CSAI and placebo. The study population included PD patients with motor fluctuations that persisted despite optimized oral/transdermal medication. Results of the 12-week double-blind phase showed a clinically meaningful OFF time reduction with CSAI versus placebo, mirrored by an increase in “good quality” ON time.33 The 52-week open-label phase confirmed that these effects were sustained with long-term treatment.45 QoL of the106 PD patients (at least 3 years since diagnosis) treated with CSAI was assessed using the PDQ-8 instrument. The change in QoL between baseline and week 12 was not found to be significantly different between the treatment groups. The authors suggested that this lack of effect on QoL might be due to the relatively limited time for dose adjustment and the short study duration, plus insufficient study power, despite the significant benefit observed for CSAI on Patient Global Impression of Change scores.33 Similarly, no substantial changes were seen for PDQ-8 scores when comparing the double-blind phase baseline and the end of the open-label phase (week 64).45 The lack of effect of CSAI on QoL observed in TOLEDO contrasts with other studies discussed here that have shown significant QoL improvements but following a longer duration of treatment.42,44,64,65
A prospective analysis of the effect of the effect of CSAI on motor and non-motor symptoms, cognitive function and QoL was undertaken in 22 patients with early-stage advanced PD, defined as <71 years of age with a diagnosis of advanced PD for <3 years.48 After 6 months of CSAI treatment, mean PDQ-39 scores were found to be significantly reduced from baseline (p ≤ 0.001). The same research team also evaluated sequential use of CSAI followed by DBS in 20 PD patients. After 6 months of CSAI treatment PDQ-39 scores were reduced by 39.6% compared with the optimized medical treatment baseline values (p ≤ 0.001).46
Long-term improvement in QoL based on >1 year of follow-up has also been reported in studies of CSAI. An observational study of 36 consecutive PD patients treated with CSAI over a 10-year period (2004 to 2014) used the neurologist’s assessment of Clinical Global Impression to evaluate QoL.43 Mean CSAI treatment duration in these patients was 21.65 months with motor symptoms and QoL rated as “much improved” or better in 83%.43 In a 2-year follow-up of 110 patients with advanced PD, continued treatment with CSAI was associated with stable QoL scores, assessed using PDQ-39, and a sustained reduction in motor fluctuations.47
The APOMORPHEE trial was a randomized, double-blind, placebo-controlled study conducted in 11 centers in France that specialized in PD and sleep.29 Forty-six PD patients with motor fluctuations and moderate-to-severe insomnia (Insomnia Severity Index score≥ 15) were treated with either night-time CSAI (up to 5 mg/h) or matching placebo, and then crossed over to the other treatment after a 14-night washout period. For each treatment period, there was a 10-night titration phase followed by a 7-night fixed-dose phase. Mean change in Parkinson’s Disease Sleep Scale (PDSS) scores from the beginning to the end of the treatment period was significantly greater with night-time CSAI than with placebo (p = 0.041). However, more frequent dizziness was reported for CSAI treatment than placebo (p-0.041).29
Ongoing Studies
Several ongoing studies incorporate QoL outcome measures (Table 2). The AGAPO study (NCT03693872) will evaluate CSAI with and without dopaminergic agonists in patients with PD (who are suitable for CSAI therapy). QoL is a primary outcome variable and will be evaluated over 6 months using PDQ-39.
The Phase 3 EARLY-PUMP study (NCT02864004) will evaluate CSAI versus oral medication alone in patients with early PD when motor complications have just emerged. Again, QoL is a primary outcome variable and will be assessed using PDQ-39.
A long-term safety study of CSAI in patients with advanced PD that is insufficiently controlled on optimized oral therapy is ongoing (INFUS-ON; NCT02339064) in which QoL is included as a secondary patient-reported outcome measure.
