Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Impact of Coexisting Dementia on Inpatient Outcomes for Patients Admitted with a COPD Exacerbation
Authors Gupta A, McKeever TM , Hutchinson JP, Bolton CE
Received 25 October 2021
Accepted for publication 3 February 2022
Published 10 March 2022 Volume 2022:17 Pages 535—544
DOI https://doi.org/10.2147/COPD.S345751
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Ayushman Gupta,1 Tricia M McKeever,1,2 John P Hutchinson,3 Charlotte E Bolton1
1NIHR Nottingham BRC Respiratory Theme, Translational Medical Sciences, School of Medicine, University of Nottingham, Nottingham, UK; 2Division of Epidemiology and Public Health, School of Medicine, University of Nottingham, Nottingham, UK; 3Respiratory Medicine, Sherwood Forest Hospitals NHS Foundation Trust, Sutton-in-Ashfield, UK
Correspondence: Charlotte E Bolton, B22, NIHR Nottingham BRC respiratory theme, Translational Medical Sciences, School of Medicine, University of Nottingham, Clinical Sciences Building, City Hospital Campus, Hucknall road, Nottingham, NG5 1PB, UK, Email [email protected]
Purpose: People with COPD are at a higher risk of cognitive dysfunction than the general population. However, the additional impact of dementia amongst such patients is not well understood, particularly in those admitted with a COPD exacerbation. We assessed the impact of coexisting dementia on inpatient mortality and length of stay (LOS) in patients admitted to hospital with a COPD exacerbation, using the United States based National Inpatient Sample database.
Patients and Methods: Patients aged over 40 years and hospitalised with a primary diagnosis of COPD exacerbation from 2011 to 2015 were included. Cases were grouped into patients with and without dementia. Multivariable logistic regression analysis, stratified by age, was used to assess risk of inpatient deaths. Cox regression was carried out to compare death rates and competing risk analysis gave estimates of discharge rates with time to death a competing variable.
Results: A total of 576,381 patients were included into the analysis, of which 35,372 (6.1%) had co-existent dementia. There were 6413 (1.1%) deaths recorded. The odds of inpatient death were significantly greater in younger patients with dementia (41– 64 years) [OR (95% CI) dementia vs without: 1.75 (1.04– 2.92), p=0.03]. Cases with dementia also had a higher inpatient mortality rate in the first 4 days [HR (95% CI) dementia vs without: 1.23 (1.08– 1.41), p=0.002] and a longer LOS [sub-hazard ratio (95% CI) dementia vs without: 0.93 (0.92– 0.94), p< 0.001].
Conclusion: Dementia as a comorbidity is associated with worse outcomes based on inpatient deaths and LOS in patients admitted with COPD exacerbations.
Keywords: chronic obstructive pulmonary disease, exacerbation, morbidity, length of stay
Plain Language Summary
People with COPD, a smoking related lung disease, are more at risk of developing dementia, but not much is known about what effects this has in such people. We looked to assess the impacts of dementia in people who were admitted to hospital with a flare up of COPD. Patients admitted with a flare-up who also had dementia were more at risk of death and had a longer length of stay when compared to those without dementia. This is an important finding, as it highlights there is a need to recognise this at-risk group of patients and possibly develop a more tailored care plan.
Introduction
Chronic obstructive pulmonary disease (COPD) is a complex, multisystem disorder that is associated with an increasing morbidity and mortality.1 It is clear that comorbidities in COPD contribute to the disease burden.2 Therefore, systematic research into the effects of co-existing conditions in COPD is of great importance, particularly as it may help guide long term management.
