Back to Journals » Journal of Inflammation Research » Volume 16
Impact of Early Natural Killer Cell Reconstitution on the Outcomes of T Cell-Replete Allogeneic Hematopoietic Stem Cell Transplantation
Authors Zhou Z, Liu X, Zhang X , Wen S, Hua H, Wang Z, Xu Z, Lu Y, Wang F
Received 11 April 2023
Accepted for publication 4 July 2023
Published 19 July 2023 Volume 2023:16 Pages 2993—3008
DOI https://doi.org/10.2147/JIR.S416708
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Ziwei Zhou, Xuan Liu, Xuejun Zhang, Shupeng Wen, Huan Hua, Zhenzhen Wang, Zheng Xu, Yu Lu, Fuxu Wang
Department of Hematology, The Second Hospital of Hebei Medical University, Hebei Key Laboratory of Hematology, Shijiazhuang, Hebei 050000, People’s Republic of China
Correspondence: Fuxu Wang, Department of Hematology, The Second Hospital of Hebei Medical University, Hebei Key Laboratory of Hematology, No. 215 West Heping Road, Shijiazhuang, Hebei, 050000, People’s Republic of China, Tel +86 13931100360, Fax +86 031166003936, Email [email protected]
Background: Early immune reconstitution is crucial to successful outcomes after allogeneic stem cell transplantation (allo-HSCT). However, in T cell-replete HSCT, the impact of natural killer (NK) cells on transplantation outcome and the factors influencing early NK cell reconstitution remain unclear.
Methods: In this retrospective study, we analyzed 128 patients with hematological malignancies who received the first T cell-replete allo-HSCT between May 2019 and September 2021. After application of a conditioning regimen, prophylaxis for graft versus host disease (GVHD), and engraftment, the patients received prevention and treatment procedures for cytomegalovirus (CMV) reactivation. NK cells, T lymphocytes and B lymphocytes in peripheral blood were collected and analyzed at 30, 60, 90, 135 and 180 days after transplantation to observe immune cell reconstitution. Overall survival (OS), relapse-free survival (RFS), minimal residual disease (MRD), relapse, and non-relapse mortality (NRM) were evaluated. SPSS 25.0 and R version 4.2.1 were used for statistical analysis.
Results: In patients with rapid NK recovery (NK cell count at 30 days post-HSCT [NK30] > 165/μL and 60 days post-HSCT [NK60] > 265/μL), we observed lower rates of NRM, CMV reactivation and acute GVHD (aGVHD). Multivariate analysis indicated that a lower NK30 (≤ 165/μL) was an independent factor associated with inferior OS and RFS. The NK30 and NK60 in patients with CMV reactivation and aGVHD after transplantation were significantly lower than those in patients without these complications. In addition, CD107a expression in NK cells was also significantly lower in patients who experienced aGVHD. Correlation analysis did not find an inhibitory effect of T-lymphocyte subset reconstitution on NK cells in the early stage after transplantation.
Conclusion: Rapid NK cell reconstitution early after allo-HSCT had protective effects on NRM and survival. Promoting early NK cell reconstitution represents a new approach to improving the outcomes of allo-HSCT.
Keywords: allogeneic hematopoietic stem cell transplantation, immune reconstitution, natural killer cells, T-cell replete, non-relapse mortality
Introduction
In recent years, allogeneic hematopoietic stem cell transplantation (allo-HSCT) has been the only major method to cure hematological malignancies. However, disease relapse after HSCT, graft versus host disease (GVHD), infection and other transplant-related complications continue to drive high mortality rates among patients. Ex vivo T-cell depletion (TCD) of the graft uses either positive selection or negative depletion of graft cells before infusion to effectively reduce the incidence of GVHD after transplantation. In contrast, T cell-repleted grafts consisting of non-manipulated bone marrow or peripheral blood grafts require intense in vivo GVHD prophylaxis.1,2 Rapid reconstitution of the immune system after transplantation can give full play to the graft versus leukemia (GVL) effect and reduce the incidence of related complications. Studies have demonstrated a low transplantation-related mortality (TRM) rate among patients with a high lymphocyte recovery rate.3
Natural killer (NK) cells are the first lymphocyte subset to recover after allo-HSCT. They can exert cytotoxicity against residual leukemia cells and infected cells without antigen stimulation.4,5 The killing activity of NK cells after HSCT depends mainly on the mismatch between killer cell immunoglobulin-like receptors (KIRs) on the surface of donor NK cells and the major histocompatibility complex (MHC) I class molecules in recipients.6,7 NK cells have been shown to be the main effector cells that eliminate leukemia cells in T cell-depleted allo-HSCT. Stable implantation of NK cells after HSCT can improve the prognosis of patients, as shown by an early study of 54 patients with acute myeloid leukemia (AML) who received T cell-depleted allo-HSCT that revealed lower incidence rates of acute GVHD (aGVHD), relapse, and TRM among patients with rapid early NK cell recovery.8 In recent years, additional research has shown that rapid reconstitution of NK cells in the early stage after T cell-depleted and HLA-mismatched HSCT can significantly reduce the TRM and relapse incidence, thereby improving the survival of patients.6
However, no consensus has been reached regarding the impact of NK cell reconstitution on transplantation-related complications in T cell-replete HSCT. Only a few studies have examined the reconstitution of NK cells in T cell-replete allo-HSCT, and the results have been controversial. The purpose of this retrospective study was to analyze the impact of early NK cell reconstitution on outcomes after T cell-replete allo-HSCT with a myeloablative conditioning regimen, in order to provide clinical evidence for the ability of NK cell reconstitution to aid in the prognosis of patients and in improving their survival.
