Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Impact of Comorbidity Prevalence and Cardiovascular Disease Status on the Efficacy and Safety of Nebulized Glycopyrrolate in Patients with COPD
Authors Putcha N, Ozol-Godfrey A, Sanjar S , Sharma S
Received 21 January 2021
Accepted for publication 23 March 2021
Published 19 April 2021 Volume 2021:16 Pages 1061—1073
DOI https://doi.org/10.2147/COPD.S302088
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Nirupama Putcha,1 Ayca Ozol-Godfrey,2 Shahin Sanjar,2 Sanjay Sharma2
1Division of Pulmonary and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA; 2Sunovion Pharmaceuticals Inc., Marlborough, MA, USA
Correspondence: Nirupama Putcha
Division of Pulmonary and Critical Care Medicine, Johns Hopkins University School of Medicine, 5501 Hopkins Bayview Circle, JHAAC 4B.74, Baltimore, MD, 21224, USA
Tel +1 410-550-0545
Email [email protected]
Purpose: Patients with COPD often have multiple coexisting comorbidities, affecting quality of life, morbidity and mortality. However, the prevalence and impact of comorbidities on the efficacy of bronchodilators in COPD is poorly understood.
Patients and Methods: In this post hoc analysis, pooled data from the 12-week, placebo-controlled GOLDEN 3 and 4 studies of nebulized glycopyrrolate (GLY) in individuals with moderate-to-very-severe COPD were used to quantify comorbidities and assess their impact on treatment efficacy and safety.
Results: Comorbidities that were most prevalent in the GOLDEN 3 and 4 study population were hypertension, high cholesterol and osteoarthritis. Participants were grouped based on their pre-specified comorbidity count into Group A (≤ 2 comorbidities; n=439) and Group B (> 2 comorbidities; n=854). Treatment with GLY resulted in significant improvements in forced expiratory volume in 1 second (FEV1) and St George’s Respiratory Questionnaire (SGRQ) total scores, independent of comorbidity prevalence. A higher prevalence of cardiovascular disease (CVD) comorbidities was observed among individuals in Group B, compared with Group A. In a sub-analysis based on prevalence of CVD, treatment with GLY resulted in significant FEV1 improvements independent of CVD prevalence, although values were numerically higher in the CVD group. GLY also led to higher improvements in SGRQ scores in the CVD group. GLY was well tolerated regardless of comorbidity or CVD prevalence, with a lower incidence of serious adverse events compared with placebo.
Conclusion: A simple comorbidity count demonstrated that a majority of patients with COPD in the GOLDEN 3 and 4 studies had multiple comorbidities, with CVD being common in those with high comorbidity count. Results from this post hoc analysis demonstrate that GLY improved FEV1 and SGRQ scores in individuals with COPD, independent of their comorbidities or CVD status.
Keywords: comorbidities, COPD, cardiovascular disease, LAMA, nebulized glycopyrrolate
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive disease characterized by persistent respiratory symptoms and airflow limitation.1 Comorbidities are common in patients with COPD, and can contribute to both severity of symptoms and disease progression.2 Many of these comorbidities have common risk factors (eg, tobacco use)3 and their number often increases with COPD severity.2 Comorbidities can also have an impact on COPD disease progression, exacerbation frequency, quality of life, and mortality;3–5 importantly, comorbidities can influence decisions about COPD management.3 Comorbidities that are most prevalent among patients with COPD are cardiovascular disease (CVD), lung cancer and diabetes.4 Among these comorbidities, CVD is associated with increased risk of mortality.6–8 CVD and COPD are equally prevalent and share some common risk factors (eg, obesity, tobacco use); a meta-analysis of observational studies showed that patients with COPD are 2-fold more likely to have CVD, compared with those without COPD (odds ratio [OR], 2.46 [95% CI: 2.02–3.0]).9 An understanding of the interrelationship between COPD and comorbidities may facilitate better treatment strategies.
Overall comorbidity burden has also been studied in COPD.10–12 Analysis of comorbidity, quantified by a COPD-specific comorbidity count, showed that a higher comorbidity score was associated with a worse St George’s Respiratory Questionnaire (SGRQ) total score, increased risk for exacerbations, and worse dyspnea score.10 Although prevalent in the COPD population, the prevalence of comorbidities is not commonly characterized in randomized clinical trials (RCT), as patients with significant comorbidities are usually excluded from RCTs.13,14 Thus, there are limited data on the impact of comorbidities on treatment outcomes in patients with COPD.