Cognitive impairment is a well-documented comorbid state for those with COPD. However, its impact on patients with COPD is less clear, with only a few studies having directly examined this in patients with COPD and the association reported has been inconsistent.3–6 One possibility is that cognitive impairment in such patients is often reported to be mild or subclinical and so may not contribute to the overall disease pathology.7 However, there is evidence to suggest that the presence of altered cognition impairs important aspects of disease management that in turn may result in a worse health related outcome. The management of COPD often requires individuals to learn new skills such as inhaler techniques, as well as to organize, and execute essential self-care functions.8,9 Inability to carry out these functions can result in poor adherence to medications. Cleutjens et al demonstrated patients with COPD and cognitive dysfunction had a worse dropout rate from pulmonary rehabilitation programmes compared to controls, attributing this to frailty and a limited ability to plan and execute complex functions.10
Whilst literature reporting potentially negative impacts of altered cognition in COPD exists, studies on the direct effects of dementia as a comorbidity on morbidity and mortality are lacking. Further, to our knowledge no study assessing the effects in patients with an exacerbation of COPD exist. This study assesses the impact of coexisting dementia on hospital related outcomes, such as inpatient mortality and length of stay in patients admitted with a COPD related exacerbation, using a United States (US) based national inpatient dataset.
Materials and Methods
National Inpatient Sample Database
Hospital discharge records from 2011–2015 of the National Inpatient Sample (NIS) database, part of the Healthcare Cost and Utilization Project (HCUP), were accessed to carry out retrospective analysis. The NIS is maintained by the Agency for Healthcare Research and Quality and is a large all-payer inpatient healthcare database in the United States of America (USA), consisting of records on inpatient utilisation, charges, quality, and outcomes.11 The data are collected through a multi-staged, clustered, stratified sampling method. In 2011, all discharge records from 20% of sampled hospitals were obtained and from 2012 onwards, 20% of discharge records from all US hospitals in the HCUP universe were retained. Individual records represent discharges from hospital and the lack of unique patient identifiable details mean that each inpatient admission (including readmissions) is regarded as a separate record. Each discharge record comprises up to 30 diagnoses and 15 procedures, all coded using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) for 2011–2015 (1st-3rd yearly quarters) or ICD-10-CM for 2015 (4th yearly quarter). On October 1, 2015, hospital administrative data began using ICD-10-CM codes, which is why the database contains both coding systems. The NIS data are entirely de-identified, making no Institutional Review Board approval necessary for this study. However, permission for access to the database is granted once the user(s) sign a data-use agreement and complete an online training module on data protection.
Study Population
Patients aged over 40 years who were admitted to hospital between January 1, 2011 and December 31, 2015, with a primary diagnosis of acute exacerbation of COPD (ICD-9-CM codes 490, 491.21 and 491.22 and ICD-10-CM codes J44.0, J44.1 and J44.9) were included. The ICD classification system has been validated and previously used in identification of cases with acute exacerbations of COPD.12–15 From this cohort, patients were categorised into those with a co-existent diagnosis of dementia (ICD-9-CM codes 290, 294.1–294.9, 331 and 797 and ICD-10-CM codes G30-31, F015 and F039) and without. Given the pattern of dementia associated with COPD is heterogenous, a wide range of dementia diagnosis codes were included.
Study Parameters
Outcome measures analysed included length of stay (LOS), calculated by subtracting the admission date from the discharge date, and inpatient deaths. Covariates included age, gender, comorbidity, race, year of admission and expected primary payer. Comorbidity was assessed using the updated Charlson comorbidity index, which derives scores based on the presence of the following conditions: COPD, dementia, rheumatological disease, renal disease, diabetes, congestive cardiac failure, liver disease, hemiplegia/paraplegia, malignancy, AIDS/HIV or metastatic solid tumour.16,17 Scores were derived from secondary ICD-9-CM and ICD-10-CM diagnostic codes and matched according to published algorithm.18 The Charlson comorbidity index was then modified to exclude COPD and dementia diagnoses to avoid over adjusting when adjusting for comorbidity as a covariate.