Materials and Methods
Patients
This study included 128 adult patients with malignant hematological disease who received their first allo-HSCT in the Hematological Department of the Second Hospital of Hebei Medical University between May 2019 and September 2021. Patients received granulocyte colony-stimulating factor (G-CSF)-mobilized T cell-replete HSCT. The graft sources were peripheral blood stem cells. Donor types included matched sibling donors, haplo-identical donors, and matched unrelated donors.
The inclusion criteria for patients were: 1. disease types including: acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and myelodysplastic syndromes (MDS); 2. complete hematopoietic reconstitution within 30 days after transplantation; 3. evaluation of the primary disease as complete remission (CR) with no minimal residual disease (MRD) and completely chimeric bone marrow at 30 days after transplantation; 4. regular monitoring of myelogram, MRD based on flow cytometry and genetics, and chimerism status after transplantation; and 5. performance of immune cell reconstitution detection at 30 and 60 days after transplantation. The exclusion criteria were: 1. malignant lymphoma leukemia phase in patients who received allogeneic transplantation; 2. death within 3 months after transplantation; 3. loss to follow-up within 3 months after transplantation; 4. treatment with autologous hematopoietic stem cell transplantation; and 5. treatment with CAR-T cell therapy before transplantation (Figure 1). This study was approved by the Research Ethics Committee of the Second Hospital of Hebei Medical University (approval number: 2022-R664), and all patients signed an informed consent form before participation.
 |
Figure 1 Flow chart of patient inclusion. Abbreviations: HSCT, Haematopoietic stem cell transplantation; MRD, Minimal residual disease; CAR-T, Chimeric antigen receptor (CAR)-T cell. |
Conditioning Regimen, Prophylaxis for GVHD, and Engraftment
Patients received modified busulfan (BU)/cyclophosphamide (CY) or total body irradiation (TBI)/CY as a myeloablative conditioning regimen. On this basis, patients who received transplants from mismatched related or unrelated donors and patients older than 40 years who received transplants from matched related donors were treated with rabbit anti-human thymocyte immune globulin (ATG).
GVHD prophylaxis was 2 mg/kg/d cyclosporine and 1 g/d mycophenolate mofetil9,10 from the beginning of the conditioning regimen, and then methotrexate at doses of 15 mg/m2 at 1 day and 10 mg/m2 at 3, 6, and 11 days after transplantation. GVHD was diagnosed and graded according to the Glucksberg-Seattle standard.
Granulocyte engraftment was defined as an absolute neutrophil count ≥0.5×109/L for 3 consecutive days, and platelet engraftment was defined as a platelet count ≥20×109/L for 7 consecutive days without platelet transfusion.
Prevention and Treatment of CMV Reactivation
All patients and donors were negative for CMV-DNA before conditioning. All patients regardless of history of CMV infection were given the following treatment to prevent virus reactivation starting from the beginning of the conditioning regimen: ganciclovir 5 mg/kg twice a day before reinfusion of stem cells and 0.4 g acyclovir twice a day after the reinfusion of stem cells. CMV-DNA detection was performed twice per week starting from the transplantation date. A CMV-DNA level of >1000 copies/mL was defined as CMV reactivation, and ganciclovir at a dose of 5 mg/kg was started immediately and administered twice daily until the results for CMV-DNA were negative twice in a row.
Detection of Immune Cell Reconstitution
To observe immune cell reconstitution, 4-mL samples of EDTA anticoagulant peripheral blood were collected at 30, 60, 90, 135 and 180 days after transplantation. The reconstitution of NK cells, T lymphocytes and B lymphocytes in these samples at various time points after transplantation was detected by MACSQUANT 10 flow cytometry (Miltenyi Biotec). Lymphocyte surface antigen markers included: NK cells: CD3-CD56+, T lymphocytes: CD3+, and B lymphocytes: CD3-CD19+. This study mainly analyzed the reconstitution results for NK cells and T lymphocytes in the early stage (30 and 60 days) after transplantation.
Endpoints
The final follow-up was performed on September 1, 2022. Overall survival (OS) was defined as the time interval from the transplantation date to death. Relapse-free survival (RFS) was defined as the time interval from the transplantation date to relapse or death. The definition of relapse included: 1. morphological relapse based on a proportion of leukemia cells in bone marrow exceeding 5%; 2. extramedullary relapse based on the appearance of extramedullary leukemia lesions; and 3. MRD relapse as assessed by flow cytometry and quantitative PCR. MRD relapse was diagnosed as MRD positive, and non-relapse mortality (NRM) was defined as death due to any cause other than relapse.
Statistical Analysis
SPSS 25.0 and R version 4.2.1 were used for statistical analysis. Receiver operator characteristic (ROC) curves were used to determine cut-off values. Categorical data were compared by chi-square test, and continuous variables were compared using t-test and Mann–Whitney U-test. Multivariate analysis of categorical variables was achieved using logistic regression. The Kaplan–Meier method was used for survival analysis, and comparison of survival rates was analyzed by log rank test. The Cox regression model was used for multivariate survival analysis of variables shown to be statistically significant in univariate survival analysis. Linear correlation among continuous variables was assessed using Pearson’s correlation test. The cumulative incidence rates of NRM, GVHD, CMV, relapse and competition events were compared by Fine Gray’s test. Differences for which P values were <0.05 were defined as statistically significant.