Glycopyrrolate inhalation solution (GLY; Lonhala®, Sunovion Pharmaceuticals Inc., Marlborough, MA, USA) 25 μg twice daily (BID) delivered by the eFlow® Closed System nebulizer (Magnair®, PARI Pharma GmbH, Starnberg, Germany)15 was approved by the US Food and Drug Administration (FDA) for the long-term, maintenance treatment of airflow obstruction in patients with COPD in December 2017.16 This approval was based, in part, on the outcomes from the 12-week, placebo-controlled Glycopyrrolate for Obstructive Lung Disease Via Electronic Nebulizer (GOLDEN) 3 and 4 Phase III studies (NCT02347761 and NCT02347774, respectively).17 In addition, the long-term efficacy and safety of GLY 50 μg were further supported by outcomes of the 52-week GOLDEN 5 Phase III study (NCT02276222).18
The prevalence of comorbidities in the GOLDEN 3 and 4 RCTs, as well as their impact on the efficacy and safety of GLY, were not assessed in the original analysis.17 A secondary analysis of GLY in participants with pre-existing cardiovascular (CV) risk factors showed that GLY had an acceptable safety profile and improved lung function and patient-reported outcomes (PROs), irrespective of CV risk factors.19 In contrast, an analysis of the impact of metabolic syndrome status on participants treated with GLY showed that changes in PROs were only significant in those without metabolic syndrome.20 In this post hoc analysis of pooled data from the 12-week GOLDEN 3 and 4 studies, we assessed the prevalence of comorbidities using the COPD-specific comorbidity score, and the impact of comorbidity burden on the efficacy and safety of 25 μg BID dose of GLY.
Methods
Study Design
Details of the GOLDEN 3 and 4 studies have been previously described (Figure S1).17 Briefly, in the 12-week, multicenter, placebo-controlled, double-blind studies, participants (N=1,293) were randomized in a 1:1:1 ratio to receive placebo or GLY (25 or 50 μg BID), via the eFlow® CS nebulizer. Randomization in each of the studies was stratified by background long-acting β2-agonist (LABA) use (yes/no) and by CV risk (high/low). Ipratropium bromide, as supplemental medication, and albuterol (salbutamol), as rescue medication, were permitted. Data for the GLY 50 μg BID treatment arm are not presented in this post hoc analysis in order to focus on the FDA-approved and clinically relevant GLY 25 μg BID. The data from the 50 μg BID dose was used for identifying the overall prevalence of comorbidities in the study population, but analysis of outcomes was performed using the placebo and GLY 25 μg BID arms; inclusion of the 50 μg BID data does not substantially change the prevalence or distribution of comorbidities.
Participants
Detailed inclusion and exclusion criteria have been published previously.17 Briefly, enrolled participants included males or females ≥40 years of age, current or ex-smokers with ≥10 pack-year smoking history, a clinical diagnosis of moderate-to-very-severe COPD (as defined by the GOLD 2020 Report),1 and qualifying post-bronchodilator (ipratropium 68 μg) spirometry (FEV1 ≤80% of predicted normal, FEV1 >0.7 L, and FEV1/forced vital capacity ratio [FVC] <0.70).17
Statistical Analysis
Pooled data from the GOLDEN 3 and 4 studies were analyzed. Study participants were grouped based on the median number of co-existing comorbidities (Group A: ≤2 comorbidities; Group B: >2 comorbidities), and separately by the cardiovascular disease (CVD) status, with CVD defined as the presence of any of the following preferred terms: coronary heart disease (CHD), congestive heart failure (CHF) or peripheral vascular disease (PVD). This analysis compared GLY and placebo treatment in participants grouped by their baseline comorbidities on the following endpoints: lung function, as assessed by changes from baseline in trough FEV1 at Week 12 and changes from baseline in SGRQ total and domain scores at Week 12. Safety data were analyzed using descriptive statistics;17 adverse events (AEs) and serious adverse events (SAEs) were coded according to MedDRA Version 15.1 and summarized by treatment, system organ class, and preferred term.
Change from baseline in trough FEV1 at Week 12 was analyzed using a mixed model for repeated measures. Changes from baseline in SGRQ total and domain scores at Week 12 were analyzed by analysis of covariance. The proportions of individuals with reduction in SGRQ total score ≥4 units (defined as minimum clinically important differences)21 were analyzed using a logistic regression model. For consistency with the new drug application submitted to the FDA for Lonhala® Magnair®, all models included covariates for baseline level of the appropriate outcome measure, CV risk (high/low), and background LABA use (yes/no). Efficacy analyses used the intent-to-treat (ITT) population and the safety analyses were conducted using the safety population; both populations consisted of all participants randomized to treatment and who received ≥1 dose of study drug. Only data that were measured while on randomized blinded study treatment (ie, on-treatment data) were analyzed. No multiplicity adjustments were made for the comparisons. All statistical procedures were performed using SAS® v9.2 or higher (SAS Institute Inc., Cary, NC) and p-value interpretations were made at the 5% significance level.
