Back to Journals » Journal of Inflammation Research » Volume 14
Impact of Adjunctive Diode Laser Application to Non-Surgical Periodontal Therapy on Clinical, Microbiological and Immunological Outcomes in Management of Chronic Periodontitis: A Systematic Review of Human Randomized Controlled Clinical Trials
Authors Pawelczyk-Madalińska M , Benedicenti S, Sălăgean T, Bordea IR, Hanna R
Received 6 February 2021
Accepted for publication 1 April 2021
Published 15 June 2021 Volume 2021:14 Pages 2515—2545
DOI https://doi.org/10.2147/JIR.S304946
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Magdalena Pawelczyk-Madalińska,1– 3,* Stefano Benedicenti,1 Tudor Sălăgean,4 Ioana Roxana Bordea,5 Reem Hanna1,6,*
1Department of Surgical Sciences and Integrated Diagnostics, Laser Therapy Centre, University of Genoa, Genoa, Italy; 2Department of Periodontology, Pomeranian Medical University, Szczecin, 70-204, Poland; 3FAN-DENT Centrum Stomatologii i Periodontologii, Gdańsk, 80-257, Poland; 4Department of Land Measurements and Exact Sciences, University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca, Cluj-Napoca, Romania; 5Department of Oral Rehabilitation, “Iuliu Hațieganu” University of Medicine and Pharmacy Cluj-Napoca, Cluj-Napoca, Romania; 6Department of Oral Surgery, King’s College Hospital NHS Foundation Trust, London, UK
*These authors contributed equally to this work
Correspondence: Reem Hanna
Department of Surgical Sciences and Integrated Diagnostics, Laser Therapy Centre, University of Genoa, Genoa, Italy
Tel +39 0103537446
Email [email protected]
Tudor Sălăgean
Department of Land Measurements and Exact Sciences, University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca, Cluj-Napoca, Romania
Tel +40 744707371
Email [email protected]
Background: Due to the limitations of scaling and root planing (SRP) in chronic periodontitis (CP) management, research has been focused on utilising additional therapies to enhance conventional treatment methods. The present systematic review is aimed to appraise the accessible scientific evidence of in vivo human studies to establish the effectiveness of adjunctive diode (λ 808- λ 980nm) laser treatment to SRP in CP.
Methodology: This systematic review was conducted following the PRISMA statement guidelines. The review protocol is registered in PROSPERO (CRD 42021227695). The search strategies were based on structured electronic and manual (with appropriate keywords) and were conducted to collect the applicable published data on RCTs studies (in vivo human), spanning over ten years between August 2010 and August 2020. The articles were selected to address the following research focus question: “Does diode laser (λ 808- λ 980nm) therapy have superior effects as an adjunct to SPR, compared to SRP alone, in terms of clinical or microbiological or immunological profiles in the management of CP?”
Results: Fifteen articles met the eligibility criteria and are included in this review. A wide range of discrepancies and inconsistencies were shown in the outcomes of the laser and SPR treatment modality, compared to SRP alone. The data on standardised study protocol, optimal laser parameters and outcome measurements were inconclusive, and a high risk of bias in the majority of the studies observed, which are crucial in establishing a homogenous and reproducible protocol.
Conclusion: In light of the confined evidence-based data and critical evaluation of this systematic review, the efficacy of adjunctive diode laser treatment ranging between 808 and 980nm to SRP remains debatable. The observational quality of the present systematic review was emphasised after scrutinising the available data, and an attempt to propose a laser protocol for future RCTs consideration was a great challenge due to an absence of clear and standardised recommendations in delivering a reliable laser protocol which can be replicable by future investigators. RCTs with robust methodology are warranted.
Keywords: high intensity laser, diodes laser treatment, chronic periodontitis, systematic diseases, non-surgical periodontal therapy, scaling and root planing, randomised controlled trials studies, RCTs
Summary
- Majority of included studies showed inconsistency in the outcomes with a high risk of bias. Hence, producibility of the assessment methods, as well laser protocol remains debatable due to elevated percentage of high and moderate risks of bias of the selected studies.
- Despite the variations of studies’ results, adjunctive laser therapy has added value, compared to SRP.
- Double-blind, multicenter, well-designed RCT studies with robust methodology and laser protocol, comparing diode laser with or without SRP, to SRP alone to justify effectiveness of diode laser (808–980nm) and produce a standardised laser protocol for various wavelengths are warranted.
Introduction
Chronic periodontitis (CP) pathogenicity is multifactorial related to mediated-inflammatory response periopathogens,1 in which surgical or non-surgical treatment modalities emerged. However, each of these approaches has its own advantages and limitations.2–4 The current gold standard non-surgical, mechanical instrumentation for bacterial debridement of periodontal pocket deeper than 4mm and bone loss (which are periodontitis indicators)5–8 is scaling and root planing (SRP). Many risk factors can influence the outcome of SRP such as smoking, stress and systematic diseases.2,3,5,7 However, the SPR treatment modality does not offer entirely successful long-term outcomes in the treatment of CP. This has led to various different treatment modalities to emerge, such as laser therapy of various wavelengths.9–11
A randomised controlled trial (RCT) study by Everett et al, 2017 has shown that statistically significant differences in clinical and microbiological parameters at three and six months after treatment for both groups; combined carbon dioxide laser [CO2, 10600nm, 4 W and 6 W (two passes) in continuous mode] and SRP and control (SRP alone), but no significant difference was observed between the two groups.12 Interestingly, the results of a study by Krohn-Dale et al, 2012 failed to support that Er:YAG laser debridement of recruited pocket depth (PD) ≥5 mm may be superior to conventional methods in the treatment of smokers with CP. Both treatments showed a significant decrease in PD from baseline to 12 months (p < 0.01).13 This was supported by a systematic review, which showed that the Er:YAG laser can be significant in reducing clinical parameters in the short term for patients with CP.14 This concept was supported by a study by Yanli et al, 2017 in terms of reduction and control of the periopathogen proliferation in CP.15 A meta-analysis by Jia et al, 2020 evaluated clinical attachment level (CAL) gain of Er:YAG, Er, Cr;YSGG, Nd:YAG and diode laser, as monotherapy or adjunctive to SRP of CP.16 The data extraction was up to 2018. The authors concluded that the influence of the following wavelengths ranking from best to worse on CAL gain at three months: Er:YAG as monotherapy, adjunctive diode laser to SRP, Adjunctive Er:YAG to SRP, Er,Cr;YSGG, as monotherapy, adjunctive Nd:YAG to SRP, and SRP, whereas, the CAL gain at six months, the ranking wavelengths on results were as follows: Adjunctive diode laser to SRP, adjunctive Nd: YAG to SRP, SRP, adjunctive Er:YAG to SRP, and Er:YAG as monotherapy.16
It is important to note from the above-mentioned evidence that diode laser-assisted periodontal treatment could be superior to SRP alone and could serve as a significant adjunctive treatment tool, compared to SPR alone.
The photothermal properties of diode laser photonic energy facilitates ablation of the graduation tissue and inflamed periodontal tissue (sulcular debridement) and coagulation at the same time, which can be achieved at 60°C, leading to a protein denaturation and reduction in the proinflammatory cytokines.17 As the diode laser family is predominantly absorbed by mainly haemoglobin and pigmented bacteria and poorly absorbed by hydroxyapatite in teeth and bone, it is considered as a safe and suitable treatment modality in sulcular debridement.18 The photonic energy of an ablative diode laser in the diseased periodontal pocket results in various beneficial effects:19–23
- A reduction in the bacterial volume of Aggregatibacter actinomycetemcomitans (A.a) (periodontal pocket-violet complex) and Porphyromonas gingivalis (P.g) (Red Complex),21,22 which can easily penetrate the sulcular epithelium without damaging the underlying connective tissues (bactericidal effect), which are the prime pathogens in periodontitis. The desirable clinical outcome can be achieved when the photonic energy of specific laser wavelengths is absorbed by brown/black-pigmented anaerobic bacteria. Also, it exerts a debridement effect through removal of the inflammatory products.
- A reduction in the inflammatory markers and an increase in cell proliferation and lymphatic circulation lead to periodontal attachment improvement (Regenerative effect).
- Post-operative pain alleviation (Quasi photobiomodulation (PBM) effect).
Proinflammatory cytokines contribute significantly in periodontal tissue damage, especially interleukin 1 (IL-1), IL-6 and tumour necrosis factor-α (TNF-α).24,25 The RANKL, ligand RANKL and its soluble counterpart osteoprotegerin (OPG) are the major regulatory pathways of osteoclasts activity.26 Several studies have shown an increase in RANKL expression in diseased periodontal tissues.19,24 Metalloproteinases degrade the extracellular matrix and increase in its activity is one of the predisposing factors in periodontal disease.27,28
The diode laser family ranging from 800–980nm (near-infrared) can eradicate the inflamed tissue from the pocket and decrease the amount of pro-inflammatory agents to encourage prompt healing.29–34 On this note, understudying the mechanism of transition from healthy periodontium to diseased and subsequently to various stages of disease progression is crucial in order to develop effective approaches toward disease prevention and therapy.35
Data has shown that adjunctive diode laser treatment to the conventional methods has proven to reduce the bacterial load in periodontal pocket.36,37 Controversially, a study by Slot et al, 2009 failed to support this.38 It’s noteworthy that due to a wide methodological heterogeneity in the available literature data, laser treatment efficacy in CP management, as a monotherapy or as an adjunct to non-surgical periodontal therapy (NSPT) has been a challenge to interpret.
Due to the controversial above-mentioned notes in the efficacy of adjunctive diode lasers to SRP in CP treatment, as well a lack of long-term follow-up, the present systematic review aimed to scrutinise and evaluate the effectiveness of adjunctive diode-laser treatment (λ 808- λ 980nm) to SPR, compared to SRP alone in CP treatment. The objectives were as follows: to propose a laser protocol, to highlight the appropriate methodology to achieve optimal therapeutic outcomes (sulcular debridement) and to determine the critical level of the periodontal disease severity, which can be considered for this therapeutic protocol.
Materials and Methods
Protocol
Identification and critical evaluation of accessible literature was performed. A systematic review was implemented without meta-analysis due to heterogeneity of the available data and study outcomes. In accordance with the PRISMA statement guidelines, this systematic review was conducted39 (See Supplementary materials, Appendix 1) and the protocol is published in Prospective Register Of Systematic Reviews (PROSPERO) (www.crd.york.ac.uk/PROSPERO/; ref CRD 42021227695).