Levodopa-Carbidopa Intestinal Gel (LCIG) Infusion
Published Studies
Improvement in QoL using a variety of tools (PDQ-8, PDQ-39, EQ-5D VAS, SEIQoL-Q and PWI-A) in patients with advanced PD treated with LCIG have been reported in both prospective and “real-world” studies.49–51,53–55,57,60,62
The HORIZON study was a pivotal 12-week, double-blind, double-dummy, randomized clinical trial investigating the efficacy and safety of LCIG infusion.32 It was undertaken in 26 centers in Germany, New Zealand, and the USA and included assessment of QoL using the PDQ-39 and EQ-5D instruments. Seventy-one with advanced PD patients received treatment with either LCIG infusion plus placebo oral immediate-release levodopa–carbidopa (LC-IR) capsules (n = 37) or LC-IR capsules plus placebo LCIG infusion (n = 34). Results showed a statistically significantly greater improvement in QoL with LCIG versus LC-IR in patients with advanced PD. A subsequent 52-week open-label phase examined long-term safety, efficacy and QoL of LCIG treatment.52 In patients already established on LCIG treatment, sustained improvements in the PDQ-39 summary index, EQ-5D summary index and EQ-5D VAS scores were observed. However, no significant improvements in QoL measures were observed from baseline to week 52 in LCIG-naïve patients.
The registry-based GLORIA study (Global Long-term Registry: Duodopa® in patients with Advanced PD) was established to collect long-term clinical outcomes data from a large cohort of advanced PD patients being treated with LCIG in routine clinical practice in 18 different countries.66 LCIG effects on motor and non-motor symptoms and impact on QoL, assessed using PDQ-8 and EQ-5D, was evaluated over 24 months. A total of 375 patients from 75 movement disorder centers were enrolled in the registry and 258 patients completed the study.56 LCIG treatment over 24 months resulted in significantly reduced PDQ-8 scores from baseline in this cohort of advanced PD patients.56
The TANDEM study was undertaken at 17 movement disorder centers in Italy as a joint initiative of members of the Italian Academy for Parkinson’s Disease and Movement Disorders.59 The study evaluated the efficacy and tolerability of LCIG infusion in 159 advanced PD patients in routine clinical care. LCIG treatment achieved sustained improvements in QoL, assessed using PDQ-8 or PDQ-39, during 12 months of follow-up.59
The impact of long-term LCIG treatment has been evaluated in a 52-week, open-label, Phase 3, multinational study that included patients who had previously been enrolled in three other Phase 3 trials58 – the HORIZON double-blind and open-label phases,32,52 plus another 54-week open-label study.51 Overall, 262 patients were included in the analysis with a mean total duration of exposure to LCIG infusion of 4.1 years. Assessments were undertaken every 6 months. It was observed that the improvements in activities of daily living and QoL observed in the initial studies were not sustained, which the authors suggest may be due in part to disease progression.
The COSMOS study is a “real-world”, multinational observational study that aimed to investigate comedication use with LCIG treatment, to determine if it might be a monotherapy option for advanced PD patients, thereby simplifying the treatment regimen.67 A sub-analysis of study data from a cohort of Romanian patients highlighted that those treated with LCIG monotherapy had comparable non-motor symptom scores and PDQ-8 QoL scores to those of patients treated with LCIG plus add-on medication at 12 months from the start of treatment.61
The typical LCIG treatment duration is for 16 waking hours, and dosages are calculated according to formulas based on experience with this infusion timeframe.68,69 Day-time and night-time LCIG administration is also possible when there is a medically justified reason. Additional benefits of 24-hour LCIG administration on motor and nonmotor complications have been reported in several case series and small clinical studies.70 Analysis of the main reasons considered decisive for the administration of LCIG on a 24-hour basis shows that that severe night-time akinesia/bradykinesia and early morning akinesia as well as sleep disorders are the most common.71 The recently published sub-analysis of the COSMOS study demonstrated that use of 24-hour LCIG infusion for ≥12 months resulted in improvements in specific motor symptoms, non-motor symptoms and treatment-related symptoms in advanced PD patients. In this analysis, adverse effects were found to be in line with the established safety profile of LCIG.72 To our knowledge, there are no targeted clinical studies that demonstrate how these undoubted clinical benefits translate to improvements in QoL.
Ongoing Studies
The INSIGHTs study (NCT0254909) is an ongoing Phase 3, open-label, randomized, parallel group, 6-month study of LCIG versus optimized medical therapy (OMT) in advanced PD patients. The primary outcome variable is non-motor symptom scores and QoL will be evaluated as a secondary outcome measure using PDQ-8.