Results
Participant Demographics and Baseline Characteristics
Using self-reported or physician-diagnosed health data (Table S1), we identified comorbidities that were most common among participants in the GOLDEN 3 and 4 trials. The prevalence of comorbidities in the GOLDEN 3 and 4 trials were generally similar to those observed in the COPDGene® and SPIROMICS studies (Table S2).10–12 However, compared with COPDGene® and SPIROMICS, a higher prevalence of hypertension and osteoarthritis was observed in the GOLDEN 3 and 4 population. When assessing baseline characteristics among participants in the GOLDEN 3 and 4 trials with specific comorbidities (Table S3), individuals with depression and anxiety had a lower median age (~61 years), whereas those with osteoporosis had the oldest median age (68 years). Participants with CHD and obstructive sleep apnea (OSA) included a greater proportion of males (65.3–66.9%), whereas those with osteoporosis, depression, and anxiety were predominantly female (60.5–84.0%). Participants with any of obesity, OSA, diabetes, or CHF as a comorbidity had the highest BMIs (32.2–35.9 kg/m2).
Pooled data from participants (N=1,293) in GOLDEN 3 and 4, were grouped based on the number of comorbidities (Figure S2), with 2 comorbidities considered as a median cut-off between the groups. The 14 comorbidities shown in Table 1 were used for grouping individuals into Group A (≤2 comorbidities; N=439) and Group B (>2 comorbidities; N=854).
 |
Table 1 Prevalence of Comorbidities in the Pooled Patient Population from GOLDEN 3 and 4 |
Baseline demographics and disease characteristics are presented in Table 2. Participants in Groups A and B had similar median age and the majority were males, although the proportion of males was greater in Group A. The proportion of current smokers was higher in Group A compared with Group B, although the number of pack-years was similar between the two groups.
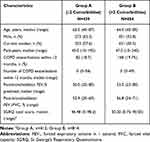 |
Table 2 Demographics and Baseline Characteristics for the Baseline Comorbidity Prevalence Groups |
Baseline lung function was similar between the two comorbidity prevalence groups (Table 2) and across treatment groups (Table 3), whereas baseline SGRQ total and domain scores were higher in Group B compared with Group A, indicating worse health status among participants with high number of comorbidities (Table 2). Baseline SGRQ were higher in patients receiving GLY compared with placebo in both comorbidity groups (Table 3).
 |
Table 3 Baseline FEV1, FVC, SGRQ Total and Domain Scores in the Comorbidity Groups, by Treatment |
Cardiovascular Comorbidities
We observed a higher prevalence of CVD comorbidities (CHD, CHF and PVD), among participants with >2 comorbidities compared with those with ≤2 comorbidities (Table 1). To understand the impact of CVD comorbidities on the efficacy of GLY 25 μg BID in participants with moderate-to-very-severe COPD, we performed a sub-analysis using pooled data (N=861) grouped by baseline CVD status (Table 4; Figure S2).
 |
Table 4 Participant Distribution by Baseline CVD Status |
Both non-CVD and CVD groups were generally similar in age and BMI. However, there were more males in the CVD group compared with the non-CVD group (Table 5). The percentage of current smokers was lower in the CVD group compared with the non-CVD group; however, pack-years were similar across groups. Baseline SGRQ scores were highest in participants receiving GLY in the CVD group but were similar between treatments in the non-CVD group.
 |
Table 5 Baseline Demographics and Disease Characteristics by CVD Status |
Concomitant medication use was higher among individuals in the CVD group compared with those in the non-CVD group (Table S4), particularly the use of acetylsalicylic acid (aspirin; CVD: 64.7% vs non-CVD: 22.1%), ACE inhibitors (CVD: 37.6% vs non-CVD: 21.2%) and β-blocking agents (CVD: 45.9% vs non-CVD: 12.3%).
Efficacy
FEV1 in Overall Comorbidity Prevalence Groups
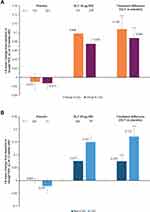
At 12 weeks, treatment with GLY 25 μg BID resulted in clinically significant improvements from baseline in trough FEV1 compared with placebo in both comorbidity groups. The placebo-adjusted least squares (LS) mean change from baseline in trough FEV1 were 108 mL and 88 mL in Group A and Group B, respectively (p<0.0001 vs placebo for both groups; Figure 1A).