Other variables forming part of the baseline patient characteristics included use of non-invasive ventilation (ICD-9-CM codes 93.9 and ICD-10-CM codes 5A09357, 5A09358, 5A09457, 5A09458, 5A09557 and 5A09558) and mechanical ventilation (ICD-9-CM codes 96.7 and ICD-10-CM codes 5A0935Z, 5A0945Z, 5A0955Z, 0BH17EZ, 0BH18EZ and 0BH13EZ) carried out following respiratory compromise (respiratory failure, insufficiency or arrest) (ICD-9-CM codes 518.81, 518.82 and 518.84 and ICD-10-CM codes J96.0, 96.2, J96.9, R09.2 and J80) during inpatient stay.
Statistical Analysis
All statistical analyses were conducted using Stata SE V.15. Continuous variables were represented as median and interquartile range (IQR) and categorical data as frequency and percentages. Mann–Whitney–Wilcoxon test was used to compare continuous variables and chi squared for categorical variables.
Logistic regression analysis was carried out to assess inpatient deaths in patients with an exacerbation who had co-existing dementia compared to those without dementia. Cox proportional hazard regression analysis was used to assess the impact of cognitive impairment on inpatient death rates, with inpatient stay as the time variable (days). Participants were also censored at discharge or if the information was unknown. The Schoenfeld test was used to test for violation of the proportional hazard assumption, with the group being stratified by time points where the hazard rates were proportional, if the assumption was violated.
Competing risk analysis was used to determine differences in length of stay in patients with and without dementia, with inpatient discharge rate considered as the primary event and patients deceased a competing variable, using Fine and Gray regression model.19 The Fine and Gray model allows estimation of the effect of covariates on the incidence of an event (ie cumulative incidence function) whilst accounting for competing risks, reporting sub-distribution hazard ratios. For analysis in this study, it gave an indication of length of stay, ie lower discharge rates (lower sub-distribution hazards) would be in keeping with a longer hospital stay.
Age, gender, modified Charlson comorbidity index, year of admission, race and primary payer were considered a priori confounders and were included in the regression analysis model. Interactions between variables and outcome measures were evaluated using likelihood ratio tests and models with statistically significant interactions were further estimated in strata. Results from the different models were represented as odds ratios (OR), proportional hazard ratios or sub-distribution hazard ratios. P<0.05 was considered statistically significant. Only discharge records with complete datasets were included in the analysis, ie records with missing values were excluded.
Results
Baseline Characteristics
A total of 576,381 patients with a primary discharge diagnosis of COPD exacerbation were identified between 2011 and 2015, after excluding patients who were 40 years old or younger and with missing values (Figure 1).
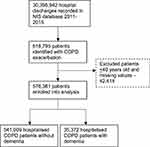 |
Figure 1 Flow chart of patients included in the study. |
The baseline characteristics are shown in Table 1. The prevalence of diagnosed dementia in admitted patients with COPD exacerbations was 6.1%. The median (IQR) age for COPD patients with dementia was 82 (76–87) years vs 68 (59–77) years for those without. Patients with COPD and dementia had a higher burden of comorbidities. Fewer patients with coexisting dementia had reported respiratory compromise but of those that did, there was no difference in receiving NIV or mechanical ventilation.
 |
Table 1 Baseline Characteristics for Patients Admitted with a COPD Exacerbation with and without Dementia |
Inpatient Deaths
A total number of 6413 (1.1%) inpatient deaths in patients with COPD occurred between 2011 and 2015. The proportion of inpatient deaths were significantly greater in patients with co-existing dementia compared to those without [n (%) COPD with dementia vs COPD without dementia: 581 (1.6) vs 5832 (1.1); p<0.001]. There was a significant interaction with age (p=0.02 likelihood ratio test of interaction) and the odds of death were therefore stratified according to age (Table 2). Patients with COPD and dementia in the youngest age category (41–64 years) had a significantly increased risk of inpatient death compared to patients without (Adjusted OR 1.75 95% CI 1.04 to 2.92). However, there were no significant differences observed in the older age strata.