Levodopa-Carbidopa-Entacapone Intestinal Gel (LECIG) Infusion
Published Studies
LECIG infusion is the latest addition to the range of DAT options available to patients with advanced PD.73,74 It requires the same surgical procedure as LCIG to insert a PEG-J system that enables intrajejunal infusion. LECIG infusion is reported to have equivalent motor efficacy to LCIG infusion, but in LECIG the inclusion of entacapone increases the bioavailability of levodopa so that effective levodopa plasma concentrations can be achieved, but using lower overall doses.34
LECIG has received marketing authorization in several European countries for the treatment of PD patients who have motor complications that cannot be adequately controlled by optimized oral/transdermal PD medication.75 In a study of a small cohort of patients with advanced PD treated with LECIG in Sweden, 62% (13/21 patients) reported an improvement in their QoL using a self-rated scale of “improved”, “unchanged”, “worsened”, or “I do not know” after a median treatment duration of 305 days.63
Ongoing Studies
Recognizing the importance of collecting “real world” data to support information obtained from controlled clinical trials, the prospective, non-interventional ELEGANCE study (NCT05043103) was established. This aims to obtain routine clinical practice data on the efficacy and safety of LECIG and is being undertaken at various centers in around 16 countries across Europe. The study cohort will comprise patients with advanced PD who have been prescribed treatment with LECIG (either de novo or switching from another therapy) due to severe motor fluctuations and hyperkinesia or dyskinesia despite taking optimized oral/transdermal PD therapy. QoL will be evaluated using the PDQ8 and PDQ39 over 24 months.
Studies Comparing Device-Aided Therapies
Although no head-to-head randomized, controlled studies comparing the different DATs have been undertaken, a range of observational and real-world studies have investigated their comparative clinical efficacy and impact on QoL.
The EuroInf 1 study was an open-label, prospective, observational, 6-month, multicenter study comparing 43 advanced PD patients treated with CSAI and 44 with LCIG infusion.64 In suitably selected patients, both CSAI and LCIG infusions had beneficial effects on QoL (assessed using PDQ-8), as judged by the effect size of the particular treatment. In the EuroInf 2 study, three DATs were compared in a “real world” clinical practice setting: CSAI, LCIG infusion and DBS.65 All three treatments were associated with improved QoL assessed using PDQ-8, however the greatest relative change and largest effect size was observed for CSAI after a 6-month follow-up.
A retrospective cohort analysis has compared LCIG infusion (34 patients) with DBS (97 patients).26 After 12 months of treatment, a significantly greater reduction in daily pill burden (p ≤ 0.0001), with more patients being able to discontinue oral medication completely, was observed in the LCIG infusion-treated group. Currently, LCIG and DBS are also being compared in the INfusion VErsus STimulation in Parkinson’s Disease (INVEST; NCT02480803) study in patients with PD. QoL will be evaluated using the PDQ-39 at 12 months.
Recently, a network meta-analysis compared the efficacy of CSAI, LCIG infusion, DBS and best medical therapy (BMT) in resolving OFF-time and improving QoL in advanced PD patients.76 A total of 22 studies including 2063 patients fulfilled the inclusion criteria for analysis. They comprised four randomized, controlled trials, as well as 16 single-armed, one 2-armed and one 3-armed prospective studies. In this analysis, after 6 months of treatment all three DATs provided greater improvements in PD-specific QoL than BMT, however LCIG and DBS were associated with significantly greater improvement QoL scores than CSAI or BMT.
Conclusion
Effective management of persistent PD motor complications (fluctuations, dyskinesias) with infusion therapies has been shown to improve QoL compared with optimised oral therapy. Ameliorating the decline in QoL for patients with PD as their disease advances may have beneficial effects on the QoL for family members and carers. Suitable PD patients should be therefore considered for infusion therapies as soon as symptoms dictate so a good QoL can be maintained for as long as possible.