FEV1 by CVD Prevalence
Treatment with GLY 25 μg BID resulted in significant improvements from baseline in trough FEV1 compared with placebo in both the CVD and non-CVD groups (Figure 1B). The placebo-adjusted change from baseline was numerically greater in the CVD group compared with the non-CVD group (172 mL vs 76 mL, respectively; p<0.0001 vs placebo for both groups).
In the subgroup with CHD, the placebo-adjusted LS mean change from baseline in trough FEV1 was 173.2 mL (p<0.0001 vs placebo; Table S5). While improvements in FEV1 were also observed with GLY 25 μg among participants with CHF and PVD, fewer patients were represented in the analysis of these categories.
SGRQ Total Score and Responders
Placebo-adjusted improvements from baseline in SGRQ total score with GLY 25 μg BID at 12 weeks were significant in both comorbidity prevalence groups (Figure 2A). This pattern was consistent across all of the SGRQ domain scores (activity, symptoms and impacts) in both groups; the greatest differences between comorbidity prevalence groups were observed in the symptoms and impacts domain scores, both of which showed numerically greater improvements from baseline in group A compared with group B. The odds of being an SGRQ responder (≥4-unit reduction)21 were significantly greater with GLY 25 μg BID treatment compared with placebo in either comorbidity prevalence group (Figure 3A).
At 12 weeks, GLY treatment resulted in significant improvements from baseline in SGRQ total scores compared with placebo, regardless of baseline CVD status (Figure 2B), although the improvements were numerically greater in the CVD group. This pattern was consistent across all SGRQ domain scores in both groups, with greatest improvements observed in the symptoms domain. The odds of being an SGRQ responder were significantly greater with GLY 25 μg BID treatment compared with placebo in both CVD and non-CVD groups (CVD: p<0.01; non-CVD: p<0.05; Figure 3B).
In the subgroup of participants who had comorbid CHD (placebo, n=64; GLY, n=62), we observed a significant improvement in SGRQ total scores with GLY 25 µg BID (Table S5). The placebo-adjusted LS mean difference (SE) for the CHD group was –4.27 (1.85; p=0.02 vs placebo). The odds of being an SGRQ responder were significantly greater with GLY 25 μg BID treatment compared with placebo in patients with CHD (OR [95% CI]: 2.95 [1.27, 6.89], p=0.012). Similar results were observed in the subgroup of participants with comorbid CHF (placebo, n=19; GLY, n=10), where GLY 25 µg BID resulted in significant improvements in total SGRQ scores: placebo-adjusted LS mean difference (SE) was –11.06 (5.41; p=0.048 vs placebo). The odds of being an SGRQ responder were significantly greater with GLY 25 μg BID treatment compared with placebo in patients with CHF (OR [95% CI]: 12.57 [1.64, 96.5], p=0.015).
Safety
Comorbidity Prevalence Groups
GLY was generally well tolerated regardless of comorbidity prevalence (Table 6). Overall incidence of AEs was similar across treatments and comorbidity prevalence groups; incidence of SAEs was greater among participants in the high comorbidity prevalence group (Group B), compared with the low comorbidity prevalence group (Group A). The most common AEs across treatment groups were worsening of COPD, cough, and dyspnea; incidences of these AEs were generally similar across the comorbidity prevalence groups. In the low comorbidity prevalence group (Group A), cardiac disorders were observed in 1 patient (0.6%) receiving GLY (2 events, 1 case each of unstable angina and coronary artery stenosis) and 2 patients (1.4%) receiving placebo (3 events; 1 case each of sinus tachycardia, atrial flutter, and cardiomyopathy). In the high comorbidity prevalence group (Group B), cardiac disorders were observed in 5 patients (1.8%) receiving GLY (5 events; 3 cases of angina pectoris, and 1 case each of coronary artery disease and palpitations) and 8 patients (2.7%) receiving placebo (9 events; 2 cases of atrial fibrillation and 1 case each of the following: unstable angina, coronary artery disease, palpitations, acute myocardial infarction, cardiomegaly, tachycardia, and ventricular tachycardia).