 |
Table 2 Inpatient Deaths Stratified by Age for Patients with and without Dementia |
Inpatient Death Rates
The death rates are shown in Table 3 stratified by 2 time points (<5 days and 5–100 days), where the hazard rates were proportional (Schoenfeld test <5 days p=0.53; 5–100 days p=0.09). Patients with COPD and dementia who were admitted with an exacerbation had a significantly greater death rate compared to patients without dementia within the first 4 days of admission. Furthermore, from 5 days onwards the mortality rate was lower, indicating that those patients with dementia that had survived the initial few days of admission were less likely to die during the rest of their inpatient stay. Age as a covariate did not interact with death rates (p=0.53).
 |
Table 3 Proportional Hazard Ratios of Death Rates Stratified by Inpatient Length of Stay for Patients with and without Dementia |
Length of Stay
The median LOS for patients with COPD and dementia was longer than those without dementia [median (IQR) LOS with dementia vs without dementia:4 (3–6) vs 3; (2–5) p<0.001]. The discharge rate was also significantly lower for patients with co-existent dementia, as indicated by the sub-distribution hazard ratio (SHR), therefore demonstrating a longer length of stay (Table 4).
 |
Table 4 Sub-Distribution Hazard Ratios of Inpatient Discharges for Patients with and without Dementia |
Discussion
COPD is frequently associated with comorbidities that adversely contribute to the disease burden and symptomatology. Understanding the effects of such co-existing conditions is imperative to better characterise the full clinical spectrum of COPD and help guide management. The presence of cognitive impairment in patients with COPD is well reported, yet evidence on its impact is lacking. This large nationwide study reported the impact of dementia in patients admitted to hospital with a COPD exacerbation. It showed that patients with COPD who had co-existing dementia had worse outcomes based on inpatient deaths and LOS.
Many studies, which have shown an association of coexisting dementia and COPD with adverse hospital related outcomes, have consisted of a smaller sample size in comparison to the current work, with few investigating effects of dementia directly and none focussing on patients admitted with an exacerbation of COPD.3,4,20,21 The results from this study strengthen the previous, albeit limited available literature. Liao et al reported an increased risk of inpatient mortality and prolonged length of stay, as well as acute organ dysfunction and severe sepsis in patients admitted to hospital who had a diagnosis of dementia and COPD.3 However, this encompassed all hospitalised patients with a diagnosis of COPD rather than focussing on individuals admitted primarily with a COPD exacerbation.
One possibility for the adverse outcomes observed is that dementia is associated with increased disability and mortality in all hospitalised patients22 and would have an additive adverse effect in patients admitted with COPD exacerbations. A systematic review evaluating the effects of dementia on length of stay reported that majority of the studies showed an increased stay associated with dementia, ranging up to 22 days for different primary pathologies.23 A variety of medical, social and organizational factors may influence this, such as increased risk of inpatient complications or greater disability requiring more social input. Similarly, Shen et al suggested dementia had a 28% increased risk of mortality in hospitalized older patients after adjusting for confounders.24 For patients with COPD, in particular, cognitive deficits may delay discharge by further worsening functional limitations and increasing the need for assistance in safety, transportation and personal care.25
Dementia may contribute to overall worsening COPD disease severity and therefore worse outcomes in hospitalised patients with COPD. Cognitive impairment can hinder complex tasks in COPD, such as appropriate inhaler technique and self-management strategies, predisposing patients to disease progression and frequent exacerbations.9,10,26,27 Increased exacerbation frequency is also associated with more adverse hospital related outcomes, possibly owing to the fact that repeated exacerbations are associated with rapid decline in lung function, reduced exercise capacity and higher burden of symptomatology, all of which further contribute to disease progression.28 Alternatively, cognitive dysfunction in COPD may occur through pathways that represent a sequelae due to worsening lung function, and so the effects seen on morbidity and mortality may be as a result of worsening disease severity.29
It is also important to note that withholding invasive life support measures in patients with physiological decompensation, particularly if it is not clinically appropriate eg in frail and comorbid patients, may also contribute to inpatient mortality. It can be argued that the presence of dementia is associated with a higher burden of frailty. However, the evidence for the use of invasive therapeutic measures in patients with dementia is mixed; Richardson et al stated patients with dementia admitted acutely are treated significantly less aggressively than those without dementia.30 Conversely, a US based study reported that between 2001 and 2011 the utilization of invasive mechanical ventilation increased fourfold in patients with dementia than in those without dementia.31 In this study, there was no difference in the utilisation of mechanical and non-invasive ventilation in patients with and without dementia that had respiratory compromise, suggesting that this did not play a role in the hospital related outcomes. However, what is unclear is whether less blood gases to investigate respiratory compromise were conducted.