The perception that infusion therapies should be reserved for end-stage disease is unwarranted and needs to change. Indeed, the range of DAT options, including infusion therapies, should be discussed with patients at an early stage in the course of their PD to enable them to make an informed choice about the best therapy to suit their personal circumstance as their disease progresses. The latest clinical trial evidence suggests a potential benefit for timely intervention with infusion therapies when motor fluctuations can no longer be controlled with optimized oral or transdermal treatment but before patient QoL and functioning have been significantly compromised.
Acknowledgments
Editorial assistance in the preparation of this manuscript was provided by Dr Karen Wolstencroft (manuscript drafting, collation of author comments, literature searches), who was financially supported by Britannia Pharmaceuticals Limited.
Disclosure
VAC has received personal fees from AbbVie and Stada, outside the submitted work. JAS has previously received consultancy and speaking honoraria from Britannia Pharmaceuticals Limited. AOD reports consultancy and speaker honoraria from AbbVie and Stada, outside the submitted work. The other authors report no conflicts of interest in this work.
References
1. Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386(9996):896–912. doi:10.1016/S0140-6736(14)61393-3
2. de Bie RMA, Clarke CE, Espay AJ, et al. Initiation of pharmacological therapy in Parkinson’s disease: when, why, and how. Lancet Neurol. 2020;19(5):452–461. doi:10.1016/S1474-4422(20)30036-3
3. Abbruzzese G, Barone P, Bonuccelli U, et al. Continuous intestinal infusion of levodopa/carbidopa in advanced Parkinson’s disease: efficacy, safety and patient selection. Funct Neurol. 2012;27(3):147–154.
4. Gaire S, Kafle S, Bastakoti S, et al. Continuous subcutaneous apomorphine infusion in advanced Parkinson’s disease: a systematic review. Cureus. 2021;13(9):e17949. doi:10.7759/cureus.17949
5. Kumaresan M, Khan S. Spectrum of non-motor symptoms in Parkinson’s disease. Cureus. 2021;13(2):e13275. doi:10.7759/cureus.13275
6. Chapuis S, Ouchchane L, Metz O, et al. Impact of the motor complications of Parkinson’s disease on the quality of life. Mov Disord. 2005;20(2):224–230. doi:10.1002/mds.20279
7. Savci C, Sendir M. Evaluation of health related quality of life in patients with Parkinson’s disease. Neurosciences. 2009;14(1):60–66.
8. Chaudhuri KR, Antonini A, Robieson WZ, et al. Burden of non-motor symptoms in Parkinson’s disease patients predicts improvement in quality of life during treatment with levodopa-carbidopa intestinal gel. Eur J Neurol. 2019;26(4):581–e543. doi:10.1111/ene.13847
9. Kovacs N, Bergmann L, Anca-Herschkovitsch M, et al. Outcomes impacting quality of life in advanced Parkinson’s disease patients treated with levodopa-carbidopa intestinal gel. J Parkinsons Dis. 2022;12(3):917–926. doi:10.3233/JPD-212979
10. Szasz JA, Jianu DC, Simu MA, et al. Characterizing advanced Parkinson’s disease: Romanian subanalysis from the OBSERVE-PD Study. Parkinsons Dis. 2021;2021:6635618. doi:10.1155/2021/6635618
11. Thach A, Jones E, Pappert E, et al. Real-world assessment of the impact of “OFF” episodes on health-related quality of life among patients with Parkinson’s disease in the United States. BMC Neurol. 2021;21(1):46. doi:10.1186/s12883-021-02074-2
12. Gokcal E, Gur VE, Selvitop R, et al. motor and non-motor symptoms in Parkinson’s disease: effects on quality of life. Noro Psikiyatr Ars. 2017;54(2):143–148. doi:10.5152/npa.2016.12758
13. Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci. 2017;18(7):435–450. doi:10.1038/nrn.2017.62