 |
Table 6 Summary of AEs and SAEs, Including Individual AEs with Incidence ≥3% in Any Treatment Group, by Baseline Comorbidity Prevalence Group (Safety Population) |
CVD Subgroups
Overall, GLY was generally well tolerated regardless of CVD status and incidence of all AEs and SAEs was lower with GLY treatment compared with placebo (Table 7). The most common AEs across treatment groups were cough, worsening of COPD and dyspnea (all <10% across CVD treatment groups). In the CVD group, cardiac disorders were observed in 2 patients (2.6%) receiving GLY (2 events; both angina pectoris), and 5 patients (5.4%) receiving placebo (5 events; 1 case each of the following: unstable angina, atrial fibrillation, cardiomegaly, coronary artery disease, and tachycardia). In the non-CVD group, cardiac disorders were observed in 4 patients (1.1%) receiving GLY (5 events; 1 case each of the following: angina pectoris, unstable angina, coronary artery disease, coronary artery stenosis, and palpitations) and 5 patients (1.5%) receiving placebo (7 events; 1 case each of the following: atrial flutter, palpitations, sinus tachycardia, acute myocardial infarction, atrial fibrillation, cardiomyopathy, and ventricular tachycardia).
 |
Table 7 Summary of AEs and SAEs, Including Individual AEs with Incidence ≥3% in Any Treatment Group, by Baseline CVD (Safety Population) |
Incidence of cardiovascular and major adverse cardiac events was low (<5%) in both CVD and non-CVD subgroups (Table 8). There were only 2 MACE recorded, both of which were non-fatal myocardial infarction and occurred in the placebo arm among non-CVD patients.
 |
Table 8 Summary of Cardiovascular AESIs and MACE in Any Treatment Group, by Baseline CVD (Safety Population) |
Discussion
This post hoc analysis of pooled data from the GOLDEN 3 and 4 trials demonstrated a high prevalence of comorbidities, especially CVD, among individuals with moderate-to-very-severe COPD. Despite the high prevalence of comorbidities and CVD in this population, significant improvements in lung function and SGRQ total scores were observed with GLY 25 μg BID compared with placebo. Further, the odds of being an SGRQ responder were significantly higher with GLY 25 μg BID compared with placebo, regardless of comorbidity or CVD status at baseline. Treatment was well tolerated, with a similar incidence of AEs and SAEs with GLY or placebo, regardless of comorbidity burden.
COPD is a complex, heterogeneous, and multicomponent disorder, and disease severity can be further exacerbated by the presence of coexisting conditions.4,22 Comorbidities are frequent in patients with COPD, and affect overall prognosis and treatment options.3 An analysis of data from 20,296 patients with COPD showed that patients at GOLD stages 3 and 4 were more likely to have 1–3 comorbidities compared with patients with normal lung function (GOLD stage 0).8 Data from large clinical trials such as TORCH and UPLIFT have shown that comorbidities such as CVD and lung cancer are common contributors to mortality among patients with COPD.4,23,24 Similarly, a longitudinal, observational study of 1,664 patients with COPD identified 79 comorbidities, 12 of which (including lung cancer and CVD) were associated with an increased risk of death. In this observational study, the average number of comorbidities were ~6 per patient and tended to be significantly higher among non-survivors, compared with survivors.4 In addition, studies have shown that lung function impairments are associated with higher risk of comorbidities, resulting in higher mortality and hospitalization.8 In spite of this evidence, assessment and consideration of comorbidities in RCTs is limited. This may be, in part, due to the methods of identifying and categorizing comorbidities, as well as stringent recruitment criteria which may exclude patients with comorbidities. Some studies have used cluster analysis to analyze comorbidities; such analyses have been informative,2,22,25 but these may not be feasible for smaller studies and lack broad applicability.
A simple comorbidity count method was previously developed to identify and characterize comorbidities in COPD; analysis of two large COPD cohorts, COPDGene® and SPIROMICS using this method showed that determining a comorbidity score could provide insight into clinical trial readouts including SGRQ total score, exacerbation risk, and dyspnea score.10 The use of this simple comorbidity count10 with the pooled population from the GOLDEN 3 and 4 clinical trials showed similarities in the prevalence of comorbidities among patients with COPD between the GOLDEN trial, COPDGene®, and SPRIOMICS cohorts. Not surprisingly, the existence of a greater number of comorbidities (ie, a higher comorbidity score) was associated with a higher prevalence of CVD. Additionally, demographic characteristics of study participants were generally similar following grouping based on comorbidity count (Table S6). Across all three cohorts, patients with >2 comorbidities tended to have higher pack-years and were clinically obese, compared with their counterparts in the ≤2 group. Despite similar lung function across cohorts, patients from the GOLDEN trials had a higher proportion of current smokers and higher baseline SGRQ scores, reflective of worse disease in this population; a higher prevalence of anxiety, depression and insomnia was observed in the GOLDEN trials. These results show that the comorbidity burden observed in the GOLDEN trials was generally representative of that demonstrated in large epidemiologic COPD cohort studies.