One explanation for the odds of inpatient mortality being higher in younger patients with COPD and co-existing dementia could be that cognitive impairment contributed to a greater risk of frailty in the younger cohort compared to the older patients. Furthermore, aspiration, disability and sedentary lifestyle are important consequences of dementia and related causes of death.32 However, these risk factors are also more common with increasing age.33 Therefore, the difference in the degree of effect seen in patients with dementia vs without dementia at a younger age may reflect the disparity in prevalence of factors such as aspiration between the groups, which diminishes with age. This pattern of mortality between age groups is in line with another nationwide study investigating mortality outcome in all hospitalised patients ≥65 years with and without dementia.34 Similarly, Feary et al identified an increased risk of acute ischaemic events in patients with COPD with the biggest effect observed in the younger patients.35
The fact that mortality rate was greater in the patients with dementia in the early stage of their admission may indicate that such patients initially present with more severe exacerbations or have less of a physiological reserve to survive the acute illness. One plausible explanation for the lower mortality rate seen after 5 days is that patients with dementia who survive the early days of admission are medically stable but have a prolonged inpatient stay because of increased need for social support, such as discharge to nursing facility. However, patients without dementia would be less likely to require a further package of care and therefore, any prolonged length of stay in these patients may be due to in-hospital complications or deterioration physiological function, all of which are associated with increased mortality. This is in contrast to Chang et al who reported a higher overall mortality rate in patients with COPD and dementia compared to those with COPD alone.4 However, it should be noted that the follow up time period in their study was considerably longer than the NIS database and extended to within the community, rather than being a snapshot of their hospital admission.
One point to note is that the proportion of females admitted with a COPD exacerbation was higher than males. Certainly, the prevalence of COPD is reported to be higher in males.36 However, it has been previously suggested that females may be more susceptible to exacerbations and therefore more frequent hospitalisations.15,37 Gender plays an important role in determining hospital related outcomes in patients with COPD, such that men are associated with a poorer outcome.38 This is possibly owing to differences in disease severity, behaviors towards healthcare utilization and response to available therapeutic modalities.39,40 Similarly, there are also gender disparities reported amongst patients with dementia.41 This study revealed poorer outcomes associated with COPD and dementia even after adjusting for sex as a comorbidity.
