Back to Journals » OncoTargets and Therapy » Volume 16
Immunotherapy Plus Radiotherapy for the Treatment of Sarcomas: Is There a Potential for Synergism?
Authors Wang J , Ge H , Tian Z
Received 1 March 2023
Accepted for publication 25 May 2023
Published 7 June 2023 Volume 2023:16 Pages 385—397
DOI https://doi.org/10.2147/OTT.S410693
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Arseniy Yuzhalin
Jiaqiang Wang,1 Hong Ge,2 Zhichao Tian1
1Department of Bone and Soft Tissue, the Affiliated Cancer Hospital of Zhengzhou University and Henan Cancer Hospital, Zhengzhou, Henan Province, 450008, People’s Republic of China; 2Department of Radiotherapy, the Affiliated Cancer Hospital of Zhengzhou University and Henan Cancer Hospital, Zhengzhou, Henan Province, 450008, People’s Republic of China
Correspondence: Zhichao Tian, Department of Bone and Soft Tissue, the Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, 450008, Henan Province, People’s Republic of China, Email [email protected]
Abstract: Soft tissue sarcoma (STS) is a highly heterogeneous malignant tumor derived from mesenchymal tissue. Advanced STS has a poor response to the current anti-cancer therapeutic options, with a median overall survival of less than two years. Thus, new and more effective treatment methods for STS are needed. Increasing evidence has shown that immunotherapy and radiotherapy have synergistic therapeutic effects against malignant tumors. In addition, immunoradiotherapy has yielded positive results in clinical trials for various cancers. In this review, we discuss the synergistic mechanism of immunoradiotherapy in cancer treatment and the application of this combined regimen for the treatment of several cancers. In addition, we summarize the existing evidence on the use of immunoradiotherapy for the treatment of STS and the relevant clinical trials that are currently ongoing. Furthermore, we identify challenges in the use of immunoradiotherapy for the treatment of sarcomas and propose methods and precautions for overcoming these challenges. Lastly, we propose clinical research strategies and future research directions to help in the research and treatment of STS.
Keywords: PD-1 inhibitor, PD-L1 inhibitor, immune checkpoint inhibitor, radiotherapy, sarcoma, immunoradiotherapy
Introduction
Soft tissue sarcoma (STS) is a highly heterogeneous (more than 70 subtypes) malignant tumor derived from mesenchymal tissue.1–3 Although the incidence of STS is low, hundreds of thousands of advanced STS cases are recorded worldwide each year. Chemotherapy is the most effective treatment for advanced STS, with the response rate of only 15–20%.4,5 This poor efficacy resulting in a median overall survival of less than two years.6–8 Therefore, new therapeutic options are needed for the effective treatment of advanced STS.
Immunotherapies are treatments that kill tumor cells by activating or promoting the body’s anti-tumor immunity.9,10 At present, the widely used immunotherapies in clinical practice include programmed death receptor‐1 (PD‐1)/programmed death protein ligand‐1 (PD‐L1) inhibitors and cytotoxic T-lymphocyte antigen 4 (CTLA-4) inhibitors, and the new types of immunotherapy include adoptive cellular therapies and cancer vaccines.11,12 PD-1/L1 and CTLA-4 inhibitors have promising efficacy (with a response rate of 10–28%) in the treatment of a few pathological subtypes of STS (undifferentiated pleomorphic sarcoma, dedifferentiated liposarcoma, alveolar soft part sarcoma and angiosarcoma), and their efficacy in the treatment of other subtypes of STS are limited.11,13–15 Among approaches of adaptive cellular therapies, only engineered T-cell receptor (TCR) therapy has achieved remarkable efficacy in synovial sarcoma, with a response rate of over 50%.16,17 The efficacy of adoptive cellular therapies in other subtypes of STS are limited.11 Similarly, cancer vaccines only have promising efficacy in synovial sarcoma and myxoid/round cell liposarcoma.18 Overall, the efficacy of immunotherapy alone in STS is disappointing. Increasingly combined therapies have been used to improve the efficacy of immunotherapy for malignant tumors and achieve synergistic sensitization.19–21 Among these combined treatment strategies, the combination of immunotherapy and radiotherapy (immunoradiotherapy) has been extensively studied and is expected to have significantly improved treatment effects on malignant tumors, including STS.22–26
In this review, we summarize the synergistic mechanism of immunoradiotherapy for the treatment of various malignant tumors, the clinical research on and progress in the application of immunoradiotherapy for the treatment of various malignant tumors, the existing evidence on the use of immunoradiotherapy for the treatment of STS, and key ongoing clinical trials. In addition, we propose clinical research strategies and future research directions to help in the research and treatment of STS.