Population, Intervention, Comparison, and Outcomes – PICO
- Population: Patients diagnosed with CP, according to the 1999 AAP Classification of Periodontal Diseases and Conditions.11
- Intervention: Diode surgical laser (λ808- λ980nm) treatment, as an adjunct to SRP.
- Comparison: SPR alone (non-surgical approach).
- Outcomes: Evaluation of clinical parameters or microbiological or immunological profiles.
Focused Research Question
Does diode laser (λ 808- λ 980nm) treatment have superior effects as an adjunct to SPR, compared to SRP alone, in terms of clinical, microbiological or immunological profiles in CP management?
Search Strategy
MEDLINE (NCBI PubMed and PMC), Cochrane Central Register of Controlled Trials (CCRCT), Scopus, Science Direct, Google Scholar, EMBASE, EBSCO were scanned. The following journals were hand searched: Photochemistry and Photobiology B: Biology, Lasers in Medical Science, Clinical Periodontology and Photomedicine and Laser Surgery. In order to detect unpublished studies, grey literature sources were screened. The search strategy included only terms related to or describing the study domains and interventions spanning ten years between August 2010-August 2020. Different combinations of the following keywords were used: high intensity laser, diodes laser treatment AND chronic periodontitis, smoking, systematic diseases, non-surgical periodontal therapy AND scaling and root planing, Randomised Controlled Trials. The search was performed by two independent authors (MM and RH). Whereas the utilised MeSH terms were as follows: Chronic periodontitis OR Non-surgical periodontal treatment OR Scaling root planing therapy OR Surgical diode laser treatment OR Sulcular debridement OR Clinical periodontal parameters OR Periodontal microbial profile OR Periodontal immunological markers.
Eligibility Criteria
- Full-text articles related to CP.
- Subjects >18-year-old diagnosed with CP according to 1999 American Academy of Periodontology (AAP) Classification of Periodontal Diseases and Conditions.11
- Studies that utilised diode laser-assisted therapy ranging from 808 to 980nm for single or multiple procedures.
- Studies which utilised diode laser tips inside the pocket for debridement.
- RCTs studies (Split mouth/parallel studies) which utilised combined laser therapy and SRP (hand and ultrasound instrumentations) therapy.
- Studies which treated PD ≥ 4mm and < 10mm, including residual pockets.
- Studies with a minimum follow-up period of at least one month (four weeks) after treatment.
- Systematic diseases.
- Smoking/non-smoking subjects.
- Studies in English language only.
- Electronic search databases from August 2010-August 2020.
- In vitro, animal in vivo, clinical non-RCTs studies, case series and case reports.
- Studies presented in conferences, books, editorial report, short communications, systematic and narrative reviews.
- Studies which utilised antibiotics for less than three months.
- Subjects who had periodontal therapy in last month prior to enrolling in RCTs.
- Subjects on anti-inflammatory, hormonal medications or on the substance used drugs.
- Pregnant/breastfeeding patients.
- Subjects with PD >10mm.
- Studies which utilised wavelength < 808nm.
- Studies utilising light-emitting diodes (LEDs), as a light source.
- Studies employing antimicrobial photodynamic therapy (a-PDT) and PBM therapy.
- Studies utilising mouth rinsing during the course of treatment (Povidone-iodine, methylene blue or chlorhexidine) protocols just prior to lasing or during treatment time.
- Studies with the abstract or title only.
- Studies with no outcome variable of interest.
- Studies employed subjects diagnosed with aggressive periodontitis.
Treatment Outcome Measures
Primary Outcomes
- To assess and evaluate the effectiveness of adjunctive diode laser treatment (808–980nm) treatment to SRP in CP management.
- To examine the clinical periodontal parameters PD/probing pocket depth (PPD), clinical attachment level (CAL), bleeding on probing (BOP), plaque index (PI), gingival index (GI), modified gingival index (MGI).
- To evaluate the microbiological profile and immunohistochemistry markers obtained from gingival crevicular fluid (GCF) samples or polymerase chain reaction (PCR) or plasma level of reactive oxygen metabolites (ROM) or HbA1c.
Secondary Outcomes
- To propose a laser protocol according to severity of the disease.
- To highlight the appropriate methodology to achieve optimal therapeutic outcomes (sulcular debridement).
- To determine the critical level of periodontal disease severity, which can be considered for this therapeutic protocol.
Data Extraction
Two blind reviewers (MM and RH) independently selected eligible studies from the search. They performed the review, assessment and data extraction for each eligible study. Data that they considered relevant to the present systematic review was extracted from all eligible articles and a tabular representation of the same was prepared. The main extracted domains are listed below:
- Study type, year, origin.
- Sample size.
- Participants’ gender and age.
- SRP protocol and irrigation episodes.
- PD measurements prior to treatment.
- Laser treatment protocol: laser paraments, dosimetry, number of sessions, treatment duration, tip movement, laser tip status (initiated and noninitiated) and power meter utilisation.
- Systematic diseases.
- Smokers and non-smokers.
- Clinical variables.
- Duration of follow-up time-point.
- Implemented investigations.
- Performed Statistical analysis.
- Results and conclusion.
Quality Analysis
Quality evaluation of all included studies of this systematic review were assessed to appraise their methodological and clinical outcomes via the information given in the original full text publications. The assessment was performed using RoB tool for Randomised trials, Version 2.0 (RoB 2)40,41 to evaluate each study quality by two independent blind reviewers (MM &RH). The assessment criteria under the following headings performed as follows: bias arising from the randomisation process, bias due to deviations from intended intervention, bias due to missing outcome data, bias in measurement of the outcome, bias in selection of the reported result.
Additionally, a pragmatic approach and quality measured assessment of important items were considered for each eligible study as follows:
- Industry funding
- Conflict of interest
- Ethical approval
- Description of the study procedure
- Reported blindness process and how
- Sample size and evidence of treated pockets calculation
Each study was deemed as low, moderate or high RoB. Consensus for inter-reviewer disagreements was obtained by discussion with a third author (SB) as well as a “discrepancy check” featured in RoB 2 tool.
Results
Study Selection
Figure 1 represents the PRISMA flow diagram for the search strategy utilised in this systematic review. A total of 1895 study titles were obtained from a combined electronic and manual search. Thirteen study titles were identified from cross-references. From 1908 articles, we excluded 22 duplicates. Consequently, a total of 1886 study titles were included in preliminary screened by two independent authors (MM and RH). 1860 articles were excluded, due to the following reasons: case series and case report (n= 150), in vitro and in vivo studies (n=700), systematic and literature reviews (n=140), articles not in English language (n=77), abstract and title (n=660), articles published more than 10 years ago (n=133). The remaining 26 articles were further evaluated based on the review eligibility criteria. Additionally, 11 articles were excluded based on the following reasons: inappropriate study design (n=5), PBMT and a-PDT (n=6). The remaining 15 articles42–56 were included and analysed in this review. In order to reduce bias and human error, two authors independently extracted the data (MM and RH).
 |
Figure 1 PRISMA flow-chart of the study selection criteria for the included article reports.Notes: Adapted from Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. © 2009 Moher et al. Creative Commons Attribution License.39 |
Country of Origin
An extensive diversity in the country of origin was noted amongst the included papers (Table 1). The distribution of the studies was as follows: three in India,42,53,56 two in Brazil,43,44 five in Turkey45,46,49–51 and one study noted in each of the following countries: Italy,52 Iraq,47 USA,48 Croatia54 and Iran.55
Number of Participants, Age and Gender Distribution
The range of the participant number were as follows: from 11 to 20 in two studies,45,47 from 21 to 50 in eleven studies42–44,46,48,49,51,53–56 and from 51 to 60 participants in two studies50,52 (Table 1), whereas the distribution of the subjects’ number according to gender is illustrated in Figure 2 for only 11 of the 15 studies, whereas four studies failed to document the gender of their participants.45,47,52,56
 |
Figure 2 Illustrates the percentage of the gender distribution according to the number of the recruited subjects. |
Study Design
Eight of the 15 studies conducted a split-mouth study design43–45,47,48,52,54,55 (53.33%), whereas the remaining seven studies conducted a parallel group study design (46.67%),42,46,49–51,53,56 in which 60% published in a peer-reviewed journal and 26.66% failed to report ethical approval (Table 1).
Selection Criteria
All the studies identified their subjects diagnosed with CP in their eligibility criteria, according to the 1999 AAP.11
Smoking Status
A total of 12 of the 15 studies excluded smokers in their eligibility criteria,42–46,49–51,53–56 while the other two studies included them,48,52 in which one study included smokers and non-smokers,48 whereas one of the 15 studies has not reported47 (Table 1).
Systemic Disease Status
Four of the 15 studies included subjects with well-controlled NIDDM in their eligibility criteria (26.66%),42,46,47,50 whereas one study included systematic diseases without specifying their nature, but excluded bleeding disorders in its eligibility criteria (6.6%).55 Therefore, a total of 33% of the included studies in this systematic review represents subjects with systematic diseases, which might influence the outcomes. The remaining 66.66% recruited fit and healthy subjects (Table 1).
Utilised Wavelength
Various ranges of single laser wavelength were utilised from 808nm to 980nm. Three studies used 808nm,42–44 whereas two employed λ 810 nm.45,46 Ten of the 15 studies utilised various wavelengths ranging from λ 940nm- λ 980nm, in which six studies used 940nm,47–52 one study employed λ 970 ±15nm,53 and three studies utilised 980nm54–56 (Table 2) (Figure 3).
 |
Table 2 Shows a Tabular Representation of Diode Laser-Assisted Treatment Parameters Reported in the Included Eligible Randomized Controlled Trials Studies |
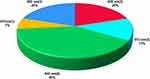 |
Figure 3 Illustrates the percentage of various wavelengths utilised in the selected studies and the number between brackets shows the number of the studies in which wavelength used. |
Number of Sessions
Laser-assisted treatment was applied in 10 of the 15 studies, immediately after SRP on the same session.42,45,46,48–53,55 Additionally, five of the 15 studies43,44,47,54,56 utilised multiple procedures (lasing), ranging from two to three sessions for two weeks (healthy subjects and with systematic conditions), in which two studies applied three sessions based on laser-assisted therapy at 0-day, 7th and 14th day after SRP47 and 0-day, 3rd and 7th day after SRP.54 However, two studies applied two sessions of laser-assisted therapy in which at day 0, which was immediately after SRP and the another one at the 7th day after SRP43,44 and the last one was study applied two sessions of laser-assisted therapy in which at day seven after SRP and the another one at the 14th day after SRP56 (Table 1).