The main limitation with the NIS database was the lack of unique patient identifiers. As a result, it was not possible to explore re-admissions due to multiple exacerbations or discharge failure. This is of particular importance, given that if one group had more readmissions there was a greater chance that patients would be included more than once in the analysis for that group. However, the large number of discharge records should have helped negate this issue. In addition, it was only possible to assess in-hospital mortality, as cases were not followed up once discharged. Therefore, it did not reflect the 30-day mortality and may have underestimated the mortality rates, particularly if a high proportion of patients in one group were discharged with end of life care plans for example. There are also limitations with the reliability of clinical coding, as with many other studies. It has previously highlighted that dementia is usually underdiagnosed in patients with COPD.42 This may have been the case in this study, where the prevalence of dementia in patients with COPD reported was 6%, despite including a large range of dementia related diagnostic codes. This is considerably less than other studies; Zarowitz et al reported a prevalence of 37% of concurrent dementia amongst nursing home residents with COPD.43 Furthermore, the use of ICD classification system alone may lead to misidentification of some acute exacerbation cases. Therefore, the difference in observed outcomes may have been underestimated. However, this reinforces the argument that there is a need to screen and identify patients with cognitive impairment in such a cohort of patients. Furthermore, some of the known prognostic factors of COPD, including a history of cigarette smoking, lung function and severity of dementia were not available from the claims database. The inability to adjust for these prognostic factors might have affected the results, though adjusting for comorbidity index was a reasonable approach to addressing this issue. The exclusion of cases with missing values may introduce a bias into the analysis but given the large sample size, this is not expected to be the case. Finally, the lack of reliable data on medications, particularly when considering polypharmacy and determinants of frailty, both of which are associated with adverse outcomes, meant that their associations with mortality and LOS in patients with COPD and dementia could not be ascertained.
Conclusion
The findings of this study have important clinical implications. Identifying pre-existing dementia in patients with a COPD exacerbation and deploying pre-emptive strategies such as clear treatment escalation plans and timely involvement of multidisciplinary teams to facilitate discharge planning, may improve inpatient mortality and length of stay. Clinicians should be alert to developing a more tailored care plan for this subpopulation, taking into account patients’ specific dementia care needs and considering involvement of dementia specialist teams.
Abbreviation
COPD, chronic obstructive pulmonary disease; NIS, national inpatient sample database; LOS, length of stay; ICD, international classification of diseases.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by the National Institute for Health Research (NIHR) Nottingham Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Disclosure
Professor Charlotte E Bolton reports grants from AZ, Boehringer, Chiesi, GSK, Novartis, BLF, personal fees from AZ, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. British Lung Foundation. Chronic obstructive pulmonary disease (COPD) statistics; 2019. Available from: https://statistics.blf.org.uk/copd.
2. Cavaillès A, Brinchault-Rabin G, Dixmier A, et al. Comorbidities of COPD. Eur Respir Rev. 2013;22(130):454–475. doi:10.1183/09059180.00008612
3. Liao K-M, Lin T-C, Li C-Y, Yang Y-HK. Dementia increases severe sepsis and mortality in hospitalized patients with chronic obstructive pulmonary disease. Medicine. 2015;94(23):e967–e968. doi:10.1097/MD.0000000000000967
4. Chang SS, Chen S, McAvay GJ, Tinetti ME. Effect of coexisting chronic obstructive pulmonary disease and cognitive impairment on health outcomes in older adults. J Am Geriatr Soc. 2012;60(10):1839–1846. doi:10.1111/j.1532-5415.2012.04171.x
5. Antonelli R, Pedone C, Onder G, Pahor M, Carbonin P. Predicting length of stay of older patients with exacerbated chronic obstructive pulmonary disease. Aging Clin Exp Res. 2001;13(1):49–57. doi:10.1007/BF03351494
6. Ranieri P, Bianchetti A, Margiotta A, Virgillo A, Clini EM, Trabucchi M. Predictors of 6-month mortality in elderly patients with mild chronic obstructive pulmonary disease discharged from a medical ward after acute nonacidotic exacerbation. J Am Geriatr Soc. 2008;56(5):909–913. doi:10.1111/j.1532-5415.2008.01683.x
7. Lima O, Oliveira-Souza R, Santos O, Moraes P, Sá L, Nascimento OJ. Subclinical encephalopathy in chronic obstructive pulmonary disease. Arq Neuropsiquiatr. 2007;65(4B):1154–1157. doi:10.1590/S0004-282X2007000700012
8. Incalzi RA, Gemma A, Marra C, Capparella O, Fuso L, Carbonin P. Verbal memory impairment in COPD: its mechanisms and clinical relevance. Chest. 1997;112(6):1506–1513. doi:10.1378/chest.112.6.1506
9. Baird C, Lovell J, Johnson M, Shiell K, Ibrahim JE. The impact of cognitive impairment on self-management in chronic obstructive pulmonary disease: a systematic review. Respir Med. 2017;129:130–139. doi:10.1016/j.rmed.2017.06.006
10. Cleutjens F, Spruit MA, Ponds R, et al. The impact of cognitive impairment on efficacy of pulmonary rehabilitation in patients with COPD. J Am Med Dir Assoc. 2017;18(5):420–426. doi:10.1016/j.jamda.2016.11.016
11. Databases H. Healthcare Cost and Utilization Project (HCUP); 2014. Available from: www.hcup-us.ahrq.gov/databases.jsp.