Results
Characteristics of Patients
Among the 128 patients, 68 were male and 60 were female, and the median age was 34 (18–69) years. The disease types included 62 cases of AML, 49 cases of ALL, and 17 cases of MDS. Before transplantation, 99 cases were categorized as CR and 29 cases as non-CR. Among the cases in CR, 25 were positive for MRD and 74 were negative for MRD. Donor types included haplo-identical donor in 74 cases, matched sibling donor in 50 cases, and matched unrelated donor in 4 cases. The median mononuclear cell count in the grafts was 9.50 (4.86–22.01) ×108/kg, and the median CD34+ cell count was 4.17 (1.58–8.83) × 106/kg. The median engraftment times for granulocytes and platelets were 12 (8–25) days and 15.5 (8–29) days, respectively.
ROC curve analysis showed that the NK cell count at 30 days after HSCT (NK30) was associated with the overall mortality of patients after transplantation, with a cut-off value of 165/μL (area under the curve [AUC]=0.743, 95% confidence interval [CI]: 0.655–0.831; Figure 2). When the 128 patients were dichotomized into two groups according to this cut-off value, 74 patients had NK30 >165/μL and 54 patients had NK30 ≤165/μL. No significant differences in age, sex, disease type, risk stratification, MRD before transplantation, blood type matching, donor type, etc. were found between the two groups (Table 1). The median NK cell count 60 days after transplantation (NK60) was 265/μL, and patients also were dichotomized according to this median in further analyses.
 |
Table 1 Clinical Characteristics of 128 Patients with High and Low NK Cell Count at 30 Days After HSCT |
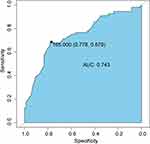 |
Figure 2 ROC curve for NK30 as a predictor of all-cause mortality. Abbreviations: ROC, Receiver operator characteristic; AUC, Area under the ROC curve. |
OS and RFS According to NK Cell Reconstitution
After a median follow-up of 23.6 (12.2–40.8) months. 34 patients were diagnosed with relapse. Analysis of the cumulative incidence of relapse with death as a competing event showed no statistical difference between the cumulative incidence of relapse between patients with high NK30 versus low NK30, and the same result was found in the comparison of patients with high and low NK60 (both P>0.05; Figure 3).
Twenty-nine patients died during the following-up period, 18 of whom died from relapse. Analysis of NRM with death caused by relapse as a competing event confirmed that the cumulative incidence rates of NRM among patients with high NK30 and NK60 were significantly lower than the respective rates in patients with low NK30 and NK60 (P=0.03; Figure 4).
Survival analysis demonstrated that the 1-year OS rates of patients with high NK30 and NK60 were higher than those of patients with low NK30 and NK60, respectively (90.50%±3.40% vs 70.30%±6.20%, P=0.001; 87.5%±4.10% vs 76.50%±5.30%, P=0.006; Figure 5A and B). In addition, the RFS rates of patients with high NK30 and NK60 were significantly better than those of patients with low NK30 and NK60, respectively (Figure 5C and D). These findings show that rapid reconstitution of NK cells in the early stage after transplantation can improve patient survival.
Survival analysis also was performed according to disease types, and the results showed that high NK30 and NK60 in 79 AML/MDS patients were significantly associated with superior OS and RFS (all P<0.05). In contrast, the analysis results for 49 ALL patients showed that NK30 and NK60 had no effect on OS and RFS in these patients (all P>0.05).
We further analyzed other factors related to transplantation outcomes. On univariate analysis, high risk stratification, positive MRD before transplantation, low NK30, and low NK60 were related to inferior OS and RFS (Table 2). A Cox regression model using significant variables from the univariate analysis was employed to conduct the multivariate analysis. The results showed that low NK30 and positive MRD before transplantation were independently associated with poor OS and RFS. These results indicate that rapid reconstitution of NK cells early (especially within 30 days) after transplantation is an independent protective factor for the survival of patients (Table 3).
 |
Table 2 Univariate Analysis of Factors Associated with OS and RFS After HSCT |
 |
Table 3 Multivariate Analysis of Factors Associated with OS and RFS After HSCT |
CMV Reactivation
CMV reactivation after HSCT was detected in 56 of 128 patients (43.7%), and the median time to reactivation was 35 (7–360) days after transplantation. The cumulative incidence of CMV reactivation was determined by taking death as a competing event. Patients with high NK30 and NK60 had significantly lower incidence rates of CMV reactivation than patients who had low NK30 and NK60 (P<0.01, P=0.005, respectively; Figure 6A and B).
Both univariate and multivariate analyses of early NK cell recovery together with other factors related to CMV reactivation after HSCT, such as age, ATG in conditioning regimen, and T-cell subset count at 30 days after HSCT were performed, and the results identified low NK30 and the use of ATG in conditioning were independent factors related to a high incidence of CMV reactivation (Table 4 and Table 5).
 |
Table 4 Univariate Analysis of Risk Factors for CMV Reactivation |
 |
Table 5 Multivariate Analysis of Risk Factors for CMV Reactivation |
CMV reactivation can also affect the reconstitution of NK cells after HCST. Our research showed that the NK30 and NK60 in patients with CMV reactivation were significantly lower than those in patients without CMV reactivation (NK30: 186.61±181.23/µL vs 333.28±216.15/µL, P<0.01; NK60: 296.16±274.37/µL vs 414.65±311.15/µL, P=0.026; Figure 6C). However, no significant difference in the level of CD107a expression by NK cells was observed between patients with and without CMV reactivation (P>0.05; Figure 6D).