Reported Laser Parameters
Table 2 shows the reported laser parameters of the chosen studies while the missing data is illustrated in Figure 4. Laser emission mode reported in all included studies, in which 60% utilised continuous mode (CW),42–45,47,48,52,55,56 whereas 33.33% used gated emission mode46,49–51,54 and 6.67% pulsed mode.52 Additionally, all the eligible studies reported power output that ranged from 0.8W to 3W, while the average power for gated mode ranged from 0.5–2W. Only four of the 15 studies documented the irradiance43,44,53,56 and energy density was reported only in six studies.48–53 Three studies stated that the laser fibre was initiated,48,52,53 in which one of them was indicative via photograph,53 while the other 12 studies failed to report.42–47,49–51,54,56 Only one study reported the tip speed movement.55 Additionally, only three studies utilised a power meter (20%)43,44,53 while only one study failed to provide spot size/fibre diameter information.48 Only one study calculated the total energy,56 whilst eight of the 14 studies failed to report this, despite it being easy to calculate; however, it was difficult to calculate it in the remaining six studies due to a lack of data (Table 2).
 |
Figure 4 Shows the percentage of the recruited pocket depth measurements in relation to the utilized wavelengths. |
Four of the 15 studies failed to document the laser treatment exposure time per pocket.42,48,52,55 In Table 2, 11 of the 15 studies reported the laser treatment exposure time per pocket, ranging between 40 and 60 seconds,43,44,46,53,56 in which two studies applied 30 seconds twice with 60 seconds interval time (thermal relaxation),53,56 whereas one study reported only 10 seconds relaxation time when the pocket lasing time was >30 seconds, whereas six studies reported 20 seconds45,47,49–51,54 (Table 2).
Six of the 15 selected studies45,48,55 reported the number of treated pockets, which represented sample size, ranging from 56 to 207, whereas the probing sites ranged from 925 to 1650 reported in two studies52–54 (Table 1). Four studies47,53–55 reported PD ≥ 4mm, eight studies43–45,48–51,56 reported PD ≥ 5mm and three studies42,46,52 reported PD ≥ 4 and ≥ 5. Figure 4 shows the pocket measurement in relation to the utilised wavelengths. Only two studies utilised saline for pocket irrigation after laser treatment.42,49 The percentage of data documentation in the selected articles that can assist us in understanding the laser protocol was about 40%, whereas 60% was missing (Table 1). It is noteworthy that approximately 50.9% of the laser parameters were missing (unreported) (Table 2) (Figure 5).
 |
Figure 5 Shows the percentage of the missing data related to the laser parameters. |
Methods of Analysis
Multiple parameter assessments were conducted in most of the selected studies. The distribution of the clinical assessments in the selected studies was as follows: PD,42–56 CPD,44 PPD,56 BOP,42–44,46–51,53–56 CAL,42,46–51,53–56 cementoenamel junction (CEJ) in one study,44 while PI measured in ten studies,42,44–46,49–52,56 whereas the GI evaluated in eight studies,42,45,46,49–52,56 MGI assessed in one study,55 as well as GL,55 API54 and PCR.56 Interestingly, the microbiological analysis utilised GCF in four studies45,48–50 and CFU in two studies.42,43 In terms of the immunological analysis, CRP evaluated in one study46 and ROM employed in one study.53 The HbA1c blood test assessed in three studies.42,46,50 Table 3 outlines the summary of the clinical parameters, microbiological and immuno-biochemical markers utilised in the included studies.
The Treatment Outcomes
Table 4 illustrates the outcomes of the clinical indices, microbiological and immunological markers at various follow-up time-points, ranging from four weeks up to six months. Table 5 illustrates the summary of the significance and non-significance values of the clinical, microbiological and immune-biochemical for laser + SRP and the SRP alone groups, as well between both groups. This specifies that the selected studies utilised the major clinical parameters; however, 20% of the microbiological and immune-biochemical markers have not been utilized in the selected studies, whereas the remaining 80% utilised one of two of the markers.
 |  |  |
Table 4 Illustrates the Outcomes and the Follow-Up Period of the Eligible Studies |
RoB Assessment
The quality assessment of eligible studies of this review was performed, using the RoB 2 tool, designed for in vivo human RCTs illustrated in Figures 6–9. In terms of deviations from intended interventions, 40% moderate, 33.33% high risk and 26.66% low risk. All the chosen articles have reported extensive evidence of missing outcome data; however, they were at a low risk. The majority of the chosen studies were bias-free, arising from reporting outcome measurement (60%), whereas 6.66% at a high risk. Selective reporting of the results showed 13.33% high risk, 60% low risk, and 26.66% moderate risk (Figures 6 and 7). Overall, 40% of eligible studies reported at high risk, 33.33% low risk and 26.66% moderate risk (Figure 7).
 |
Figure 6 Quality assessment of all the included eligible studies (n=15) in 4 domains and overall bias. Studies were graded as low risk (green), moderate risk (some concerns, yellow) or high risk (red) for each domain. There is no summation across fields. The assessment was performed using RoB tool for randomised trials, Version 2.0 (RoB 2).40,41 |
 |
Figure 7 Risk of bias assessment graph of all the included studies expressed as percentages for 4 main domains as well overall bias, based on the agreed answers across two independent authors (MM and RH). The assessment was performed using RoB tool for randomised trials, Version 2.0 (RoB 2).40,41 |
 |
Figure 8 Shows the summary of the risk of Bias assessment of all the domains of the included studies based on agreed answers across two independent authors (MM &RH). The assessment was performed using RoB tool for randomised trials, Version 2.0 (RoB 2).40,41 |
 |
Figure 9 Risk of bias assessment graph of all the included studies for all the domains expressed as percentages, based on agreed answers across two independent authors (MM and RH). The assessment was performed using RoB tool for randomised trials, Version 2.0 (RoB 2).40,41 |
The sample size and evidence of treated-pocket calculation domains showed 40% high risk, 26.66% moderate risk and 33.33% low risk, whereas inadequate randomisation represented 26.66% high risk, 60% low risk and 13.33% moderate risk. Study procedure description and reported study blindness shared 60% of moderate risk (Figure 8). Overall RoB domains revealed 40% high risk, 33.33% moderate and 26.66% low risk (Figure 9). The gathered information from these figures represents the agreed answers confirmed, using a discrepancy check feature of RoB2 tool across two independent authors (MM and RH).
Figure 8 uses a traffic light system to show an illustrative representation of the quality of key aspects for each study mentioned above, including the criteria mentioned in Figure 6. Allowing for the overall quality the items assigned in red are where the study does not meet the standard (high risk) and items assigned in green for meeting the standard (low risk). For standards where we considered it possible for them to be partially met (some concerns) were assigned in yellow. This was performed for every eligible study individually by two independent reviewers (MM and RH), in order to reduce the bias and human error. Upon fulfilment of all the above-mentioned criteria, the study was determined as a low RoB. However, where one or more criteria were partly met, the studies exhibited a moderate risk of bias. A high risk was assigned to a study when one or more criteria were not met. Any disagreement resolved by discussion as well discrepancy check feature of the RoB 2 tool used, in order to obtain agreed answers.
Discussion
Despite NSPT being the worldwide acceptable treatment regimen of CP, it has been assessed critically on many circumstances over the past decade for its limitations.1,57 As a result of this, various assisted treatment modalities have emerged.58 Hence, the utilisation of diode laser-assisted treatment as an adjunct therapy, which can be beneficial in NSPT cases unable to offer adequate optimal outcome or in compromised medical health conditions.59
There is a general consensus that conventional SPR cannot provide complete eradication of the bacteria and their toxins form the root surface within the periodontal pockets, which offers the strongest rationale to utilise photonic therapies, there are extensive controversies related to the advantages and benefits of replacing SRP with laser-assisted debridement.18,60
Due to the confined research focused to explore the efficacy of adjunctive diode laser treatment to NSPT and consistent inconsistency in the existing outcomes, this systematic review was performed and grounded on the hypothesis that diode laser-assisted treatment, as an adjunct to SRP improves the clinical, microbiological and immunohistochemistry outcomes and promotes faster healing of inflamed periodontal tissues. Owing to the heterogeneity in the available data of this systematic review, a meta-analysis of the included papers was considered unachievable. Thus, a critical appraisal of the noteworthy points has been exhibited as follows:
Assessment Methods and Their Implications
The aetiology of the CP is a complex and multifactorial inflammatory disease, affecting the teeth and supporting structures.61 It appeared that the efficacy of CP treatment is almost dependent on the clinical parameters only (BOP, PPD). It is critically important to have an additional tool for periodontal evaluation, especially in the early stages of chronic periodontitis. Parallel to AAP guidelines,62 the predictability of periodontal treatment outcomes can be evaluated by an improvement in the clinical parameters of inflamed tissue such as BOP and measurable of PI level where it is ultimately compatible with the level of healthy gingivae.63 Furthermore, a reduction in CAL is an indicator of a disease progression, which is ultimately considered a reliable parameter for treatment outcome assessments.64 Nevertheless, the process of culturing the anaerobic bacteria is very challenging which may influence the outcomes.65 In view of the fact that the number of studies which conducted microbial analyses is limited, the diversity in treatment protocols and methodologies used for microbiological analysis have become problematic. The majority of these studies used various microbial analyses which have coincided with pathogen reduction. A number of these studies compared adjunctive λ805nm diode laser to the control in CP treatment showed a significant reduction in Prevotella intermedia (P.i) and P.g in the laser group. A study by Saglam et al, 2014 conducted a quantitative analysis of P.g, Tannerella forsythia (T.f), and Treponema denticola (T.d), using real-time PCR (RT-PCR).49 The volume of the following bacteria: P.g, T.f, and T.d were significantly reduced in all treatment groups after one month (P<0.05). Nonetheless, the was no statistically significant differences detected among the groups for microbiological parameters at any follow-up time points (P >0.05).