12. Stein BD, Bautista A, Schumock GT, et al. The validity of international classification of diseases, ninth revision, clinical modification diagnosis codes for identifying patients hospitalized for COPD exacerbations. Chest. 2012;141(1):87–93. doi:10.1378/chest.11-0024
13. Mehta AB, Douglas IS, Walkey AJ. Hospital noninvasive ventilation case volume and outcomes of acute exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13(10):1752–1759. doi:10.1513/AnnalsATS.201610-777LE
14. Stanford RH, Engel-Nitz NM, Bancroft T, Essoi B. The identification and cost of acute chronic obstructive pulmonary disease exacerbations in a United States population healthcare claims database. Copd. 2020;17(5):499–508. doi:10.1080/15412555.2020.1817357
15. Patil SP, Krishnan JA, Lechtzin N, Diette GB. In-hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003;163(10):1180–1186. doi:10.1001/archinte.163.10.1180
16. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi:10.1093/aje/kwq433
17. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
18. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi:10.1097/01.mlr.0000182534.19832.83
19. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. doi:10.1080/01621459.1999.10474144
20. García-Sanz M-T, Cánive-Gómez J-C, Senín-Rial L, et al. One-year and long-term mortality in patients hospitalized for chronic obstructive pulmonary disease. J Thorac Dis. 2017;9(3):636–645. doi:10.21037/jtd.2017.03.34
21. Dodd JW, Charlton RA, van den Broek MD, Jones PW. Cognitive dysfunction in patients hospitalized with acute exacerbation of COPD. Chest. 2013;144(1):119–127. doi:10.1378/chest.12-2099
22. Campbell SE, Seymour DG, Primrose WR. A systematic literature review of factors affecting outcome in older medical patients admitted to hospital. Age Ageing. 2004;33(2):110–115. doi:10.1093/ageing/afh036
23. Möllers T, Stocker H, Wei W, Perna L, Brenner H. Length of hospital stay and dementia: a systematic review of observational studies. Int J Geriatr Psychiatry. 2019;34(1):8–21. doi:10.1002/gps.4993
24. Shen H-N, Lu C-L, Li C-Y. Correction: dementia increases the risks of acute organ dysfunction, severe sepsis and mortality in hospitalized older patients: a national population-based study. PLoS One. 2012;7(8). doi:10.1371/journal.pone.0042751
25. Greenlund KJ, Liu Y, Deokar AJ, Wheaton AG, Croft JB. Association of chronic obstructive pulmonary disease with increased confusion or memory loss and functional limitations among adults in 21 states, 2011 behavioral risk factor surveillance system; 2016.
26. Turan O, Turan PA, Mirici A. Parameters affecting inhalation therapy adherence in elderly patients with chronic obstructive lung disease and asthma. Geriatr Gerontol Int. 2017;17(6):999–1005. doi:10.1111/ggi.12823
27. Allen SC, Jain M, Ragab S, Malik N. Acquisition and short term retention of inhaler techniques require intact executive function in elderly subjects. Age Ageing. 2003;32(3):299–302. doi:10.1093/ageing/32.3.299
28. Anzueto A. Impact of exacerbations on COPD. Eur Respir Rev. 2010;19(116):113–118. doi:10.1183/09059180.00002610
29. Samareh Fekri M, Hashemi-Bajgani S-M, Naghibzadeh-Tahami A, Arabnejad F. Cognitive impairment among patients with chronic obstructive pulmonary disease compared to normal individuals. Tanaffos. 2017;16(1):34–39.