Process of Anti-Tumor Immunity
Tumor immune response is a dynamic process of interaction between anti-tumor and pro-tumor immune responses. Anti-tumor immune cells include natural killer (NK) cells, dendritic (DC) cells, T cells (including cytotoxic CD8+ T cells and effector CD4+ T cells), and M1 phenotype tumor-associated macrophages (TAMs).27,28 Pro-tumor immune cells mainly include myeloid-derived suppressor cells (MDSCs), regulatory T (Treg) cells, TAMs (M2 phenotype), cancer-associated fibroblasts (CAFs), and group 2 innate lymphoid cells.28–30 Non-cellular factors (cytokines, chemokines, metabolites) related to tumor immunity can also be divided into anti-tumor and pro-tumor factors.28,31
The anti-tumor immune response, which mainly occurs in the tumor microenvironment (TME), is basically the recognition and killing of tumor cells.32–34 Tumor cells that produce various immune antigens are first recognized by NK and DC cells. Activated NK cells not only initiate cytotoxic reactions to directly kill tumor cells, but also secrete a variety of anti-cancer cytokines to recruit, stimulate, and regulate a variety of anti-tumor immune cells to further activate the anti-tumor immune response.35,36 After DC cells are activated by tumor antigens or NK cells, they mainly activate CD8+ T cells to induce anti-tumor immune responses of specific cytotoxic T lymphocytes (CTL], enhance the activities of NK cells and CD4+ T cells, and improve the anti-tumor immune response through various mechanisms.37,38 After CD4+ T cells are activated by the activated DC cells, they can directly recognize and kill some tumor cells; recruit and activate more NK cells, DC cells, and CD8+ T cells; and enhance the ability of CTL to kill tumor cells.39 Finally, CD8+ T cells are activated into CTL, becoming the main force that kills tumor cells.40,41 This anti-tumor immune response process is negatively regulated by MDSCs, Treg cells, TAMs (M2 phenotype), and CAFs.29,30,42
Effects of Radiotherapy on Anti-Tumor Immunity
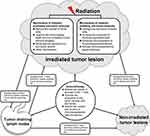
Radiotherapy is a local treatment for tumor lesions. The effect of radiotherapy on tumor immunity is mainly limited to the local TME of target tumor lesions.43,44 In addition, the promotion and inhibition of the effects of radiotherapy on anti-tumor immune responses are entire processes and dynamic changes that occur in the TME of target lesions (Figure 1).45 Radiotherapy may promote or inhibit anti-tumor immune responses depending on the total radiation dose and fractionations administered.25,46
There are several mechanisms underlying the promotion of anti-tumor immune response using radiotherapy. First, radiotherapy leads to a decrease in the number of tumor cells. Data from studies of many cancers suggest that a smaller tumor burden is associated with better treatment outcomes of immunotherapy.47 The decrease in the tumor volume and the number of tumor cells caused by radiotherapy can reduce the pressure on the anti-tumor immune system and reduce the chances of tumor cell mutation and immune escape. Second, radiotherapy leads to stress and apoptosis (immunogenic death) of some tumor cells, resulting in the deposition of a large number of different types of tumor immune antigens on the tumor cell surface and in the TME. These antigens promote the activation and expansion of NK and DC cells, ultimately leading to the production of a large number of activated CD8+T cells for the achievement of an anti-tumor immune response.24,48–50 Third, radiotherapy enhances the expression and secretion of anti-tumor immune-related cytokines, chemokines, and growth factors, thereby activating and enhancing anti-tumor immune response.22,25,51 Furthermore, radiotherapy can induce the normalization of blood vessels in tumor tissues and increase the permeability of local blood vessels to anti-tumor immune cells, thus increasing the infiltration density of immune cells in the TME.25,45 Other mechanisms behind the effect of radiotherapy on anti-tumor immune response have not been fully explored. For example, it has been reported that radiotherapy can reduce the abundance of tumor-induced erythroid progenitor cells in a manner dependent on interferon and CD8+ T cells, thereby inhibiting tumor growth.52 In addition, radiation-induced exosomes can stimulate or inhibit anti-tumor immune responses through a series of mechanisms, such as metastasis of tumor antigens.25
In some cases (usually at large radiation doses), radiotherapy will inhibit the anti-tumor immune response in the TME of the irradiated lesion (Figure 1). The mechanisms underlying this effect are as follows: 1) damage to anti-tumor immune cells in the irradiated TME, resulting in a reduction of the number of cells and impairment of their functions;22,25 2) increase in the numbers and proportions of immunosuppressive cells, such as MDSCs, Treg cells, TAMs (M2 phenotype), CAFs, and tumor-associated neutrophils (N2 phenotype) in the irradiated local TME;25,53 3) induction of high expression of anti-tumor immunosuppressive cytokines and chemokines in the local TME;22,25,54 and 4) activation of the immunosuppressive signaling pathway (including the PD-1/L1 pathway), which inhibits the anti-tumor activity of CD8+ T cells.55,56