Both studies by Euzebio Alves et al, 201343 and De Micheli et al, 201144 used adjunctive λ 808 nm laser to SRP compared to SPR alone, evaluating the changes in P.g and P.i pathogens (especially black-pigmented bacteria) with the microbial culture method. The microbiological analysis revealed a significant bacterial reduction in both groups, despite the fact that no significant differences were noted between both groups. They reported that the laser did not cause a significant pathogen reduction, compared to SRP. It is noteworthy that a study by Euzebio Alves et al, 201343 examined samples related to single-rooted teeth, which usually respond well to conventional therapies, due to their morphology and adequate access; hence, the results can be anticipated.
It’s important to note that a strong reduction in periodontal pathogens, especially orange and red-complex bacteria is considered one of the key influences in NSPT clinical success. Previous reports have shown that Gram−ve bacteria in PD are difficult to eliminate.66,67
RT-PCR is considered a reliable diagnostic tool for the detection and quantification of P.g, which also play a pivotal role in the initiation and progression of CP. Euzebio Alves et al, 201343 and De Micheli et al, 201144 studies in this systematic review employed λ 808 nm diode laser, as an adjunct to SRP have examined the changes of the P.g and P.i pathogens in microbial culture. A study by Euzebio Alves et al, 201343 recruited two preselected contralateral single-rooted teeth with a PD ≥ 5 mm, which were randomly assigned to the test or control group. However, only the samples of the deepest site of two single-rooted teeth were checked for RT-PCR, whereas laser irradiation only at one-site of the pocket. This can ultimately lead to predictable conclusions. Hence, adjunctive laser irradiation has not added extra benefits to the NSPT, in terms of the clinical parameters and microbiological profile. Moreover, this study failed to report the performed protocol of RT-PCR analysis. Similarly, a study by De Micheli et al, 2011 reported that laser treatment + SPR therapy has not shown significant pathogen reduction, compared SPR alone.44 It is noteworthy that a study by Euzebio Alves et al, 2013 used samples of single-rooted teeth, knowing their simple morphology, easy access and that they respond well to conventional NSPT.43 Therefore, the outcomes were predictable. This coincided with a study by Caruso et al, 2008 who utilised RT-PCR method, but only descriptively reported the cultured samples, which were pathogen free, without performing statistical analysis on the changes in the periodontal pathogens, knowing that the RT-PCR test lacks comparability for pathogen numbers.68 Balasubramaniam et al, 2014 assessed the short-term effectiveness of diode laser in addition to SRP in patients with CP on the following clinical parameters: PPD, BOP, PI, CAL and ROM.53 The serum level of ROM was significantly reduced in both groups (SRP alone and SPR + laser). However, no significant differences were observed between both groups. Conversely, a study by Uslu et al, 201869 showed that Myeloperoxidase (MPO) level was less in the laser + SRP group, whereas it was increased in the SPR alone group. The results are indicative of the fact that laser was shown to have positive effects on the oxidative stress in reducing inflammation.
Based on the above notes, our observations revealed that the discrepancy in the results of the above-mentioned studies could be related to the immunobiochemistry analysis utilised in these studies, as many analysed different biochemical parameters; however, there is evidence of a lack of knowledge of the biochemical markers, which are considered very sensitive. Despite the results of the majority of the chosen studies in the present review indicating that diode laser-assisted therapy is an effective adjunctive treatment modality over SRP alone, they have shown a degree of discrepancy in the level of significance amongst the following clinical parameters findings: PD/PPD, CAL, BOP, PI, API, and GI (Table 4). As a result, there is significant concern in obtaining a reproducible methodology. Additionally, the discrepancy in immunobiochemical marker assessment has reflected on the following reporting outcomes: IL-1, IL-6, IL-8, intercellular adhesion molecule-1 in GCF and HbA1c levels. This could be due to utilisation of the unstandardised RT-PCR technique protocols used in analysing the data. It is important to emphasise that all the studies in the present review focused on analysing one or more of the clinical parameters but utilising the immunological and microbiological markers lacked in most included studies (Table 3). Future studies are warranted to address robust outcome assessments.
Representation of the Treatment Outcomes
Despite the authors of this systematic review having attempted to follow a prudent and pragmatic approach in presenting the data, there was profound inconsistency or discrepancy noted in the methods of assessment of the efficacy of adjunctive diode laser-assisted approach. Additionally, the conflicting results among most of the included studies governed by several discrepancies such as lack of description of disease severity, small sample size, lack of power meter used, inconsistent and short-term follow-up duration and contradictory description of significance level of results. Significant variations in duration of the follow-up were noted, most of the selected studies from four weeks up to six months, as described in Table 4. Moreover, there is a discrepancy in the statistical significance in SPR + laser and SPR alone and between both groups (Table 5). There is a necessity for a follow-up period longer than six months to justify the effectiveness of adjunctive diode laser treatment, compared to SPR alone, taking into consideration clinical, microbiological and immunohistochemical assessment markers.
Impact of Single or Multiple Laser Sessions After SRP on Outcomes
A study by Dakhil et al, 2019 employed multiple diode laser procedures after SRP (three sessions at 0-day, 7th and 14th day), in which both groups (Laser + SRP and SRP alone) revealed a significant improvement in BOP and CAL. Nonetheless, no significant differences were shown between both groups after a three-month follow-up.47 It is important to highlight that this study only reported patients with PD > 4mm without indicating the number of the deep pockets evaluated. At the time of the procedure, the purpose of using saline irrigation between treatment were unreported.
Two studies utilised λ980 nm diode laser as an adjunct treatment modality to SRP. One of them was by Yadwad et al, 2017 where a single pocket was treated twice for 30 seconds with 60 seconds time interval on first visit and this protocol was repeated after one week, in which no additional effect SRP + laser group, compared SRP alone group on bacterial reduction was observed.56 In contrary to the other study by Dukić et al, 2013 which utilised multiple applications of λ 980nm irradiation after SRP on 0-day, 3rd, 7th day after SRP. The irradiation time was 20 seconds. It is important to note that this protocol was effective in improving PD only in moderate PD, ranging between 4 and 6 mm.54 This laser treatment regime was the closest to a study by Samulak et al, 2020 when 20 seconds of laser irradiation was applied on the tooth side and repeated twice within two weeks after the initial treatment.70
Interestingly, Saglam et al, 2014 study utilised a single laser irradiation application immediately after SRP which led to additional benefits in PD reduction (0.63 mm, p < 0.01, I2 = 0%) and CAL gain (0.52 mm, p = 0.02, I2 = 0%), compared to SRP alone.49 Conversely, another subgroup analysis evaluated the effect of multiple diode laser applications within the first week after SRP which did not confirm an advantage of laser application in combination with SPR. In terms of two applications of laser therapy after SRP, De Micheli et al, 2011 study showed the results of two laser treatment applications (at 0-day and 7th-day after SRP) were similar in PI and BOP improvement, for which additional laser therapy added no extra value.44 This coincided with Euzebio Alves et al, 2013 study when a protocol of λ 808nm irradiation protocol with two applications at 0-day and 7th day after SRP employed. All clinical parameters (BOP, PD, CAL) in both laser + SRP and SRP alone groups were improved but no statistically significant difference was observed between both groups.43 It is essential to note that only one study by Balasubramaniam et al, 2014 in this systematic review documented two examiners having measured the PD and were comparable.53
Based on the above-mentioned notes, it appears that there is a discrepancy in the studies’ results utilising different laser irradiation protocols, highlighting that multiple laser treatment sessions after SRP have not added any additional values to the outcomes.
Role of Influencing Factors on the Outcomes
Smoking is a significant contributing risk factor in the deterioration of periodontal diseases and debilitating the healing outcome after both surgical and NSPT, which is well documented in the literature.71 Long-term tobacco smoking habits can compromise the outcomes of any periodontal treatment. It’s tempting to speculate that adjunctive diode laser treatment to SRP outcomes would be compromised in the smokers’ cohort over the non-smokers. A study by Dakhil et al, 2019 included smokers and non-smokers, which might imply outcome bias.47 Two studies included smokers in their eligibility criteria,47,52 out of which one study conducted by Crispino et al, 2015 reporting a statistically significant reduction in the following parameters in the laser group versus (Vs) SRP alone: average of GI level (80% vs 44%), PI (67% vs 57%) and PD (76% vs 58%).52 In contrast, the second study conducted by Nguyen et al, 2015 has not shown a significant effect of adjunctive diode laser treatment to SRP on PD improvement, compared to the SRP alone group. It is important to note that λ 940nm irradiation combined SRP at 0.8W is shown to be insufficient for therapeutic purposes in the smokers’ cohort.48 It is a challenge to outline a laser proposal highlighting whether diode laser treatment can add any extra beneficial value in improving the clinical parameters in smokers with CP compared to a non-smoker cohort. Hence, RCTs with extensive data sample size combined with a robust laser protocol are warranted. It has been reported in literature that the link between CP and NIDDM can be bidirectional, in which the latter can be considered as a predisposing factor in developing CP and in severe periodontitis it can affect the glycaemic control in diabetic patients.72 It’s tempting to assume that using diode laser-assisted treatment with SRP can add value in improving the clinical parameters, microbiological and biochemical markers. In this systematic review, four of the 15 studies utilised participants with NIDDM. The results of a study by Chandra et al, 2019 revealed a statistically significant improvement of the clinical parameters and microbiological profile in the laser + SRP group compared to SRP from baseline to three-month follow-up timepoint, as well the biochemical markers (HbA1c). The study laser protocol was as follows: λ 808nm at 1.5W-1.8W in a CW. The reduction in HbA1c level was 16.25% in laser + SRP group versus 9.76% in SRP alone group; however, no statistically significant improvement between both groups was noted.42 Nonetheless, a study by Koçak et al 201650 has shown significant (P < 0.05) reduction in the following cytokines levels in the GCF at 3 months: IL-1, IL-6, IL-8, intercellular adhesion molecule and vascular cell adhesion molecule, as well HbA1c levels after treatment.50 It’s noteworthy that the utilisation of 940nm at 3 W in a gated emission mode (average power 1.5 W) has reduced the HbA1c levels more significantly (P < 0.05) in laser group, compared to SRP alone (0.41 vs 0.22% respectively). A greater improvement was noted in CAL and PD of moderate PD, ranging between 5–6 mm in diabetic patients, compared SRP alone group.50 Controversially, a study by Dakhil et al, 2019 that used λ 940nm at 0.8 W in a CW has shown a statistically significant gain in the CAL and reduction in BOP in both group at three-month recall, but no statistically significant differences were reported between both groups. It is noteworthy that a low power is shown to be insufficient to improve the clinical parameters and reduce the pathogens.47 This coincided with the results of Dengizek et al, 2018 study when 810nm laser, at 1W in gated mode (500ms on/500ms off) utilised. We have calculated the energy 500mJ per pulse with a peak irradiance of 796 W/cm2 and the average irradiance was 398W/cm2. The peak power of 1W in gated mode was sufficient to significantly reduce (P < 0.05) GI, BOP and PD in SRP + laser group, compared to SRP alone group. However, no significant differences (P > 0.05) in the HbA1c and serum CRP levels observed between both groups.46 The authors concluded that this laser protocol was efficient in contributing to reducing local inflammation and enhancing periodontal healing without beneficial effects on systematic inflammatory response and glycaemic control.46
As per the above, we can extrapolate the impact of the wavelength and the power output on the clinical periodontal parameters and the microbiological and biochemical markers, especially the HbA1c level, in utilising laser-assisted treatment in diabetic patients with CP. Moreover, the various power output utilised led to inconsistency and controversy in the outcomes.