14. Bailey GA, Hubbard EK, Fasano A, et al. Sleep disturbance in movement disorders: insights, treatments and challenges. J Neurol Neurosurg Psychiatry. 2021;92(7):723–736. doi:10.1136/jnnp-2020-325546
15. Bang Y, Lim J, Choi HJ. Recent advances in the pathology of prodromal non-motor symptoms olfactory deficit and depression in Parkinson’s disease: clues to early diagnosis and effective treatment. Arch Pharm Res. 2021;44(6):588–604. doi:10.1007/s12272-021-01337-3
16. Duncan GW, Khoo TK, Yarnall AJ, et al. Health-related quality of life in early Parkinson’s disease: the impact of nonmotor symptoms. Mov Disord. 2014;29(2):195–202. doi:10.1002/mds.25664
17. Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3(1):17013. doi:10.1038/nrdp.2017.13
18. Antonini A, Moro E, Godeiro C, et al. Medical and surgical management of advanced Parkinson’s disease. Mov Disord. 2018;33(6):900–908. doi:10.1002/mds.27340
19. Antonini A, Stoessl AJ, Kleinman LS, et al. Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: a multi-country Delphi-panel approach. Curr Med Res Opin. 2018;34(12):2063–2073. doi:10.1080/03007995.2018.1502165
20. Santos-Garcia D, de Deus Fonticoba T, Bartolome CC, et al. Motor fluctuations development is associated with non-motor symptoms burden progression in Parkinson’s disease patients: a 2-year follow-up study. Diagnostics. 2022;12(5):1147. doi:10.3390/diagnostics12051147
21. Szasz JA, Orban-Kis K, Constantin VA, et al. Therapeutic strategies in the early stages of Parkinson’s disease: a cross-sectional evaluation of 15 years’ experience with a large cohort of Romanian patients. Neuropsychiatr Dis Treat. 2019;15:831–838. doi:10.2147/NDT.S197630
22. Hechtner MC, Vogt T, Zollner Y, et al. Quality of life in Parkinson’s disease patients with motor fluctuations and dyskinesias in five European countries. Parkinsonism Relat Disord. 2014;20(9):969–974. doi:10.1016/j.parkreldis.2014.06.001
23. Group PDMC, Gray R, Ives N, et al. Long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson’s disease (PD MED): a large, open-label, pragmatic randomised trial. Lancet. 2014;384(9949):1196–1205. doi:10.1016/S0140-6736(14)60683-8
24. Verschuur CVM, Suwijn SR, Boel JA, et al. Randomized delayed-start trial of levodopa in Parkinson’s disease. N Engl J Med. 2019;380(4):315–324. doi:10.1056/NEJMoa1809983
25. Olanow CW, Obeso JA, Stocchi F. Continuous dopamine-receptor treatment of Parkinson’s disease: scientific rationale and clinical implications. Lancet Neurol. 2006;5(8):677–687. doi:10.1016/S1474-4422(06)70521-X
26. Soileau MJ, Pagan F, Fasano A, et al. Comparative effectiveness of carbidopa-levodopa enteral suspension and deep brain stimulation on Parkinson’s disease-related pill burden reduction in advanced Parkinson’s disease: a retrospective real-world cohort study. Neurol Ther. 2022;11(2):851–861. doi:10.1007/s40120-022-00351-x
27. Malek N, Grosset DG. Medication adherence in patients with Parkinson’s disease. CNS Drugs. 2015;29(1):47–53. doi:10.1007/s40263-014-0220-0
28. Szasz JA, Constantin VA, Orban-Kis K, et al. Levodopa-carbidopa intestinal gel in advanced Parkinson’s disease: observations and dilemmas after 10 years of real-life experience. Pharmaceutics. 2022;14(6). doi:10.3390/pharmaceutics14061115
29. De Cock VC, Dodet P, Leu-Semenescu S, et al. Safety and efficacy of subcutaneous night-time only apomorphine infusion to treat insomnia in patients with Parkinson’s disease (APOMORPHEE): a multicentre, randomised, controlled, double-blind crossover study. Lancet Neurol. 2022;21(5):428–437. doi:10.1016/S1474-4422(22)00085-0