In addition to its influence on the anti-tumor immune response in the local TME of the irradiated lesion, radiotherapy can also induce the abscopal effect (Figure 1). The abscopal effect is a phenomenon seen when irradiation at a distinct anatomic site induces a systemic antitumor response throughout the body.57,58 The abscopal effect describes the shrinkage of unirradiated tumors that occurs concurrently with irradiated tumors in patients with multiple tumors. The basic principle of the abscopal effect is that the activated NK cells, DC cells, and CTL, as well as related non-cellular factors in the local TME of the target lesion, circularly move to the tumor tissue at the non-irradiated site to produce an anti-tumor effect.59,60 Therefore, the main agents that drive the occurrence of the abscopal effect include CTL, NK cells, and cytokines that promote anti-tumor immunity activated in irradiated lesions.61,62 Traditionally, these drivers are believed to migrate through the blood system to distant non-irradiated lesions. Recent research shows that tumor-draining lymph nodes (TDLN) and tumor-derived exosomes play key roles in the abscopal effect. TDLN are essential for effective anti-tumor immunity induced by radiotherapy. TDLN promote effective anti-tumor immune response by inducing the infiltration of CD8+T cells and increase in M1 phenotype TAMs. The loss of bilateral TDLN weakens the enrichment and cytotoxicity of CD8+T cells, leading to the weakening of the anti-tumor response induced by radiotherapy.63 Another mechanism underlying the abscopal effect is the immunomodulatory effect of tumor-derived exosomes. Tumor-derived exosomes contain genetic material and immunosuppressive molecules. These exosomes may carry these signals to distant locations and interact with other immune cells, leading to abscopal effects.22,25 Theoretically, when a target lesion is irradiated, the cytokines and immune cells in the whole body, including distant non-irradiated lesions, will also change; thus, the incidence of abscopal effects can reach 100%.22 However, in clinical practice, the incidence of abscopal effects with clinical efficacy is low. This shows that the majority of abscopal effects are subclinical.64–66 Thus, the “radscopal” technique was proposed to improve the abscopal effect of radiotherapy. The radscopal technique refers to the enhancement of the anti-tumor effect on the whole body through the administration of low-dose radiotherapy for treatment of multiple additional lesions based on conventional radiotherapy for target lesions.67 Conventional radiotherapy may produce powerful immunosuppressive factors, whereas low-dose radiotherapy can reprogram and reactivate the TME, thus increasing the incidence of the abscopal effect.22,67
In summary, the effect of radiotherapy on the TME is two-sided; that is, it can promote or inhibit anti-tumor immune responses. The dose and fractionation of radiotherapy and the pathological classification of the tumor are key factors in the promotion or suppression of anti-tumor immunity.
Synergistic Mechanisms of Immunoradiotherapy
Immunotherapy can produce a series of synergistic effects when administered in combination with radiotherapy. These synergistic effects occur in irradiated and non-irradiated tumor lesions (Figure 1). The synergistic mechanisms of immunoradiotherapy are as follows: 1) restoration of the anti-tumor activity of activated CD8+ T cell;68 2) promotion of the production of more anti-tumor immune factors and T cells;69 and 3) reduction in the numbers and functions of Treg cells and other immunosuppressive cells and factors and increase in the ratio of CD8+ T cells to Treg cells, ultimately leading to the extension of the killing ability to tumor cells.68,70,71
In summary, recent preclinical studies have demonstrated that immunoradiotherapy has great synergistic potential. The generation of this synergistic effect depends on the radiotherapy dose and fraction mode, as well as the timing and sequence of immunotherapy administration.72,73 However, the treatment responses of different pathological types of tumors vary significantly.68
Efficacy of Immunoradiotherapy for Different Cancers
There are several potential synergistic mechanisms of immunoradiotherapy. However, these mechanisms are meaningful only when significant efficacy is achieved in clinical practice. Some representative clinical trials on the use of immunoradiotherapy for the treatment of some cancers have been completed (Table 1). Some of these trials achieved promising efficacy.
 |
Table 1 Representative Clinical Trials on Immunoradiotherapy for the Treatment of Different Cancers |
The results of a Phase 1 clinical trial reported in 2018 showed that multisite stereotactic body radiation therapy (SBRT) (30–50 Gy in three to five fractions to 2–4 lesions), followed by pembrolizumab, is well tolerated and has acceptable toxicity in patients with advanced solid tumors.78 To our knowledge, this was the first report of multisite SBRT combined with immunotherapy. The results of a new clinical trial showed that multisite stereotactic ablative body radiosurgery (SABR) (single fraction of 20 Gy or 10 fractions of 3 Gy to all metastatic sites) and short-course pembrolizumab for the treatment of oligometastatic clear cell renal cell carcinoma is well tolerated and has excellent local control. These findings support the synergistic anti-tumor activity of immunoradiotherapy.74 Several other representative clinical trials on the safety and efficacy of immunoradiotherapy for the treatment of multiple malignancies involved the use of PD-1 inhibitors combined with single-lesion radiation.75–77,84 Although the synergistic anti-tumor effect of immunoradiotherapy was believed to have been achieved in these trials, no significant abscopal effect was observed in these studies.