Role of Laser Parameter Protocol on Outcomes
Laser wavelengths ranging between λ805- λ810nm have a high absorption affinity to haemoglobin, which can be associated with an increase in the risk of thermal damage when blood covers the root surface. On this note, a study by Crispino et al, 2015 suggested a laser regime allowing a few days interval between laser irradiation or the use of saline irrigation of the pocket before irradiation to remove blood from the pocket. At the two-month follow-up period, a statistically significant improvement in BOP in both groups was reported. The results showed (Table 4) that diode laser treatment can add benefits to SRP, compared to SRP alone as a considered routine use of adjunctive diode laser to SRP in the treatment of moderate-to-severe CP.52 However, within this context, a study by Lin et al, 2009 did not support this concept, when λ 810nm at 2W output in a CW delivered with 400µm fiber was utilised. Chlorhexidine gluconate solution was used to irrigate the pocket after SRP73 but no significant differences were observed between both groups (laser + SRP and SPR alone). Diode laser-assisted subgingival curettage resulted in statistically significant reduction in PD, SBI, and GI and CAL gain, compared to SPR alone group at a four-week follow-up recall.
The likelihood of the harmful effects of λ 808 nm laser on the periodontium was demonstrated by the results of De Micheli et al, 2011 study.44 In terms of the deterioration in PD and CAL in the laser + SRP group was based on the following laser protocol, at 0-day and 7th day after SRP: λ 808 nm, 1.5W, CW, 1193.7 W/cm2, 20 seconds exposure time per pocket. However, no differences between both groups was noted in terms of Pl and BOP parameters and total bacterial load of P.g., A.a., P.i. levels.44 Equally, a study by Euzebio Alves et al, 2013 utilised the same laser protocol and the latter study has shown no antibacterial effect in the laser group.43 Controversially, a study by Bansal et al, 2019 (808 nm, 0.4 W, CW with 20 seconds exposure time per site and 0.8 W in pulsed mode with 10 seconds exposure time per tooth site)74 and Giannelli et al, 2012 study (810 nm, 1 W, CW, 353.4 W/cm2, 66.7 J/cm2)75 have shown a significant reduction in the perio-pathogens in the laser + SRP group.
It is important to note that three of the 15 eligible studies (De Micheli et al, 2011,44 Euzebio Alves et al, 2013,43 Balasubbramani et al, 201453 have reported the use of power meter and suggested its importance, however, there is no documentation on the utilised therapeutic power that was measured with a power meter, which is quite confusing. Therefore, many conflicting pieces of evidence from various clinical trials are observed in literature.17
A study by Chandra et al, 2019 was unclear whether 1.5W or 1.8W or the value between was utilised. Nevertheless, this higher power output setting of λ 808nm diode laser delivered in a CW with a 300mm fibre showed a reduction in A.a and P.g colonies significantly and further reduction in the HbA1c level of 6.49% in laser + SPR group observed at three-month follow-up.42 This coincided with the positive correlation suggested in a study by Yadward et al, 2017. They observed that utilisation of 980nm at 2W in CW has a reduction in the levels of P.g colonies and clinical parameters evaluated at baseline, 4–6 weeks and 12–14 weeks follow-up period in both groups.56 This suggests that P.g levels play a vital role in the induction and progression of CP.76,77
A study by Zare et al, 2014 that utilised λ 980nm (1W power, CW, 400 μm fibre) has shown a reduction in BOP without significant negative impacts on root surfaces and gingival recession. The results in both groups have shown a statistically significant improvement (p<0.001) in GL (recession) and MGI without statistically significant differences between both groups in the above clinical parameters of p = 0.903 and p = 0.379, respectively.55 This is confirmed by Kreisler et al, 2002 study, when 1W power output had no or little effect on the root surface and the attachment level of the periodontal tissue.78 In contrast, a power output of 1.5 W and higher can cause thermal damage and attachment loss. This was demonstrated by an in vivo animal study conducted by Romanos et al, 2004 where the results have shown a complete removal of sulcular epithelium and connective tissue when λ 980nm utilised at 2W in CW with 15 second laser irradiation per pocket, whereas hand instruments used to eliminate the remnant of epithelial tissue in treated sites.79 Moreover, the treated sites with 4W showed necrosis signs, which subsequently can delay healing and compromise desirable outcomes.79
From the above notes, it is possible to extrapolate that utilisation of a high-power output of diode laser, as an adjunct to the NSPT has not added additional benefits, compared to SPR alone.
Two studies utilised λ 940nm at 0.8 W in CW.47,48 One of them was a study by Dakhil et al, 2019 which showed no statistically significant difference in the clinical parameters (PD, CAL and BOP) between both groups at three-month recall.47 While the other study was by Nguyen et al, 2015, which concluded that SRP + laser did not enhance clinical outcomes compared to SRP alone in the treatment of inflamed sites with ≥5 mm PD, in periodontal maintenance patients. There was a statistically significant reduction in PD and BOP and CAL gain, in both groups (SRP + laser and SRP alone), but no evidence of statistically significant difference between both groups.48 Similarly, the GCF and IL-1β levels showed no statistically significant differences between both groups.
Our observations from the above two studies are that it seems the peak power of 0.8 W in CW was ineffective in eradicating the bacteria in the pocket and eliminating the inflamed epithelium, which this coincided with by Zingle et al, 2012 study.80 Controversially, the Saglam et al, 2014 study utilised 940nm at 1.5W in a gated mode (20 msec on/20msec off) (average power 0.75 W), 300 micrometre tip revealed an improvement in the laser + SPR group, compared to SPR alone, in the following clinical parameters, respectively: CAL gains (1mm versus 0.9mm), reduction in the PD (1.9mm vs 0.8mm) and BOP (62% vs 52%). However, the levels of IL-1β, IL6, Matrix metalloproteinase (MMP)1, MMP8, and tissue inhibitor of metalloproteinase reduced in both groups, whereas IL8 level was increased; however, the latter exhibited a further significant increase in laser+ SRP group, compared to SRP alone group at the first month follow-up.49 Indeed, these conflicting results would have a great impact on employing these laser protocols for future studies.
A study by Meseli et al, 201745 utilised λ 810nm at a power output of 1 W, CW, 20 seconds per pocket, which showed a significant improvement in the following parameters: PI, PD, CAL, GI, BOP and GCF volume in both groups (laser + SRP and SRP alone), knowing that all SRP treatments should be in deeper than regular pockets of PD ≥ 3 mm, not only in PD ≥ 5 for the entire study, in order to achieve deeper decontamination. The bacteria from residual pockets have higher virulency than others. However, this was unclear in this study. This is another drawback of this study in not being vigilant in documentation.45
A study by Romanos et al, 2004 revealed that instrumentation of the periodontal tissues with 980nm laser led to a complete epithelial elimination when compared to NSPT with hand instruments.79 With an appropriate therapeutic power setting, diode laser wavelength of desirable penetration depth can reach target tissue, ranging from 0.5mm to 3mm.81 Hence, the laser bactericidal effects have a great impact on the residual pathogenic bacteria in the pocket epithelium. This can lead to complete de-epithelisation of the inflamed tissue in the PD, compared to mechanical NSPT alone and a better clinical connective tissue (CT) attachment, as well a reduction in the PD.82 Laser therapy can have a great influence on the molecular levels by increasing the vascular endothelial growth factor, transferring growth factor β, and mRNA expression of insulin growth factor on human gingival fibroblasts, which can subsequently modulate the CT turnover towards enhancing the healing process.83–85 After the laser therapy, coagulation at the site and blood clot stabilisation has been documented.86
A study by Hatipoğlu et al, 2017 utilised λ 940nm at average power output of 1.5W showed to be statistically significant in both groups in the following paraments and follow-up time points: at 1st-month significant improvement (p<0.05) in GI with no significant improvement in PD while the significant gain in CAL (p<0.05), at 3rd month: significant improvement in PI, but not in the PD between groups, and at 6th months both groups showed a more significant improvement (p<0.05) in PI, GI, BOP and CAL but not in PD.51 A study by Koçak et al, 2016 utilising the following laser protocol: λ 940nm, 1.5W, 20ms on, 20ms off, 20 J/cm2, exposure time: 20 seconds per pocket, in NIDDM participants with PD ≥ 5mm has shown a better improvement in the clinical parameters and HbA1c levels in SRP+ laser group, compared to SRP alone.50
It is important to highlight that PD reduction can be achieved after SRP via retraction of inflamed periodontal tissue. Nevertheless, adjunctive laser treatment can probably demonstrate a more significant reduction in PD due to laser properties in enhancing the healing process. However, it remains debatable due to a lack of reporting results in the literature.