30. Georgiev D, Delalic S, Zupancic Kriznar N, et al. Switching and combining device-aided therapies in advanced Parkinson’s disease: a double centre retrospective study. Brain Sci. 2022;12(3):343. doi:10.3390/brainsci12030343
31. Schuepbach WM, Rau J, Knudsen K, et al. Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med. 2013;368(7):610–622. doi:10.1056/NEJMoa1205158
32. Olanow CW, Kieburtz K, Odin P, et al. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol. 2014;13(2):141–149. doi:10.1016/S1474-4422(13)70293-X
33. Katzenschlager R, Poewe W, Rascol O, et al. Apomorphine subcutaneous infusion in patients with Parkinson’s disease with persistent motor fluctuations (Toledo): a multicentre, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2018;17(9):749–759. doi:10.1016/S1474-4422(18)30239-4
34. Senek M, Nielsen EI, Nyholm D. Levodopa-entacapone-carbidopa intestinal gel in Parkinson’s disease: a randomized crossover study. Mov Disord. 2017;32(2):283–286. doi:10.1002/mds.26855
35. Sensi M, Preda F, Trevisani L, et al. Emerging issues on selection criteria of levodopa carbidopa infusion therapy: considerations on outcome of 28 consecutive patients. J Neural Transm. 2014;121(6):633–642. doi:10.1007/s00702-013-1153-3
36. Calandrella D, Romito LM, Elia AE, et al. Causes of withdrawal of duodenal levodopa infusion in advanced Parkinson disease. Neurology. 2015;84(16):1669–1672. doi:10.1212/WNL.0000000000001500
37. Regidor I, Santos-Garcia D, Catalan MIJ, et al. Impact of disease duration in effectiveness of treatment with levodopa-carbidopa intestinal gel and factors leading to discontinuation. J Parkinsons Dis. 2019;9(1):173–182. doi:10.3233/JPD-181324
38. Constantin VA, Szasz JA, Orban-Kis K, et al. Levodopa-carbidopa intestinal gel infusion therapy discontinuation: a ten-year retrospective analysis of 204 treated patients. Neuropsychiatr Dis Treat. 2020;16:1835–1844. doi:10.2147/NDT.S256988
39. Szasz JA, Constantin VA, Orban-Kis K, et al. Profile of patients with advanced Parkinson’s disease suitable for device-aided therapies: retrospective data of a large cohort of Romanian patients. Neuropsychiatr Dis Treat. 2019;15:3187–3195. doi:10.2147/NDT.S230052
40. Weiss D, Ebersbach G, Moller JC, et al. Do we start too late? Insights from the real-world non-interventional BALANCE study on the present use of levodopa/carbidopa intestinal gel in advanced Parkinson’s disease in Germany and Switzerland. Parkinsonism Relat Disord. 2022;103:85–91. doi:10.1016/j.parkreldis.2022.08.018
41. Berardi A, Regoli E, Tofani M, et al. Tools to assess the quality of life in patients with Parkinson’s disease: a systematic review. Expert Rev Pharmacoecon Outcomes Res. 2021;21(1):55–68. doi:10.1080/14737167.2021.1841638
42. Drapier S, Eusebio A, Degos B, et al. Quality of life in Parkinson’s disease improved by apomorphine pump: the OPTIPUMP cohort study. J Neurol. 2016;263(6):1111–1119. doi:10.1007/s00415-016-8106-3
43. Kimber TE, Fang J, Huddy LJ, et al. Long-term adherence to apomorphine infusion in patients with Parkinson disease: a 10-year observational study. Intern Med J. 2017;47(5):570–573. doi:10.1111/imj.13378
44. Houvenaghel JF, Drapier S, Duprez J, et al. Effects of continuous subcutaneous apomorphine infusion in Parkinson’s disease without cognitive impairment on motor, cognitive, psychiatric symptoms and quality of life. J Neurol Sci. 2018;395:113–118. doi:10.1016/j.jns.2018.10.010
45. Katzenschlager R, Poewe W, Rascol O, et al. Long-term safety and efficacy of apomorphine infusion in Parkinson’s disease patients with persistent motor fluctuations: results of the open-label phase of the Toledo study. Parkinsonism Relat Disord. 2021;83:79–85. doi:10.1016/j.parkreldis.2020.12.024