GVHD
In this study, 49 patients were diagnosed with aGVHD during the follow-up period, and the median time to aGVHD was 27 (15–96) days after HSCT. Among these cases, 20 patients experienced grade I aGVHD and 29 patients experienced grade II–IV aGVHD. Chronic GVHD (cGVHD) occurred in 27 cases with all grades observed. The cumulative incidence of aGVHD in patients with low NK30 was significantly higher than that in patients with high NK30. Similarly, the incidence of aGVHD was significantly lower in patients with high NK60 than in patients with low NK60 (P<0.05; Figure 7A and B). Our results did not demonstrate a correlation between early NK cell recovery and cGVHD (P>0.05).
Both univariate and multivariate analyses showed that low NK30 was an independent factor related to a high incidence of aGVHD (Table 6 and Table 7). The NK30 and NK60 of patients with aGVHD after HSCT were significantly lower than those of patients without aGVHD (NK30: 178.37±162.19/µL vs 325.39±223.18/µL, P<0.01; NK60: 294.90±291.78/µL vs 404.94±299.65/µL, P=0.043; Figure 7C). At the same time, the study found that the level of CD107a expression on NK cells 30 and 60 days after transplantation in patients with aGVHD was significantly lower than that in patients without aGVHD (17.79%±11.83% vs 24.06%±17.05%, P=0.026; 15.31%±12.49% vs 21.12%±13.76%, P=0.018; Figure 7D).
 |
Table 6 Univariate Analysis of Risk Factors for aGVHD |
 |
Table 7 Multivariate Analysis of Risk Factors for aGVHD |
Considering that the appearance of aGVHD within 30 days after transplantation and the use of corticosteroids affect early NK cell reconstitution,11,12 we excluded the 25 patients who developed aGVHD within 30 days and still found that the cumulative incidence of aGVHD was significantly higher in patients with a low NK30 than in those with a high NK30 (P=0.01).
Correlation of NK Cell and T Cell Reconstitution
The NK cell count and T lymphocyte count 30 and 60 days after HSCT were analyzed as continuous variables using linear correlation. The results showed that there were no significant correlations between T lymphocyte count and NK cell count (Figure 8).
Discussion
NK cells are potent effectors for GVL effect and fighting viral infection after allo-HSCT. NK cells have been reported to attenuate GVHD by killing activated T lymphocytes and antigen-presenting cells (APCs) in recipients.13–15 Previous studies have shown that rapid reconstitution of NK cells can reduce the incidence of transplantation-related complications and improve survival in T cell-depleted HSCT.8,16,17 Because multiple players are involved in the outcome of transplantation, such as age, resource of graft, donor type, conditioning regime and so on, the influence of early NK cell reconstitution on the prognosis of non-T cell-depleted allo-HSCT remains controversial.18–20
Our results demonstrated that the cumulative incidence rates of NRM, CMV reactivation, and aGVHD in patients with rapid NK cell reconstitution (high NK30 and NK60) early after allo-HSCT were significantly reduced. Better OS and RFS rates were achieved in patients with high NK30 and NK60 compared with corresponding controls. We identified high NK30 as an independent factor related to superior OS and RFS in these patients. This is in line with a recent single-center clinical study. Zhao et al21 found that rapid NK cell reconstitution was associated with significantly higher 3-year OS and RFS rates in AML or MDS patients received non-depleted T-cell transplantation. However, they did not demonstrate the effect of NK cell reconstitution on NRM. In our study, a high incidence of NRM was found in patients with delayed NK cell reconstitution.
We further analyzed the effects of NK recovery rate on patient survival rates based on disease type. The results revealed that among 79 patients with AML or MDS, those with rapid NK cell reconstitution still had better OS and RFS, while no such difference was observed in ALL patients. This suggests that NK cells have a more significant killing effect after transplantation in patients with AML or MDS. This may be because the GVL effect of NK cells is influenced by other factors such as conditioning regimen, post-transplantation immunosuppressant application, and donor type.