Treatment with GLY resulted in clinically significant improvements in FEV1 independent of baseline comorbidity or CVD prevalence. Of note, improvements in FEV1 from baseline in patients with CVD were numerically greater than those observed in non-CVD patients, with ~100 mL difference between the two subgroups, despite similar FEV1 at baseline. Additional analysis of potential factors associated with CVD and lung function are needed to understand the differences in FEV1 improvements observed between the CVD and non-CVD groups. Improvements in cardiac function and output, and pulmonary microvascular blood flow have been observed with bronchodilators in a few smaller studies of patients with COPD.26–28 Bronchodilators can also reduce pulmonary hyperinflation, as demonstrated in several clinical studies of COPD.29,30 Changes in hyperinflation after administration of tiotropium (TIO; a long-acting muscarinic antagonist [LAMA]) or budesonide/formoterol (inhaled corticosteroid/LABA) were measured in 20 patients with stable COPD; while improvements in spirometry and hyperinflation were observed in both treatment groups, the impact of TIO on hyperinflation was greater, compared with budesonide/formoterol.30 Treatment with 150 µg indacaterol (a LABA) in 40 patients with stable COPD showed significant reductions in hyperinflation in acute conditions, compared with placebo; further, this was associated with improvements in cardiac function.31 Intermediate cardiac endpoints and hyperinflation were not analyzed in the current study; it is likely that GLY-mediated improvements in FEV1 in the CVD group could also improve cardiac function and hyperinflation, and future studies could explore this as a powered endpoint.
Presence of comorbidities may contribute to variability in SGRQ responses with GLY 25 µg BID. A recent post hoc analysis of pooled data from the GOLDEN trials demonstrated differential treatment effects by presence of metabolic syndrome at baseline.20 While changes from baseline in SGRQ scores were significantly improved compared with placebo regardless of their baseline comorbidity or CVD status, the magnitude of changes in SGRQ total and domain scores was greater in individuals with low comorbidity count and in patients with CVD. These differences are not likely to be driven by COPD severity, as the baseline lung function was similar between comorbidity and CVD groups. The consistently significant improvements in SGRQ with the GLY 25 μg BID dose are encouraging, confirming the positive changes in PROs observed in the overall population,17 and support the use of this treatment in patients, regardless of the presence of comorbidities or CVD.
The safety profile of GLY 25 μg BID was consistent in both comorbidity and CVD prevalence groups. In addition, the incidence of SAEs was lower with GLY compared with placebo in both comorbidity prevalence groups. These results further demonstrate the safety of GLY in patients with COPD, irrespective of presence of comorbidities. However, previous studies had shown a correlation between presence of comorbidities or CVD and increased patient mortality;4,6 the current analysis is limited and cannot assess the impact of comorbidities on patient mortality in the GOLDEN 3 and 4 studies due to the short, 12-week study duration and the limited 30-day safety follow-up period. A previous analysis of the 48-week GOLDEN 5 study showed that GLY 50 μg BID did not display differences in CV-related mortality between patients with low or high CV risk factors.19 Additional, long-term clinical trials or real-world data assessing the impact of GLY on mortality in patients with COPD and comorbidities are needed.
This study is limited by the post hoc nature of the patient stratification in this analysis and the lack of adjustment for multiplicity. The differences in baseline demographics and disease characteristics may have contributed to some of the observed differences in treatment responses. In addition, the recruitment criteria of the two trials led to the exclusion of patients with severe comorbidities, including unstable cardiac or pulmonary disease;17 therefore, the GOLDEN cohort may not be an accurate representation of the real-world COPD population. As such, clinical trials with less restrictive recruitment criteria, particularly in the context of comorbidities, and real-world studies are necessary to complement existing clinical trials. This would allow for a comprehensive understanding of the impact of comorbidities on treatment efficacy and safety in patients with COPD.
Conclusions
In this pooled analysis of the GOLDEN 3 and 4 studies, use of a simple comorbidity count highlighted the prevalence of comorbidities, particularly CVD, among patients with COPD in clinical trials. Treatment with GLY 25 µg BID resulted in lung function and PRO improvements independent of the comorbidity and CVD prevalence, both in terms of FEV1 and SGRQ total scores, respectively. Interestingly, improvements in FEV1 were larger in CVD compared with non-CVD patients, which warrants further analysis. Importantly, GLY was well tolerated with no differences in safety outcomes in either comorbidity or CVD prevalence group. These findings represent important clinical phenotypes in the COPD population and highlight the importance of comorbidities in the proper management of patients with COPD.