It is noteworthy that each of the tested wavelengths ranging from 808nm to 810 and 940nm to 980nm has a slightly different degree of affinity to water and haemoglobin, melanin, and porphyrins, which requires careful consideration when future laser protocols are formulated. Also, the laser irradiation per pocket’s exposure time plays a fundamental key factor in achieving optimal outcomes. The degree that the target tissue absorbs of the laser photonic energy, which is transformed into heat to achieve the desired effects depends on the exposure time duration. In this systematic review, the range of the exposure time is between 20 and 60 seconds with various implications on the outcomes, which only 11 studies reported this variable,43–47,49–56 where two of them stated the treatment time 30 seconds twice with a 60 seconds thermal relaxation time.53,56 Furthermore, the laser beam profile has a thermal effect on bacteria as they are killed, it also has the property of deactivating the deeper-seated bacterial toxins in the cementum portion of the root.87
From our observation, 12 of the 15 studies43–45,47–51,53–56 (approximately 70%) failed to provide a clear understanding of their technique in measuring the PD and their allocation in the mouth, as and the study designs were very confusing, as they mixed up single and multi-rooted teeth in their assessments. These discrepancies have a great impact on the clinical outcomes and utilised pockets >5mm without specifying if these cases were moderate periodontitis (PD 4 −6mm) or severe (PD >7mm). This can have a great influence on the healing process. However, the remaining three studies42,46,52 have stated that they utilised moderate periodontitis with a PD between 4–6mm and Saglam et al, 2014 divided the PD groups into moderate and severe in their study.49
It appears that the critical level of the periodontal disease severity has a great impact on the proposing therapeutic protocol. In CP patients with PPD ≤ 5 mm, SRP plus diode laser (λ 808- λ 980 nm) is more effective, compared to SRP alone. This depends on a robust methodology and laser protocol.
Moreover, the treatment outcome can be influenced by various risk factors such as smoking, diabetes, life style, host genetics, cardiovascular diseases, pathogenic oral microbiome or combined pathogens.17,77,88 The photonic energy of specific laser wavelengths shows high absorption affinity to brown/black-pigmented anaerobic (Gram−ve) bacteria (P.g, P.i, Prevotella nigrescens, Prevotella melaninogenica and Bacteroides), which are the prime pathogens in periodontitis.60,77,88–91 Therefore, this can be one of the key factors in reducing bacterial colony volume and enhancing the clinical outcome.
All the included studies in the present review have described the fiber movement technique inside the periodontal pocket. All the studies utilised a fibre movement from the apical to the coronal part of the pocket with a sweeping or spiral movement shown in Figure 10, except one study by Dukić et al, 2013 which employed a tip movement in a corono-apical direction (parallel).54
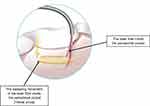 |
Figure 10 Illustrates the spiral movement of the laser fiber (arrow in yellow) inside the periodontal pocket by which 14 of the selected studies in this systematic review utilized. |
Impact of the Initiated Tip (Hot Tip) and Non-Initiation on Clinical Outcome
Since diode laser tips impact heat transfer on tissues, a lot of understanding is required for the use of initiated versus non-initiated fibre optics as well as the thermal effects on the tissues. The fibre tip initiation is achieved when the tip of the laser touches a dark chromophore. Both the power settings of the diode laser and type of initiator used, affect the degree to which the temperature of the soft tissue increases during incisions and has to be considered for safety in soft tissue applications.92 Given the high affinity of the diode group of lasers for dark pigments and supplemented with the use of an initiated tip, as soft tissue absorbs energy, it results in heat production and thus a rise in tissue temperature.52 This process helps in thorough elimination of the infected sulcular epithelium and can be achieved as when compared to conventional hand and ultrasonic instruments. Evidence-based scientific evidence shows that blue articulating paper seems to be the safest method of fibre initiation, compared with the other initiators with no initiator providing the best cutting efficiency.93
In the present systematic review, three of the 15 studies reported the tip was initiated,48,52,53 of which one study illustrated via photo but was not documented in the methodology,53 whereas the remaining 12 studies failed to report in their eligibility criteria. Nonetheless, reading through their manuscripts, it was indicative that the tip was cleaved, as required, and the concept of treatment suggested that the tip was “hot tip”; however, it is not clear to the readers, if the tip was initiated or self-initiated.
A study by Kurtzman et al, 2015 suggested a diode laser-assisted periodontal treatment protocol in which the use of an uninitiated tip (1.5–1.8W, pulsed mode) for bacterial laser reduction is followed by ultrasonic scaling and the use of a diode laser with an initiated tip (0.4 to 0.8 W in CW) for sulcular debridement.94 The final step is to irrigate the pockets with the ultrasonic unit using chlorhexidine with antimicrobial capabilities against Gram−ve and Gram+ve bacteria and fungi. The authors believe that this protocol can be utilised to decrease pocket depth following healing compared to SRP alone.94 An in vivo animal study by Romanos et al, 2018 aimed to assess potential photothermal risks that could be caused due to irradiation of a four-walled peri-implant defect using various diode lasers.93 In this study, the implant was irradiated with pulsed diode lasers of λ 940nm, λ 975nm and λ 980 nm for 30 seconds, using non-initiated, cork and blue paper-initiated tips followed by an evaluation of temperature differences at the apical and coronal regions of the implant. The authors showed that the initiator does not affect the maximum temperatures produced during dental implant surface decontamination. However, non-initiated diode laser tips may overheat faster (within 30 seconds) than initiated tips. There is minimal risk of overheating at the apical portion of the implant. In terms of overheating risk, it seems that the 940 nm diode laser is the safest of the evaluated diode laser systems.92
Üstün et al, 2014 conducted a single-blind, randomised-controlled, split-mouth clinical trial on 21 patients to determine the clinical and biochemical efficacy of an λ 810 nm diode laser as an adjunct to SRP in the management of CP patients.95 The authors have emphasised on the use of correct laser parameters since the utilisation of low energy settings may be ineffective in the complete removal of the pocket epithelium, and high energy settings may cause thermal damage to the surrounding tissues. In this study, the 810 nm laser was set at a peak power of 2.5 W, ½ duty cycle, 20 Hz, and applied with a 320μm fibre. The “hot tip” technique was used in this study because of its low tissue penetration, permitting complete removal of the gingival epithelium contaminated by intracellular periodontopathogens, with minimal injury to the underlying lamina propria which have been proven in the scientific literature.75 The fibre was introduced like a probe into the periodontal pocket. After the activation of the laser, the fibre was slowly moved from apical to coronal in a sweeping motion to avoid thermal side effects. No complications related to laser application were reported with the described parameters and technique. Furthermore, the authors believe that as the carbonised tip absorbed that wavelength and re-emitted much longer infrared wavelengths, the transmission of λ 810 nm light to the tissue was likely to be minimal if it occurred at all.7
Based on the above reported irradiation parameters, suggesting that diode lasers capitulate a complete removal of the diseased sulcular epithelium, without causing major signs of connective tissue damage. Diode laser photonic irradiation resulted in micro-vessel constriction, possibly related to direct vasomotor effects and/or deactivation of local proinflammatory cytokines and the induction of bleeding necessary for the formation of a clot and to promote postoperative haemostasis. Owing to these adjunctive benefits, the diode group of lasers can be routinely associated as potential adjuncts SR in treating periodontal pockets in patients with moderate-to-severe CP.
RoB Assessment
All eligible studies were subjected to a qualitative assessment to verify the respective study protocol and methodology. The results of this assessment have indicated that 40% of the studies had an overall high RoB, whereas 33.33% with moderate risk (some concerns). A vast majority of the bias has shown in many domains illustrated in Figures 6–9. Another key finding of this systematic review is the presence of industry funding mentioned in three of the 15 studies,43,44,48 whereas five of the 15 studies47,51–53,55 showed 33.33% of moderate risk of bias (Figure 9). After careful reading of the eligible studies, it is easy to extrapolate the presence of a potential conflict of interest. Owing to the disparity in the qualitative assessment of the studies, the results are questionable, and the methodology associated with a high risk of bias cannot be reproduced.
The following are the limitations of present systematic review: lack of the documentation on the fundamental data of chosen articles preventing us from proposing a laser protocol; due to the heterogeneity of the data, meta-analysis was not possible to conduct; and producibility of the assessment methods and laser protocol remain debatable due to the elevated percentage of high and moderate risks of bias of the selected studies.
Conclusions and Future Prespectives
In the view of the limited available literature data and critical appraisal of this systematic review, it was concluded that the efficacy of diode laser-assisted treatment of wavelengths between λ 808- λ 980nm, as an adjunctive treatment modality over SRP, remains debatable Although the results of the majority of the included studies have indicated that diode-laser treatment is effective, as an adjunctive treatment modality over the conventional NSPT, several discrepancies amongst the eligible studies were noted.
The observational nature of this systematic review highlighted after scrutinising the available data, an attempt to propose a laser protocol that can be considered for future RCTs was a great challenge due to the lack of consensus in delivering a reliable laser protocol, which can be reproducible for future studies. Double-blind, multicenter RCT studies, comparing a wide range of diode laser wavelengths with or without SRP to SRP alone to justify treatment effectiveness and extrapolate standardised laser protocols of various wavelengths.
Author Contributions
All the authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas. All the authors have drafted or written, or substantially revised or critically reviewed the article. All the authors have agreed on the journal to which the article submitted. All the authors have reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage. All authors agreed to take responsibility and be accountable for the contents of the article.
Joined last authorship: Ioana Roxana Bordea and Reem Hanna contributed eqaully.
Funding
This research received no external funding.
Disclosure
The authors declared no conflicts of interest for this work.
References
1. Smiley CJ, Tracy SL, Abt E, et al. Systematic review and meta-analysis on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J Am Dent Assoc. 2015;146:508–524.e5. doi:10.1016/j.adaj.2015.01.028
2. Bosshardt DD, Stadlinger B, Terheyden H. Cell-to-cell communication - periodontal regeneration. Clin Oral Implants Res. 2015;26(3):229–239. doi:10.1111/clr.12543
3. Al‐Shammari KF, Neiva RF, Hill RW, et al. Surgical and non‐surgical treatment of chronic periodontal disease. Int Chin J Dent. 2002;2:
4. Graziani F, Karapetsa D, Alonso B, Herrera D. Nonsurgical and surgical treatment of periodontitis: how many options for one disease? Periodontol 2000. 2017;75(1):152–188.