Interestingly, we did not find a significant difference in the relapse rate between patients with high and low NK cell counts. Whether early NK cell recovery can reduce relapse rates has been controversial. Differences in outcomes may be related to the effects of pretreatment protocol, type of disease, donor type, and graft source. A previous study showed that NK cell reconstitution did not have an effect on relapse rates in patients with malignant hematological diseases receiving non-T cell-depleted transplantation.18 However, another study showed that rapid NK cell reconstitution was associated with a significantly reduced relapse rates in myeloid malignancies, suggesting that NK cells play a more prominent role in GVL in myeloid tumors.21 Similarly, a study on non-T cell-depleted allogeneic transplantation found that patients with high NK cell counts 1 year after transplantation did have a significantly reduced relapse rate.22 However, NK cell reconstitution early post-transplantation (within 3 months) did not contribute to the reduction of relapse rate.22
In the occurrence of GVHD, donor-derived T cells are activated to release inflammatory factors, causing damage to target organs or tissues.23,24 On one hand, NK cells inhibit the proliferation and activation of donor-derived T cells and induce apoptosis of T cells, by reducing CD25 expression levels and interferon (IFN)-γ production.25 On the other hand, T lymphocytes can inhibit the reconstitution of NK cells by competitively depleting cytokines such as interleukin (IL)-15 and IL-2.26,27 Cytokines such as IL-2 and IL-15 can promote NK cell activation by potentiating NK cell- mediated antibody-dependent cellular cytotoxicity (ADCC). Additionally, stimulation with IL-15 and IL-12 together drives the expansion of a particular subset of NK cells displaying adaptive traits similar to those of memory-like NK cells, which have enhanced anti-tumor effects.28,29 We investigated the effect of aGVHD on early NK cell reconstitution. Consistent with previous findings,30,31 we found that both NK30 and NK60 were significantly lower in patients who developed aGVHD after transplantation than in the patients without aGVHD development. Moreover, our results revealed that the level of CD107a, an indicator of NK cell killing activity,32,33 was significantly reduced in NK cells among the patients with aGVHD. This finding demonstrated that the occurrence of aGVHD after transplantation likely suppressed NK cell recovery of cell killing function. In contrast, a previous report suggested that the occurrence of GVHD after non-T cell-depleted HSCT did not have an effect on the CD107a expression of NKG2A+ NK cells.31 The discrepancy might be related to the selection of patients; in this previous report, only patients with malignant hematological disease who underwent haploidentical transplantation were selected.
Several studies have demonstrated reduced GVHD with removal of T cells from grafts, but the effect of early NK cell reconstitution on aGVHD in these studies remained uncertain.34,35 Our results showed that the incidence of aGVHD was significantly lower in patients with high NK cell counts at both 30 and 60 days post-transplantation than in patients with low NK cell counts, consistent with the results of the study reported by Kim et al.36
Previous studies have proposed an association between NK cell reconstitution after HSCT and the development of cGVHD.37 However, an effect of early NK cell reconstitution on the development of cGVHD was not found in a recent study of non-T cell-depleted allo-HSCT.21 Our results showed no effect of NK cell reconstitution on cGVHD. The discrepancy in the effect of NK cell recovery on aGVHD and cGVHD could be due to the different treatment methods used for patients who develop aGVHD or cGVHD. Patients with aGVHD after allogeneic transplantation are usually treated with a variety of immunosuppressive drugs, which could inhibit the recovery of NK cells.12
CMV reactivation commonly leads to serious or even life-threatening complications in patients who receive allo-HSCT.38,39 Poor OS and high TRM have been reported for patients who develop CMV reactivation after transplantation.40 As the earliest recovered immune cells after HSCT, NK cells can play an important role in controlling CMV infection after transplantation by exerting a killing effect to remove infected cells from the body.41,42 Our results showed a significant reduction in the cumulative incidence of CMV reactivation in patients with rapid NK cell reconstitution early after transplantation (high NK30 and NK60). Consistent with previous findings,18,21 our multivariate analysis also confirmed that low NK30 was independently correlated with a high incidence of CMV reactivation. Further analysis showed that NK30 and NK60 were significantly lower in patients who developed CMV reactivation than in those who did not.
Interestingly, the level of CD107a on NK cells in the early post-transplantation period was not significantly different between CMV-reactivated and non-reactivated patients. A recent clinical study categorized post-transplantation CMV infection as CMV reactivation and CMV disease based on the manifestation of CMV infection. Their results indicated that both CD56bright and CD56dim NK cell counts in patients who experienced CMV reactivation 60 days after transplantation were higher than those in patients who did not. They also showed that the lowest NK cell counts were found in patients who experienced CMV disease.43 Another study also showed that CMV reactivation after haploid HSCT can alter the expression of NK cell receptors to promote the maturation of NK cells, thus allowing them to exert a stronger killing activity.44 Therefore, different manifestations of CMV infection after HSCT can have various effects on NK cells. CMV reactivation could exert a protective effect in transplantation patients by promoting the maturation of NK cells. Moreover, the early post-transplantation period is dominated by the reconstitution of immature NK cell subpopulations characterized by CD56bright CD16dim expression, while the mature NK cell subpopulations that mainly perform killing functions take as long as 6 months to recover.14,45,46 CMV effects on different subpopulations and receptor reconstitution of NK cells need to be further investigated.
NK cells and T lymphocytes may interact with each other after transplantation. Previous reports have likewise indicated that both T lymphocytes in grafts and reconstituted T lymphocytes after transplantation can affect NK cell recovery and inhibit KIR reconstitution.47–49 Others have suggested that donor-derived mature NK cells also affect the recovery of T cells after transplantation.26 However, our study found no significant correlation between the reconstitution of different T lymphocyte subsets and NK cells at 30 and 60 days after transplantation. This may be due to that NK cell reconstitution is influenced by conditioning regimen. In our study, the pretreatment regimens were all myeloablative. Some patients also were given ATG in the pretreatment regimen, which has been shown to have an inhibitory effect on CD4+ T-cell and CD8+ T-cell reconstitution.50,51 Alternatively, myeloablative conditioning with cyclophosphamide postposition can produce a stronger inhibitory effect on the recovery of NK cells.52 Intriguingly, other research has suggested that T lymphocytes in grafts can promote the recovery of NK cells.53 IL-2 produced by T lymphocytes was found to promote the maturation of CD56bright NK cells and enhance the ability of NK cells to secrete cytokines as well as exert cytotoxic effects.54,55 The interactions between immune cell subpopulations after allo-HSCT and the effects of different conditioning regimens on immune cells still require further confirmation.