46. Fernandez-Pajarin G, Sesar A, Ares B, et al. Continuous subcutaneous apomorphine infusion before subthalamic deep brain stimulation: a prospective, comparative study in 20 patients. Mov Disord Clin Pract. 2021;8(8):1216–1224. doi:10.1002/mdc3.13338
47. Meira B, Degos B, Corsetti E, et al. Long-term effect of apomorphine infusion in advanced Parkinson’s disease: a real-life study. NPJ Parkinsons Dis. 2021;7(1):50. doi:10.1038/s41531-021-00194-7
48. Fernandez-Pajarin G, Sesar A, Jimenez Martin I, et al. Continuous subcutaneous apomorphine infusion in the early phase of advanced Parkinson’s disease: a prospective study of 22 patients. Clin Park Relat Disord. 2022;6:100129. doi:10.1016/j.prdoa.2021.100129
49. Antonini A, Mancini F, Canesi M, et al. Duodenal levodopa infusion improves quality of life in advanced Parkinson’s disease. Neurodegener Dis. 2008;5(3–4):244–246. doi:10.1159/000113714
50. Foltynie T, Magee C, James C, et al. Impact of Duodopa on quality of life in advanced Parkinson’s disease: a UK case series. Parkinsons Dis. 2013;2013:362908. doi:10.1155/2013/362908
51. Fernandez HH, Standaert DG, Hauser RA, et al. Levodopa-carbidopa intestinal gel in advanced Parkinson’s disease: final 12-month, open-label results. Mov Disord. 2015;30(4):500–509. doi:10.1002/mds.26123
52. Slevin JT, Fernandez HH, Zadikoff C, et al. Long-term safety and maintenance of efficacy of levodopa-carbidopa intestinal gel: an open-label extension of the double-blind pivotal study in advanced Parkinson’s disease patients. J Parkinsons Dis. 2015;5(1):165–174. doi:10.3233/JPD-140456
53. Chang FC, Kwan V, van der Poorten D, et al. Intraduodenal levodopa-carbidopa intestinal gel infusion improves both motor performance and quality of life in advanced Parkinson’s disease. J Clin Neurosci. 2016;25:41–45. doi:10.1016/j.jocn.2015.05.059
54. Palhagen SE, Sydow O, Johansson A, et al. Levodopa-carbidopa intestinal gel (LCIG) treatment in routine care of patients with advanced Parkinson’s disease: an open-label prospective observational study of effectiveness, tolerability and healthcare costs. Parkinsonism Relat Disord. 2016;29:17–23. doi:10.1016/j.parkreldis.2016.06.002
55. De Fabregues O, Dot J, Abu-Suboh M, et al. Long-term safety and effectiveness of levodopa-carbidopa intestinal gel infusion. Brain Behav. 2017;7(8):e00758. doi:10.1002/brb3.758
56. Antonini A, Poewe W, Chaudhuri KR, et al. Levodopa-carbidopa intestinal gel in advanced Parkinson’s: final results of the GLORIA registry. Parkinsonism Relat Disord. 2017;45:13–20. doi:10.1016/j.parkreldis.2017.09.018
57. Kruger R, Lingor P, Doskas T, et al. An observational study of the effect of levodopa-carbidopa intestinal gel on activities of daily living and quality of life in advanced Parkinson’s disease patients. Adv Ther. 2017;34(7):1741–1752. doi:10.1007/s12325-017-0571-2
58. Fernandez HH, Boyd JT, Fung VSC, et al. Long-term safety and efficacy of levodopa-carbidopa intestinal gel in advanced Parkinson’s disease. Mov Disord. 2018;33(6):928–936. doi:10.1002/mds.27338
59. Antonini A, Abbruzzese G, Berardelli A, et al. The TANDEM investigation: efficacy and tolerability of levodopa-carbidopa intestinal gel in (LCIG) advanced Parkinson’s disease patients. J Neural Transm. 2020;127(6):881–891. doi:10.1007/s00702-020-02175-1
60. Ehlers C, Timpka J, Odin P, et al. Levodopa infusion in Parkinson’s disease: individual quality of life. Acta Neurol Scand. 2020;142(3):248–254. doi:10.1111/ane.13260
61. Simu MA, Jianu DC, Dulamea AO, et al. Advanced Parkinson’s disease treatment simplification and long-term outcomes with levodopa carbidopa intestinal gel: COSMOS Romanian subanalysis. Brain Sci. 2021;11(12):1566. doi:10.3390/brainsci11121566