5. Caton J, Armitage G, Berlundh T, et al. A new classification scheme for periodontal and peri-implant diseases and conditions-introduction and key changes from the 1999 classification. J Periodontol. 2018;89(Suppl 1):1–8. doi:10.1002/JPER.18-0157
6. Papapanou PN, Sanz M, Buduneli N, et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol. 2018;89(Suppl 1):173–182. doi:10.1002/JPER.17-0721
7. Bosshardt D, Lang N. The junctional epithelium: from health to disease. J Dent Res. 2005;84(1):9–20. doi:10.1177/154405910508400102
8. Nanci A, Bosshardt DD. Structure of periodontal tissues in health and disease. Periodontol 2000. 2006;40(1):11–28. doi:10.1111/j.1600-0757.2005.00141.x
9. Kamma JJ, Vasdekis VG, Romanos GE. The effect of diode laser (980 nm) treatment on aggressive periodontitis: eval- uation of microbial and clinical parameters. Photomed Laser Surg. 2009;27:11–19. doi:10.1089/pho.2007.2233
10. Mummolo S, D’Ercole S, Marchetti E, et al. Oral antiseptic and periodontitis: a clinical and microbiological study. Oral Health Dent Manag. 2014;13:698–702.
11. Varela VM, Heller D, Silva-Senem MX, et al. Systemic antimicrobials adjunctive to a repeated mechanical and antiseptic therapy for aggressive periodontitis: a 6-month randomized controlled trial. J Periodontol. 2011;82(8):1121–1130. doi:10.1902/jop.2011.100656
12. Everett JD, Rossmann JA, Kerns DG, Al-Hashimi I. Laser assisted non-surgical periodontal therapy: a double blind, randomized clinical trial. Open Dent J. 2017;11:79–90. doi:10.2174/1874210601711010079
13. Krohn-Dale I, Bøe OE, Enersen M, Leknes KN. Er:YAG laser in the treatment of periodontal sites with recurring chronic inflammation: a 12-month randomized, controlled clinical trial. J Clin Periodontol. 2012;39(8):745–752. doi:10.1111/j.1600-051X.2012.01912.x
14. Zhao Y, Yin Y, Tao L, Nie P, Tang Y, Zhu M. Er:YAG laser versus scaling and root planing as alternative or adjuvant for chronic periodontitis treatment: a systematic review. J Clin Periodontol. 2014;41(11):1069–1079. doi:10.1111/jcpe.12304
15. Yanli Y, Chunmei X, Yafei W, Lei Z. Clinical and microbiologic follow-up evaluations after non-surgical periodontal treatment with Nd: YAG laser and scaling and root planning. Hua Xi Kou Qiang Yi Xue Za Zhi= Huaxi Kouqiang Yixue Zazhi= West China Journal of Stomatology. 2017;35(6):618–624. Chinese. doi:10.7518/hxkq.2017.06.011
16. Jia L, Jia J, Xie M, et al. Clinical attachment level gain of lasers in scaling and root planing of chronic periodontitis: a network meta-analysis of randomized controlled clinical trials. Lasers Med Sci. 2020;35(2):473–485. doi:10.1007/s10103-019-02875-5
17. Cobb CM. Lasers in periodontics: a review of the literature. J Periodontol. 2006;77:545–564. doi:10.1902/jop.2006.050417
18. Aoki A, Sasaki KM, Watanabe H, Ishikawa I. Lasers in nonsurgical periodontal therapy. Periodontol 2000. 2004;36:59–97. doi:10.1111/j.1600-0757.2004.03679.x
19. Leibbrandt A, Penninger JM. RANK/RANKL: regulators of immune responses and bone physiology. Ann NY Acad Sci. 2008;1143:123–150. doi:10.1196/annals.1443.016
20. Mikami R, Mizutani K, Sasaki Y, Iwata T, Aoki A. Patient-reported outcomes of laser- assisted pain control following non-surgical and surgical periodontal therapy: a systematic review and meta-analysis. PLoS One. 2020;15(9):e0238659. doi:10.1371/journal.pone.0238659
21. Hannas AR, Pereira JC, Granjeiro JM, Tjäderhane L. The role of matrix metalloproteinases in the oral environment. Acta Odontol Scand. 2007;65(1):1–13. doi:10.1080/00016350600963640
22. Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology. 2003;149:279–294. doi:10.1099/mic.0.26082-0
23. Al-hebshi NN, Shuga-Aldin HM, Al-Sharabi AK, et al. Subgingival periodontal pathogens associated with chronic periodontitis in Yemenis. BMC Oral Health. 2014;14:13. doi:10.1186/1472-6831-14-13
24. Belibasakis GN, Bostanci N. The RANKL-OPG system in clinical periodontology. J Clin Periodontol. 2012;39(3):239–248. doi:10.1111/j.1600-051X.2011.01810.x
25. Hanna R, Dalvi S, Amaroli A, De Angelis N, Benedicenti S. Effects of photobiomodulation on bone defects grafted with bone substitutes: a systematic review of in vivo animal studies. J Biophotonics. 2021;14(1):e202000267. doi:10.1002/jbio.202000267
26. Udagawa N, Takahashi N, Yasuda H, et al. Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology. 2000;141(9):478–484. doi:10.1210/endo.141.9.7634
27. Majumder P, Ghosh S, Dey SK. Matrix metalloproteinase gene polymorphisms in chronic periodontitis: a case-control study in the Indian population. J Genet. 2019;98:32. doi:10.1007/s12041-019-1077-2
28. Javed F, Ahmed HB, Saeed A, Mehmood A, Bain C. Whole salivary interleukin-6 and matrix metalloproteinase-8 levels in chronic periodontitis patients with and without prediabetes. J Periodontol. 2014;85:e130–e135. doi:10.1902/jop.2013.130514
29. De Angelis N, Hanna R, Signore A, Amaroli A, Benedicenti S. Effectiveness of dual-wavelength (Diodes 980 Nm and 635 Nm) laser approach as a non-surgical modality in the management of periodontally diseased root surface: a pilot study. Biotechnol Biotechnol Equip. 2018;32(6):1575–1582. doi:10.1080/13102818.2018.1544034
30. Dalvi S, Benedicenti S, Hanna R. Effectiveness of photobiomodulation as an adjunct to nonsurgical periodontal therapy in the management of periodontitis- a systematic review of in vivo human studies. Photochem Photobiol. 2020. doi:10.1111/php.13348
31. Maisch T. A new strategy to destroy antibiotic resistant microorganisms: antimicrobial photodynamic treatment. Mini Rev Med Chem. 2009;9(8):974–983. doi:10.2174/138955709788681582
32. Quadri T, Miranda L, Tuner J, Gustafsson A. The short term effects of low-level lasers as adjunct therapy in the treatment of periodontal inflammation. J Clin Periodontol. 2005;32(7):714–719. doi:10.1111/j.1600-051X.2005.00749.x
33. Partovi F, Izatt JA, Cothren RM, et al. A model for thermal ablation of biological tissue using laser radiation. Lasers Surg Med. 1987;7(2):141–154. doi:10.1002/lsm.1900070202
34. Lobo TM, Pol DG. Evaluation of the use of a 940 nm diode laser as an adjunct in flap surgery for treatment of chronic periodontitis. J Indian Soc Periodontol. 2015;19(1):43–48. doi:10.4103/0972-124X.145808
35. Heitz‐Mayfield LJ, Schätzle M, Löe H, et al. Clinical course of chronic periodontitis. II. Incidence, characteristics and time of occurrence of the initial periodontal lesion. J Clin Periodontol. 2003;30:
36. Fontana CR, Kurachi C, Mendonca CR, Bagnato VS. Microbial reduction in periodontal pockets under exposition of a medium power diode laser: an experimental study in rats. Laser Surg. 2004;22:
37. Moritz A, Gutknecht N, Doertbudak O, et al. Bacterial reduction in periodontal pockets through irradiation with a diode laser: a pilot study. J Clin Laser Med Surg. 1997;15(1):33–37. doi:10.1089/clm.1997.15.33
38. Slot DE, Kranendonk AA, Paraskevas S, Van der Weijden F. The effect of a pulsed Nd:YAG laser in non-surgical periodontal therapy. J Periodontal. 2009;80:1041-1056. doi:10.1902/jop.2009.080571
39. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
40. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi:10.1136/bmj.l4898
41. Higgins JPT, Eldridge S, Li T. Chapter 23: including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019). Cochrane; 2019. Available from: www.training.cochrane.org/handbook.
42. Chandra S, Shashikumar P. Diode laser - a novel therapeutic approach in the treatment of chronic periodontitis in type 2 diabetes mellitus patients: a prospective randomized controlled clinical trial. J Lasers Med Sci. 2019;10(1):56–63. doi:10.15171/jlms.2019.09
43. Euzebio Alves VT, de Andrade AK, Toaliar JM, et al. Clinical and microbiological evaluation of high intensity diode laser adjutant to non-surgical periodontal treatment: a 6-month clinical trial. Clin Oral Investig. 2013;17(1):87–95. doi:10.1007/s00784-012-0703-7
44. De Micheli G, de Andrade AK, Alves VT, et al. Efficacy of high intensity diode laser as an adjunct to non-surgical periodontal treatment: a randomized controlled trial. Lasers Med Sci. 2011;26(1):43–48. doi:10.1007/s10103-009-0753-5
45. Meseli SE, Kuru B, Kuru L. Effects of 810-nanometer diode laser as an adjunct to mechanical periodontal treatment on clinical periodontal parameters and gingival crevicular fluid volume of residual periodontal pockets. Niger J Clin Pract. 2017;20(4):427–432. doi:10.4103/1119-3077.181382
46. Dengizek ES, Gursel M, Eltas A, et al. Evaluation of long‐term effects of diode laser application in periodontal treatment of poorly controlled type 2 diabetic patients with chronic periodontitis. Int J Dent Hygiene. 2019;17:292–299. doi:10.1111/idh.12384
47. Dakhil FF, Mahmood AS. Evaluation of clinical effects of a 940nm diode laser as adjunctive therapy in the treatment of periodontal pockets. Iraqi Dent J. 2017;39(1):12–15.
48. Nguyen NT, Byarlay MR, Reinhardt RA, et al. Adjunctive non-surgical therapy of inflamed periodontal pockets during maintenance therapy using diode laser: a randomized clinical trial. J Periodontol. 2015;86(10):1133–1140. doi:10.1902/jop.2015.150152
49. Saglam M, Kantarci A, Dundar N, Hakki SS. Clinical and biochemical effects of diode laser as an adjunct to nonsurgical treatment of chronic periodontitis: a randomized, controlled clinical trial. Lasers Med Sci. 2014;29(1):37–46. doi:10.1007/s10103-012-1230-0
50. Koçak E, Sağlam M, Kayış SA, et al. Nonsurgical periodontal therapy with/without diode laser modulates metabolic control of type 2 diabetics with periodontitis: a randomized clinical trial. Lasers Med Sci. 2016;31(2):343–353. doi:10.1007/s10103-016-1868-0
51. Hatipoğlu M, Aytekin Z, Daltaban Ö, et al. The effect of diode laser as an adjunct to periodontal treatment on clinical periodontal parameters and halitosis: a randomized controlled clinical trial. Cumhuriyet Dent J. 2017;20:152–160. doi:10.7126/cumudj.369035
52. Crispino A, Figliuzzi MM, Iovane C, et al. Effectiveness of a diode laser in addition to non-surgical periodontal therapy: study of intervention. Ann Stomatol. 2015;6(1):15–20.