Several limitations need to be further addressed in our study. One limitation is the relatively small number of cases and short follow-up time. An expanded sample size is needed in further studies. Another limitation is that we did not perform an analysis based on different NK cell subpopulations. We also did not include an analysis of receptor reconstitution in NK cells. The interactions between multiple factors such as GVHD and different subpopulations of NK cells and their receptor reconstitution need to be confirmed by prospective studies.
In conclusion, we found that early NK cell reconstitution was associated to the reduced occurrence of transplantation-related complications and improved long-term survival of patients. Regular monitoring of immune cell reconstitution in patients after allo-HSCT could help identify and predict the occurrence of transplantation-related complications at an early stage. Moreover, the use of effective methods to promote early NK cell reconstitution is likely to reduce the occurrence of transplantation-related complications and to improve the long-term survival of patients.
Abbreviations
allo-HSCT, allogeneic stem cell transplantation; NK, natural killer; GVL, graft versus leukemia; NRM, non-relapse mortality; CMV, cytomegalovirus; aGVHD, acute graft versus host disease; cGVHD, chronic graft versus host disease; TRM, transplantation-related mortality; KIRs, killer cell immunoglobulin-like receptors; MHC, major histocompatibility complex; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; ALL, acute lymphoblastic leukemia; OS, overall survival; RFS, relapse-free survival; G-CSF, granulocyte colony-stimulating factor; CR, complete remission; MRD, minimal residual disease; BU, busulfan; CY, cyclophosphamide; TBI, total body irradiation; ATG, anti human thymocyte immune globulin; ROC, receiver operator characteristic; HID, haplo-identical donor; MSD, matched sibling donor; MUD, matched unrelated donor; MNC, mononuclear cell; PLT, platelet; HR, hazard ratio; APCs, antigen-presenting cells; IFN, interferon; IL, interleukin.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This study compiled with the Declaration of Helsinki. This study was approved by the Research Ethics Committee of the Second Hospital of Hebei Medical University (approval number: 2022-R664), and all patients signed an informed consent form before participation.
Acknowledgments
We thank members of our department for helpful discussion. In the meantime, we are grateful for Medjaden Inc. for scientific editing of this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by The Natural Science Foundation of Hebei Province (H2020206403) and The Medical Applicable Technology Tracking Project of Hebei Province (GZ2022014).
Disclosure
We declare that we have no conflict of interest.
References
1. Xue YJ, Suo P, Huang XJ, et al. Superior survival of unmanipulated haploidentical haematopoietic stem cell transplantation compared with intensive chemotherapy as post-remission treatment for children with very high-risk Philadelphia chromosome negative B-cell acute lymphoblastic leukaemia in first complete remission. Brit J Haematol. 2019;188:757–767. doi:10.1111/bjh.16226
2. Kleinschmidt K, Lv M, Yanir A, et al. T-cell-replete versus ex vivo T-cell-depleted haploidentical haematopoietic stem cell transplantation in children with acute lymphoblastic leukaemia and other haematological malignancies. Front Pediatr. 2021;9:794541. doi:10.3389/fped.2021.794541
3. Ogonek J, Kralj Juric M, Ghimire S, et al. Immune reconstitution after allogeneic hematopoietic stem cell transplantation. Front Immunol. 2016;7:507. doi:10.3389/fimmu.2016.00507
4. Gill S, Olson JA, Negrin RS. Natural killer cells in allogeneic transplantation: effect on engraftment, graft- versus-tumor, and graft-versus-host responses. Biol Blood Marrow Transplant. 2009;15:765–776. doi:10.1016/j.bbmt.2009.01.019
5. Locatelli F, Merli P, Rutella S. At the bedside: innate immunity as an immunotherapy tool for hematological malignancies. J Leukoc Biol. 2013;94:1141–1157. doi:10.1189/jlb.0613343
6. Zaghi E, Calvi M, Di Vito C, Mavilio D. Innate immune responses in the outcome of haploidentical hematopoietic stem cell transplantation to cure hematologic malignancies. Front Immunol. 2019;10:2794. doi:10.3389/fimmu.2019.02794
7. Anfossi N, André P, Guia S, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi:10.1016/j.immuni.2006.06.013
8. Savani BN, Mielke S, Adams S, et al. Rapid natural killer cell recovery determines outcome after T-cell-depleted HLA-identical stem cell transplantation in patients with myeloid leukemias but not with acute lymphoblastic leukemia. Leukemia. 2007;21:2145–2152. doi:10.1038/sj.leu.2404892
9. Lv M, Chang YJ, Huang XJ. Update of the “Beijing Protocol” haplo-identical hematopoietic stem cell transplantation. Bone Marrow Transpl. 2019;54:703–707. doi:10.1038/s41409-019-0605-2