30. Richardson SS, Sullivan G, Hill A, Yu W. Use of aggressive medical treatments near the end of life: differences between patients with and without dementia. Health Serv Res. 2007;42(1 Pt 1):183–200. doi:10.1111/j.1475-6773.2006.00608.x
31. Lagu T, Zilberberg MD, Tjia J, Pekow PS, Lindenauer PK. Use of mechanical ventilation by patients with and without dementia, 2001 through 2011. JAMA Intern Med. 2014;174(6):999–1001. doi:10.1001/jamainternmed.2014.1179
32. Nägga K, Wattmo C, Zhang Y, Wahlund LO, Palmqvist S. Cerebral inflammation is an underlying mechanism of early death in Alzheimer’s disease: a 13-year cause-specific multivariate mortality study. Alzheimer’s Res Ther. 2014;6(4):41. doi:10.1186/alzrt271
33. Cichero JAY. Age-related changes to eating and swallowing impact frailty: aspiration, choking risk, modified food texture and autonomy of choice. Geriatrics. 2018;3(4):69. doi:10.3390/geriatrics3040069
34. Bouza C, Martínez-Alés G, López-Cuadrado T. The impact of dementia on hospital outcomes for elderly patients with sepsis: a population-based study. PLoS One. 2019;14(2):e0212196–e0212197. doi:10.1371/journal.pone.0212196
35. Feary JR, Rodrigues LC, Smith CJ, Hubbard RB, Gibson JE. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax. 2010;65(11):956–962. doi:10.1136/thx.2009.128082
36. Sørheim I-C, Johannessen A, Gulsvik A, Bakke PS, Silverman EK, DeMeo DL. Gender differences in COPD: are women more susceptible to smoking effects than men? Thorax. 2010;65(6):480–485. doi:10.1136/thx.2009.122002
37. Lisspers K, Larsson K, Janson C, et al. Gender differences among Swedish COPD patients: results from the Arctic, a real-world retrospective cohort study. NPJ Prim Care Respir Med. 2019;29(1):45. doi:10.1038/s41533-019-0157-3
38. Gonzalez AV, Suissa S, Ernst P. Gender differences in survival following hospitalisation for COPD. Thorax. 2011;66(1):38–42. doi:10.1136/thx.2010.141978
39. Aryal S, Diaz-Guzman E, Mannino DM. COPD and gender differences: an update. Transl Res. 2013;162(4):208–218. doi:10.1016/j.trsl.2013.04.003
40. Martinez FJ, Curtis JL, Sciurba F, et al. Sex differences in severe pulmonary emphysema. Am J Respir Crit Care Med. 2007;176(3):243–252. doi:10.1164/rccm.200606-828OC
41. Corrao S, Santalucia P, Argano C, et al. Gender-differences in disease distribution and outcome in hospitalized elderly: data from the REPOSI study. Eur J Intern Med. 2014;25(7):617–623. doi:10.1016/j.ejim.2014.06.027
42. Andrianopoulos V, Gloeckl R, Vogiatzis I, Kenn K. Cognitive impairment in COPD: should cognitive evaluation be part of respiratory assessment? Breathe. 2017;13(1):e1–e9. doi:10.1183/20734735.001417
43. Zarowitz BJ, O’Shea T. Chronic obstructive pulmonary disease: prevalence, characteristics, and pharmacologic treatment in nursing home residents with cognitive impairment. J Manag Care Spec. 2012;18(8):598–606. doi:10.18553/jmcp.2012.18.8.598
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