Data Sharing Statement
Sunovion Pharmaceuticals Inc. is part of a clinical trial data sharing consortium that facilitates access for qualified researchers to selected anonymized clinical trial data. For up-to-date information on data availability please visit https://www.clinicalstudydatarequest.com/Study-Sponsors.aspx and click on Sunovion.
Ethics Statement
The GOLDEN 3 (SUN101–301: project approval number 28481) and GOLDEN 4 (SUN101–302: project approval number 28482) study protocols were approved by Quorum Review IRB North American (US and Canadian) Board (Panel II) prior to patient enrollment and were conducted in accordance with the protocols, International Council for Harmonization Good Clinical Practice guidelines, and the Declaration of Helsinki. All patients provided written informed consent.
Acknowledgments
This post hoc analysis was funded by Sunovion Pharmaceuticals Inc. The authors would like to thank Shane Hornibrook from Sarepta Therapeutics, Inc., Diane Hall from Sunovion Pharmaceuticals Inc., and Rajeshwari Sammishetty from Sage Therapeutics, Inc. for support with statistical analyses performed. Medical writing support was provided by Dhivya Ramalingam, PhD and Hashem Dbouk, PhD of Ashfield MedComms, an Ashfield Health company, and funded by Sunovion Pharmaceuticals Inc. Parts of the data shown in this paper were presented at the American Thoracic Society 2019 International Conference as a poster with interim findings. The poster’s abstract was published in the “Abstracts Issue” of American Journal of Respiratory and Critical Care Medicine: https://doi.org/10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A3871.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, took part in drafting the article or revising it critically for important intellectual content, agreed to submit to the current journal; gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
NP reports grants from NIH K award, during the conduct of the study. AOG was an employee of Sunovion Pharmaceuticals Inc at the time of the study and is currently an employee of Alexion Pharmaceuticals. SSa and SSh are employees of Sunovion Pharmaceuticals Inc. The authors report no other conflicts of interest in this work.
References
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD; 2020. Available from: http://goldcopd.org/.
2. Raherison C, Ouaalaya EH, Bernady A, et al. Comorbidities and COPD severity in a clinic-based cohort. BMC Pulm Med. 2018;18(1):117. doi:10.1186/s12890-018-0684-7
3. Brown JP, Martinez CH. Chronic obstructive pulmonary disease comorbidities. Curr Opin Pulm Med. 2016;22(2):113–118. doi:10.1097/MCP.0000000000000241
4. Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–161. doi:10.1164/rccm.201201-0034OC
5. Holguin F, Folch E, Redd SC, Mannino DM. Comorbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001. Chest. 2005;128(4):2005–2011. doi:10.1378/chest.128.4.2005
6. Morgan AD, Zakeri R, Quint JK. Defining the relationship between COPD and CVD: what are the implications for clinical practice? Ther Adv Respir Dis. 2018;12:1753465817750524. doi:10.1177/1753465817750524
7. Rabe KF, Hurst JR, Suissa S. Cardiovascular disease and COPD: dangerous liaisons? Eur Respir Rev. 2018;27(149):180057. doi:10.1183/16000617.0057-2018
8. Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962–969. doi:10.1183/09031936.00012408
9. Chen W, Thomas J, Sadatsafavi M, FitzGerald JM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(8):631–639. doi:10.1016/S2213-2600(15)00241-6
10. Putcha N, Puhan MA, Drummond MB, et al. A simplified score to quantify comorbidity in COPD. PLoS One. 2014;9(12):e114438. doi:10.1371/journal.pone.0114438
11. Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. doi:10.3109/15412550903499522
12. Couper D, LaVange LM, Han M, et al. Design of the subpopulations and intermediate outcomes in COPD Study (SPIROMICS). Thorax. 2014;69(5):491–494. doi:10.1136/thoraxjnl-2013-203897
13. Lahousse L, Verhamme KM, Stricker BH, Brusselle GG. Cardiac effects of current treatments of chronic obstructive pulmonary disease. Lancet Respir Med. 2016;4(2):149–164. doi:10.1016/S2213-2600(15)00518-4
14. Tashkin DP, Amin AN, Kerwin EM. Comparing randomized controlled trials and real-world studies in chronic obstructive pulmonary disease pharmacotherapy. Int J Chron Obstruct Pulmon Dis. 2020;15:1225–1243. doi:10.2147/COPD.S244942
15. Pham S, Ferguson GT, Kerwin E, Goodin T, Wheeler A, Bauer A. In vitro characterization of the eFlow® closed system nebulizer with glycopyrrolate inhalation solution. J Aerosol Med Pulm Drug Deliv. 2018;31(3):162–169. doi:10.1089/jamp.2017.1384
16. Sunovion Pharmaceuticals Inc. Lonhala magnair (glycopyrrolate) inhalation solution: highlights of prescribing information; 2019.