10. Huang XJ, Liu DH, Liu KY, et al. Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transpl. 2006;38:291–297. doi:10.1038/sj.bmt.1705445
11. Chiossone L, Vitale C, Cottalasso F, et al. Molecular analysis of the methylprednisolone-mediated inhibition of NK-cell function: evidence for different susceptibility of IL-2- versus IL-15-activated NK cells. Blood. 2007;109:3767–3775. doi:10.1182/blood-2006-07-037846
12. Pradier A, Papaserafeim M, Li N, et al. Small-molecule immunosuppressive drugs and therapeutic immunoglobulins differentially inhibit NK cell effector functions in vitro. Front Immunol. 2019;10:556. doi:10.3389/fimmu.2019.00556
13. Vivier E, Ugolini S. Natural killer cells: from basic research to treatments. Front Immunol. 2011;2:18. doi:10.3389/fimmu.2011.00018
14. Cooley S, Parham P, Miller JS. Strategies to activate NK cells to prevent relapse and induce remission following hematopoietic stem cell transplantation. Blood. 2018;131:1053–1062. doi:10.1182/blood-2017-08-752170
15. Hüber CM, Doisne JM, Colucci F. IL-12/15/18-preactivated NK cells suppress GvHD in a mouse model of mismatched hematopoietic cell transplantation. Eur J Immunol. 2015;45:1727–1735. doi:10.1002/eji.201445200
16. Yamamoto W, Ogusa E, Matsumoto K, Maruta A, Ishigatsubo Y, Kanamori H. Recovery of natural killer cells and prognosis after cord blood transplantation. Leuk Res. 2013;37:1522–1526. doi:10.1016/j.leukres.2013.09.005
17. Pfeiffer MM, Feuchtinger T, Teltschik HM, et al. Reconstitution of natural killer cell receptors influences natural killer activity and relapse rate after haploidentical transplantation of T- and B-cell depleted grafts in children. Haematologica. 2010;95:1381–1388. doi:10.3324/haematol.2009.021121
18. Minculescu L, Marquart HV, Friis LS, et al. Early natural killer cell reconstitution predicts overall survival in T cell-replete allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2016;22:2187–2193. doi:10.1016/j.bbmt.2016.09.006
19. Gao F, Ye Y, Gao Y, Huang H, Zhao Y. Influence of KIR and NK cell reconstitution in the outcomes of hematopoietic stem cell transplantation. Front Immunol. 2020;11:2022. doi:10.3389/fimmu.2020.02022
20. Ullah MA, Hill GR, Tey SK. Functional reconstitution of natural killer cells in allogeneic hematopoietic stem cell transplantation. Front Immunol. 2016;7:144. doi:10.3389/fimmu.2016.00144
21. Zhao F, Shi Y, Chen X, et al. Higher dose of CD34(+) cells promotes early reconstitution of natural killer cells and is associated with better outcomes after unmanipulated hematopoietic stem cell transplantation for myeloid malignancies. Transplant Cell Ther. 2022;28:589.e581–589.e510. doi:10.1016/j.jtct.2022.06.007
22. Bühlmann L, Buser AS, Cantoni N, et al. Lymphocyte subset recovery and outcome after T-cell replete allogeneic hematopoietic SCT. Bone Marrow Transplant. 2011;46:1357–1362. doi:10.1038/bmt.2010.306
23. Ball LM, Egeler RM. Acute GvHD: pathogenesis and classification. Bone Marrow Transplant. 2008;41:S58–64. doi:10.1038/bmt.2008.56
24. Vacca P, Montaldo E, Croxatto D, et al. NK cells and other innate lymphoid cells in hematopoietic stem cell transplantation. Front Immunol. 2016;7:188. doi:10.3389/fimmu.2016.00188
25. Olson JA, Leveson-Gower DB, Gill S, Baker J, Beilhack A, Negrin RS. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood. 2010;115:4293–4301. doi:10.1182/blood-2009-05-222190
26. Ciurea SO, Schafer JR, Bassett R, et al. Phase 1 clinical trial using mbIL21 ex vivo-expanded donor-derived NK cells after haploidentical transplantation. Blood. 2017;130:1857–1868. doi:10.1182/blood-2017-05-785659
27. Fujisaki H, Kakuda H, Shimasaki N, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010–4017. doi:10.1158/0008-5472.can-08-3712
28. Muntasell A, Ochoa MC, Cordeiro L, et al. Targeting NK-cell checkpoints for cancer immunotherapy. Curr Opin Immunol. 2017;45:73–81. doi:10.1016/j.coi.2017.01.003
29. Romee R, Rosario M, Berrien-Elliott MM, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med. 2016;8:357ra123. doi:10.1126/scitranslmed.aaf2341
30. Ullrich E, Salzmann-Manrique E, Bakhtiar S, et al. Relation between acute GVHD and NK cell subset reconstitution following allogeneic stem cell transplantation. Front Immunol. 2016;7:595. doi:10.3389/fimmu.2016.00595
31. Hu LJ, Zhao XY, Yu XX, et al. Quantity and quality reconstitution of NKG2A(+) natural killer cells are associated with graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2019;25:1–11. doi:10.1016/j.bbmt.2018.08.008
32. Aktas E, Kucuksezer UC, Bilgic S, Erten G, Deniz G. Relationship between CD107a expression and cytotoxic activity. Cell Immunol. 2009;254:149–154. doi:10.1016/j.cellimm.2008.08.007
33. Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi:10.1016/j.jim.2004.08.008
34. Shah NN, Baird K, Delbrook CP, et al. Acute GVHD in patients receiving IL-15/4-1BBL activated NK cells following T-cell-depleted stem cell transplantation. Blood. 2015;125:784–792. doi:10.1182/blood-2014-07-592881