62. Standaert DG, Aldred J, Anca-Herschkovitsch M, et al. DUOGLOBE: one-year outcomes in a real-world study of levodopa carbidopa intestinal gel for Parkinson’s disease. Mov Disord Clin Pract. 2021;8(7):1061–1074. doi:10.1002/mdc3.13239
63. Othman M, Widman E, Nygren I, et al. Initial experience of the levodopa-entacapone-carbidopa intestinal gel in clinical practice. J Pers Med. 2021;11(4):254. doi:10.3390/jpm11040254
64. Martinez-Martin P, Reddy P, Katzenschlager R, et al. EuroInf: a multicenter comparative observational study of apomorphine and levodopa infusion in Parkinson’s disease. Mov Disord. 2015;30(4):510–516. doi:10.1002/mds.26067
65. Dafsari HS, Martinez-Martin P, Rizos A, et al. EuroInf 2: subthalamic stimulation, apomorphine, and levodopa infusion in Parkinson’s disease. Mov Disord. 2019;34(3):353–365. doi:10.1002/mds.27626
66. Antonini A, Yegin A, Preda C, et al. Global long-term study on motor and non-motor symptoms and safety of levodopa-carbidopa intestinal gel in routine care of advanced Parkinson’s disease patients; 12-month interim outcomes. Parkinsonism Relat Disord. 2015;21(3):231–235. doi:10.1016/j.parkreldis.2014.12.012
67. Fasano A, Gurevich T, Jech R, et al. Concomitant medication usage with levodopa-carbidopa intestinal gel: results from the COSMOS Study. Mov Disord. 2021;36(8):1853–1862. doi:10.1002/mds.28596
68. Tomlinson CL, Stowe R, Patel S, et al. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25(15):2649–2653. doi:10.1002/mds.23429
69. Burack M, Aldred J, Zadikoff C, et al. Implementing levodopa-carbidopa intestinal gel for Parkinson disease: insights from US practitioners. Mov Disord Clin Pract. 2018;5(4):383–393. doi:10.1002/mdc3.12630
70. Morales-Briceno H, Mahant N, Ha AD, et al. Long-term safety and efficacy of 24-hour levodopa-carbidopa intestinal gel in Parkinson’s disease. Mov Disord. 2019;34(11):1747–1748. doi:10.1002/mds.27883
71. Thakkar S, Fung VSC, Merola A, et al. 24-hour levodopa-carbidopa intestinal gel: clinical experience and practical recommendations. CNS Drugs. 2021;35(2):137–149. doi:10.1007/s40263-020-00782-w
72. Kovacs N, Szasz J, Vela-Desojo L, et al. Motor and nonmotor symptoms in patients treated with 24-hour daily levodopa-carbidopa intestinal gel infusion: analysis of the COmedication Study assessing Mono- and cOmbination therapy with levodopa-carbidopa inteStinal gel (COSMOS). Parkinsonism Relat Disord. 2022;105:139–144. doi:10.1016/j.parkreldis.2022.08.002
73. Nyholm D, Jost WH. Levodopa-entacapone-carbidopa intestinal gel infusion in advanced Parkinson’s disease: real-world experience and practical guidance. Ther Adv Neurol Disord. 2022;15:175628642211080. doi:10.1177/17562864221108018
74. Jost WH. A novel treatment option for intrajejunal levodopa administration. Expert Rev Neurother. 2023;23(1):9–13. doi:10.1080/14737175.2023.2176222
75. Gyorfi B, Balo B, Botz K, Jost WH. Levodopa-entakapon-karbidopa intestinalis gélinfúzió elôrehaladott parkinson-kórban - új kezelési lehetôség intrajejunalis levodopa adagolására [Triple combination of levodopa, carbidopa and entacapone by intrajejunal pump in advanced Parkinson’s disease]. Ideggyogy Sz. 2022;75(11–12):365–368. Hungarian. doi:10.18071/isz.75.0365
76. Antonini A, Pahwa R, Odin P, et al. Comparative effectiveness of device-aided therapies on quality of life and off-time in advanced Parkinson’s disease: a systematic review and Bayesian network meta-analysis. CNS Drugs. 2022;36(12):1269–1283. doi:10.1007/s40263-022-00963-9
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