53. Balasubramaniam AS, Thomas LJ, Ramakrishnanan T, Ambalavanan N. Short-term effects of nonsurgical periodontal treatment with and without use of diode laser (980 nm) on serum levels of reactive oxygen metabolites and clinical periodontal parameters in patients with chronic periodontitis: a randomized controlled trial. Quintessence Int. 2014;45(3):193–201. doi:10.3290/j.qi.a31206
54. Dukić W, Bago I, Aurer A, Roguljić M. Clinical effectiveness of diode laser therapy as an adjunct to non-surgical periodontal treatment: a randomized clinical study. J Periodontol. 2013;84(8):1111–1117. doi:10.1902/jop.2012.110708
55. Zare D, Haerian A, Molla R, Vaziri F. Evaluation of the effects of diode (980 nm) laser on gingival inflammation after nonsurgical periodontal therapy. J Lasers Med Sci. 2014;5(1):27–31.
56. Yadwad KJ, Veena HR, Patil SR, Shivaprasad BM. Diode laser therapy in the management of chronic periodontitis - A clinico-microbiological study. Interv Med Appl Sci. 2017;9(4):191–198. doi:10.1556/1646.9.2017.38
57. Takei HH, Han TJ, Carranza FA, et al. Flap technique for periodontal bone implants: papilla preservation technique. J Periodontol. 1985;56(4):204–210. doi:10.1902/jop.1985.56.4.204
58. Jansson H, Bratthall G, Söderholm G. Clinical outcome observed in subjects with recurrent periodontal disease following local treatment with 25% metronidazole gel. J Periodontol. 2003;74:372–377. doi:10.1902/jop.2003.74.3.372
59. Kinane DF, Attström R. Advances in the pathogenesis of periodontitis. Group B consensus report of the fifth European Workshop in Periodontology. J Clin Periodontol. 2005;32(6):130–131. doi:10.1111/j.1600-051X.2005.00823.x
60. Cobb CM. Lasers and the treatment of periodontitis: the essence and the noise. Periodontol 2000. 2017;75(1):205–295.
61. Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3:17038. doi:10.1038/nrdp.2017.38
62. American Academy of Periodontology; Parameter on chronic periodontitis with slight to moderate loss of periodontal support. J Periodontol. 2000;71(5):853–855. doi:10.1902/jop.2000.71.5-S.853
63. Takei HH. Clinical diagnosis. In: Newman MG, Takei HH, Klokkevold PR, editors. Textbook of Clinical Periodontology.
64. Greenstein G. Contemparary interpration of probing depth assessment: diagnostic and therapeutic impòlicaitons: a literature review. J Periodontol. 1997;68(12):1194–1205. doi:10.1902/jop.1997.68.12.1194
65. Bhowmick T, Varughese TA, Arakali S, Boruchoff SE. Do positive anaerobic culture results affect physicians’ clinical management decisions? Open Forum Infect Dis. 2017;4(1):ofw236. doi:10.1093/ofid/ofw236
66. Cutroneo G, Piancino MG, Ramieri G, et al. Expression of muscle-specific integrins in masseter muscle fibers during malocclusion disease. Int J Mol Med. 2012;30:235–242. doi:10.3892/ijmm.2012.986
67. Akram Z, Al-Shareef SA, Daood U, et al. Bactericidal efficacy of photodynamic therapy against periodontal pathogens in periodontal disease: a systematic review. Photomed Laser Surg. 2016;34:137–149. doi:10.1089/pho.2015.4076
68. Caruso U, Nastri L, Piccolomini R, et al. Use of diode laser 980 nm as adjunctive therapy in the treatment of chronic periodontitis. A randomized controlled clinical trial. New Microbiol. 2008;31(4):513–518.
69. Uslu MÖ, Eltas A, Marakoğlu İ, et al. Effects of diode laser application on inflammation and mpo in periodontal tissues in a rat model. J Appl Oral Sci. 2018;26:e20170266. doi:10.1590/1678-7757-2017-0266
70. Samulak R, Suwała M, Dembowska E. Nonsurgical periodontal therapy with/without 980 nm diode laser in patients after myocardial infarction: a randomized clinical trial. Lasers Med Sci. 2020. doi:10.1007/s10103-020-03136-6
71. Chang CH, Han ML, Teng NC, et al. Cigarette smoking aggravates the activity of periodontal disease by disrupting redox homeostasis- an observational study. Sci Rep. 2018;8(1):1–10. doi:10.1038/s41598-018-29163-6
72. Preshaw PM, Alba AL, Herrera D, et al. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012;55:21–31. doi:10.1007/s00125-011-2342-y
73. Lin J, Bi L, Wang L, et al. Gingival curettage study comparing a laser treatment to hand instruments. Lasers Med Sci. 2009;26(1):7–11. doi:10.1007/s10103-009-0732-x
74. Bansal V, Gupta R, Dahiya P, et al. A clinico-microbiologic study comparing the efficacy of locally delivered chlorhexidine chip and diode LASER as an adjunct to non- surgical periodontal therapy. J Oral Biol Craniofac Res. 2019;9:67–72. doi:10.1016/j.jobcr.2018.09.001
75. Giannelli M, Formigli L, Lorenzini L, Bani D. Combined photoablative and photodynamic diode laser therapy as an adjunct to non-surgical periodontal treatment: a randomized split-mouth clinical trial. J Clin Periodontol. 2012;39(10):962–970. doi:10.1111/j.1600-051X.2012.01925.x
76. Wu YM, Yan J, Chen L, Gu Z-Y. Association between infection of different strains of Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans in subgingival plaque and clinical parameters in chronic periodontitis. J Zhejiang Univ Sci. 2007;8:121–131. doi:10.1631/jzus.2007.B0121
77. Yilmaz S, Kuru B, Kuru L, et al. Effect of gallium arsenide diode laser on human periodontal disease: a microbiological and clinical study. Lasers Surg Med. 2002;30(1):60–66. doi:10.1002/lsm.10010
78. Kreisler M, Al Haj H, Daubländer M, et al. Effect of diode laser irradiation on root surfaces in vitro. J Clin Laser Med Surg. 2002;20:63–69. doi:10.1089/104454702753768034
79. Romanos GE, Henze M, Banihashemi S, et al. Removal of epithelium in periodontal pockets following diode (980 nm) laser application in the animal model: an in vitro study. Photomed Laser Surg. 2004;22(3):177–183. doi:10.1089/1549541041438597
80. Zingale J, Harpenau L, Chambers D, Lundergan W. Effectiveness of root planing with diode laser curettage for the treatment of periodontitis. J Calif Dent Assoc. 2012;40:786–793.
81. Aoki A, Mizutani K, Takasaki AA, et al. Current status of clinical laser applications in periodontal therapy. Gen Dent. 2008;56:674–687.
82. Kreisler M, Al Haj H, d’Hoedt B. Clinical efficacy of semiconductor laser application as an adjunct to conventional scaling and root planing. Lasers Surg Med. 2005;37(5):350–355.
83. Hakki SS, Bozkurt SB. Effects of different setting of diode laser on the mRNA expression of growth factors and type I collagen of human gingival fibroblasts. Lasers Med Sci. 2012;27:
84. Sume SS, Kantarci A, Lee A, et al. Epithelial to mesenchymal transition in gingival overgrowth. Am J Pathol. 2010;177:208–218. doi:10.2353/ajpath.2010.090952
85. Kantarci A, Nseir Z, Kim YS, et al. Loss of basement membrane integrity in human gingival overgrowth. J Dent Res. 2011;90:887–893. doi:10.1177/0022034511404703
86. Rastegar S, Jacques SL, Motamedi M, Kim BM. Theoretical analysis of equivalency of high-power diode laser (810 nm) and Nd: YAG laser (1064 nm) for coagulation of tissue: predictions for prostate coagulatio. SPIE. 1992;1646:150–160.
87. Pick RM, Pecaro BC, Silberman CJ. The laser gingivectomy. The use of the CO2 laser for the removal of phenytoin hyperplasia. J Periodontol. 1985;56:492–496.
88. Burns T, Wilson M, Pearson GJ. Killing of cariogenic bacteria by light from a gallium aluminium arsenide diode laser. J Dent. 1994;22(5):273–278. doi:10.1016/0300-5712(94)90056-6
89. Schoop U, Kluger W, Moritz A, et al. Bactericidal effect of different laser systems in the deep layers of dentin. Lasers Surg Med. 2004;35(2):111–116. doi:10.1002/lsm.20026
90. Dederich DN. Laser curettage: an overview. Compend Contin Educ Dent. 2002;23(11A):1097–1103.
91. Assaf M, Yilmaz S, Kuru B, et al. Effect of the diode laser on bacteremia associated with dental ultrasonic scaling: a clinical and microbiological study. Photomed Laser Surg. 2007;25(4):250–256. doi:10.1089/pho.2006.2067
92. Romanos GE, Motwani SV, Montanaro NJ, et al. Photothermal effects of defocused initiated versus noninitiated diode implant irradiation. Photobiomodul Photomed Laser Surg. 2019;37(6):356–361. doi:10.1089/photob.2018.4545
93. Romanos GE, Sacks D, Montanaro N, et al. Effect of initiators on thermal changes in soft tissues using a diode laser. Photomed Laser Surg. 2018;36(7):386–390. doi:10.1089/pho.2017.4428
94. Kurtzman GM, Hughes MK. Evolution of comprehensive care, part 2. Hygiene and periodontal considerations. Dent Today. 2015;34(2):104–109.
95. Üstün K, Erciyas K, Sezer U, et al. Clinical and biochemical effects of 810 nm diode laser as an adjunct to periodontal therapy: a randomized split-mouth clinical trial. Photomed Laser Surg. 2014;32(2):61–66. doi:10.1089/pho.2013.3506
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