35. Meinhardt K, Kroeger I, Bauer R, et al. Identification and characterization of the specific murine NK cell subset supporting graft-versus-leukemia- and reducing graft-versus-host-effects. Oncoimmunology. 2015;4:e981483. doi:10.4161/2162402x.2014.981483
36. Kim SY, Lee H, Han MS, et al. Post-transplantation natural killer cell count: a predictor of acute graft-versus-host disease and survival outcomes after allogeneic hematopoietic stem cell transplantation. Clin Lymphoma Myeloma Leuk. 2016;16:527–535.e522. doi:10.1016/j.clml.2016.06.013
37. Kheav VD, Busson M, Scieux C, et al. Favorable impact of natural killer cell reconstitution on chronic graft-versus-host disease and cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation. Haematologica. 2014;99:1860–1867. doi:10.3324/haematol.2014.108407
38. Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2017;64:87–91. doi:10.1093/cid/ciw668
39. Cho SY, Lee DG, Kim HJ. Cytomegalovirus infections after hematopoietic stem cell transplantation: current status and future immunotherapy. Int J Mol Sci. 2019;20:2666. doi:10.3390/ijms20112666
40. Ando T, Suzuki T, Ishiyama Y, et al. Impact of cytomegalovirus reactivation and natural killer reconstitution on outcomes after allogeneic hematopoietic stem cell transplantation: a single-center analysis. Biol Blood Marrow Transplant. 2020;26:171–177. doi:10.1016/j.bbmt.2019.09.028
41. Muccio L, Falco M, Bertaina A, et al. Late development of FcεRγ(neg) adaptive natural killer cells upon human cytomegalovirus reactivation in umbilical cord blood transplantation recipients. Front Immunol. 2018;9:1050. doi:10.3389/fimmu.2018.01050
42. Adams NM, Geary CD, Santosa EK, et al. Cytomegalovirus infection drives avidity selection of natural killer cells. Immunity. 2019;50:1381–1390.e1385. doi:10.1016/j.immuni.2019.04.009
43. Park KH, Ryu JH, Bae H, et al. Delayed NK cell reconstitution and reduced NK activity increased the risks of CMV disease in allogeneic-hematopoietic stem cell transplantation. Int J Mol Sci. 2020;21. doi:10.3390/ijms21103663
44. Zhao XY, Luo XY, Yu XX, et al. Recipient-donor KIR ligand matching prevents CMV reactivation post-haploidentical T cell-replete transplantation. Br J Haematol. 2017;177:766–781. doi:10.1111/bjh.14622
45. Pical-Izard C, Crocchiolo R, Granjeaud S, et al. Reconstitution of natural killer cells in HLA-matched HSCT after reduced-intensity conditioning: impact on clinical outcome. Biol Blood Marrow Transplant. 2015;21:429–439. doi:10.1016/j.bbmt.2014.11.681
46. Park BG, Park CJ, Jang S, et al. Reconstitution of lymphocyte subpopulations after hematopoietic stem cell transplantation: comparison of hematologic malignancies and donor types in event-free patients. Leuk Res. 2015;39:1334–1341. doi:10.1016/j.leukres.2015.09.010
47. Ciurea SO, Mulanovich V, Saliba RM, et al. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:1835–1844. doi:10.1016/j.bbmt.2012.07.003
48. Eissens DN, Schaap NP, Preijers FW, et al. CD3+/CD19+-depleted grafts in HLA-matched allogeneic peripheral blood stem cell transplantation lead to early NK cell cytolytic responses and reduced inhibitory activity of NKG2A. Leukemia. 2010;24:583–591. doi:10.1038/leu.2009.269
49. Cooley S, McCullar V, Wangen R, et al. KIR reconstitution is altered by T cells in the graft and correlates with clinical outcomes after unrelated donor transplantation. Blood. 2005;106:4370–4376. doi:10.1182/blood-2005-04-1644
50. Bosch M, Dhadda M, Hoegh-Petersen M, et al. Immune reconstitution after anti-thymocyte globulin-conditioned hematopoietic cell transplantation. Cytotherapy. 2012;14:1258–1275. doi:10.3109/14653249.2012.715243
51. Servais S, Menten-Dedoyart C, Beguin Y, et al. Impact of pre-transplant Anti-T Cell Globulin (ATG) on immune recovery after myeloablative allogeneic peripheral blood stem cell transplantation. PLoS One. 2015;10:e0130026. doi:10.1371/journal.pone.0130026
52. Retière C, Willem C, Guillaume T, et al. Impact on early outcomes and immune reconstitution of high-dose post-transplant cyclophosphamide vs anti-thymocyte globulin after reduced intensity conditioning peripheral blood stem cell allogeneic transplantation. Oncotarget. 2018;9:11451–11464. doi:10.18632/oncotarget.24328
53. Foley B, Cooley S, Verneris MR, et al. NK cell education after allogeneic transplantation: dissociation between recovery of cytokine-producing and cytotoxic functions. Blood. 2011;118:2784–2792. doi:10.1182/blood-2011-04-347070
54. Fehniger TA, Cooper MA, Nuovo GJ, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–3057. doi:10.1182/blood-2002-09-2876
55. Ferlazzo G, Thomas D, Lin SL, et al. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol. 2004;172:1455–1462. doi:10.4049/jimmunol.172.3.1455
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