17. Kerwin E, Donohue JF, Goodin T, Tosiello R, Wheeler A, Ferguson GT. Efficacy and safety of glycopyrrolate/eFlow® CS (nebulized glycopyrrolate) in moderate-to-very-severe COPD: results from the glycopyrrolate for obstructive lung disease via electronic nebulizer (GOLDEN) 3 and 4 randomized controlled trials. Respir Med. 2017;132:238–250. doi:10.1016/j.rmed.2017.07.011
18. Ferguson GT, Goodin T, Tosiello R, Wheeler A, Kerwin E. Long-term safety of glycopyrrolate/eFlow® CS in moderate-to-very-severe COPD: results from the Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer (GOLDEN) 5 randomized study. Respir Med. 2017;132:251–260. doi:10.1016/j.rmed.2017.08.020
19. Ferguson GT, Tosiello R, Sanjar S, Goodin T. Efficacy and safety of nebulized glycopyrrolate/eFlow® closed system in patients with moderate-to-very-severe chronic obstructive pulmonary disease with pre-existing cardiovascular risk factors. Chronic Obstr Pulm Dis. 2018;6(1):86–99. doi:10.15326/jcopdf.6.1.2018.0146
20. Carlin B, Ferguson GT, Ozol-Godfrey A, Goodin T, Sanjar S. The effect of metabolic syndrome status on lung function and patient-reported outcomes in patients with COPD receiving nebulized glycopyrrolate. Chronic Obstr Pulm Dis. 2020;7(4):315–326. doi:10.15326/jcopdf.7.4.2020.0145
21. Jones PW. St. George’s respiratory questionnaire: MCID. COPD. 2005;2(1):75–79. doi:10.1081/copd-200050513
22. Vanfleteren LE, Spruit MA, Groenen M, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(7):728–735. doi:10.1164/rccm.201209-1665OC
23. McGarvey LP, John M, Anderson JA, Zvarich M, Wise RA. Ascertainment of cause-specific mortality in COPD: operations of the TORCH clinical endpoint committee. Thorax. 2007;62(5):411–415. doi:10.1136/thx.2006.072348
24. Berry CE, Wise RA. Mortality in COPD: causes, risk factors, and prevention. COPD. 2010;7(5):375–382. doi:10.3109/15412555.2010.510160
25. Tiew PY, Ko FWS, Narayana JK, et al. “High-Risk” clinical and inflammatory clusters in COPD of Chinese descent. Chest. 2020;158(1):145–156. doi:10.1016/j.chest.2020.01.043
26. Vogel-Claussen J, Schonfeld CO, Kaireit TF, et al. Effect of indacaterol/glycopyrronium on pulmonary perfusion and ventilation in hyperinflated patients with Chronic Obstructive Pulmonary Disease (CLAIM). A Double-Blind, Randomized, Crossover Trial. Am J Respir Crit Care Med. 2019;199(9):1086–1096. doi:10.1164/rccm.201805-0995OC
27. Hohlfeld JM, Vogel-Claussen J, Biller H, et al. Effect of lung deflation with indacaterol plus glycopyrronium on ventricular filling in patients with hyperinflation and COPD (CLAIM): a double-blind, randomised, crossover, placebo-controlled, single-centre trial. Lancet Respir Med. 2018;6(5):368–378. doi:10.1016/S2213-2600(18)30054-7
28. Stone IS, Barnes NC, James WY, et al. Lung deflation and cardiovascular structure and function in chronic obstructive pulmonary disease. A Randomized Controlled Trial. Am J Respir Crit Care Med. 2016;193(7):717–726. doi:10.1164/rccm.201508-1647OC
29. O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832–840. doi:10.1183/09031936.04.00116004
30. Santus P, Centanni S, Verga M, Di Marco F, Matera MG, Cazzola M. Comparison of the acute effect of tiotropium versus a combination therapy with single inhaler budesonide/formoterol on the degree of resting pulmonary hyperinflation. Respir Med. 2006;100(7):1277–1281. doi:10.1016/j.rmed.2005.10.008
31. Santus P, Radovanovic D, Di Marco S, et al. Effect of indacaterol on lung deflation improves cardiac performance in hyperinflated COPD patients: an interventional, randomized, double-blind clinical trial. Int J Chron Obstruct Pulmon Dis. 2015;11(10):1917–1923. doi:10.2147/COPD.S91684
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.