Several recent key clinical trials on immunoradiotherapy have yielded negative results (Table 1). In these clinical trials, immunotherapy was used in combination with various forms of radiotherapy for the treatment of different cancers. However, no abscopal effect or evidence of synergy was observed.79–83 There are several possible reasons for these findings. The mode of radiotherapy, number of target lesions irradiated, dose and fraction of radiotherapy, and timing of immunotherapy administration may have important effects on the results. However, several clinical trials on the use of different radiotherapy modalities in combination with immunotherapy for the treatment of different cancers are currently ongoing.22
There are two strategies to achieve synergy between radiotherapy and immunotherapy. The first strategy is to achieve an abscopal effect by irradiating a lesion using radiotherapy while simultaneously administering immunotherapy. The other strategy is to achieve synergistic effects by administering low-dose radiation to as many lesions as possible while simultaneously administering immunotherapy. However, regarding the irradiation of a single tumor lesion, no truly significant abscopal effect has been achieved using immunoradiotherapy in completed clinical trials with large sample sizes (Table 1). Consequently, the achievement of the abscopal effect using immunoradiotherapy remains elusive. It is relatively feasible to achieve synergistic effects by delivering low doses of radiation to as many lesions as possible.
Current Research Evidence on Sarcomas
Currently, radiotherapy is only recommended for preoperative or postoperative treatment of locally resectable STS to reduce the rate of recurrence.85–88 Immunotherapy is marginalized and is only considered to have some efficacy against a few sarcoma subtypes.87,89,90 This, together with the rarity of STS, ultimately leads to a scarcity of studies on the effect of immunoradiotherapy on STS compared to other cancers. However, some preclinical studies have preliminarily clarified the effect of radiotherapy on the TME of STS. Radiotherapy first affects TME by damaging cancer cells via direct breakage of DNA and the generation of reactive oxygen species.22 The immunogenic cell death of the damaged cancer cells affects the behavior of immune cells.25,72 Cellular response driven by DNA damage also changes the immunogenicity of these irradiated cancer cells.54 Radiotherapy can also effectively increase the quantity and density of activated CD8+ T cells and PD-L1+ macrophages in sarcomas.91–94 The change in the TME of sarcomas induced by preoperative radiotherapy is related to the incidence of postoperative metastasis.92 Although radiotherapy can induce an increase in the number of tumor-infiltrating lymphocytes in sarcoma tissues, it also upregulates the expression of various immunosuppressive factors, including PD-L1.91,92,94,95 PD-1 blockade and radiotherapy successfully repolarize myeloid cells in sarcomas, transforming the immunosuppressive TME to pro-anti-tumor immune response.93 In addition, immunoradiotherapy can effectively increase the number of B cells in TME,94,96 and B cells are associated with survival and therapy response in STSs.97–99
Some clinical reports have indicated the existence of an abscopal effect related to radiotherapy in different sarcomas.100,101 Immunoradiotherapy seems to achieve the abscopal effect more frequently in sarcomas that that of radiotherapy.102–104 In a clinical case series, three patients with undifferentiated pleomorphic sarcoma, one with intimal sarcoma and one with chondroblastic sarcoma, concurrently received SBRT and pembrolizumab at ten sites of metastatic lesions. The expected high rates of local control in tumors treated using SBRT were observed. Two patients demonstrated either an enhanced local tumor regression or a possible abscopal effect.105 In the abovementioned clinical reports, most of the patients with pathological subtypes of sarcoma, including pleomorphic sarcoma, clear cell sarcoma, unclassified round cell sarcoma, and alveolar soft part sarcoma, were highly sensitive to immunotherapy or radiotherapy.106,107 This suggests that the population that most likely to benefit from immunoradiotherapy are those with subtypes of sarcomas that are sensitive to immunotherapy and/or radiotherapy, such as those with UPS or synovial sarcoma. Because UPS is a subtype of sarcoma that is sensitive to both radiotherapy and PD-1 inhibitor therapy;11,12 Synovial sarcoma is a subtype of sarcoma that is sensitive to both radiotherapy and adaptive cellular therapy or cancer vaccine.17,18,108–110
The authors of these clinical case reports claim that radiotherapy has an abscopal effect on sarcomas. However, the results of research on other malignant tumors show that it is unrealistic to attempt to produce an abscopal effect by irradiating a single lesion. It is relatively feasible to achieve synergistic effects with immunoradiotherapy by the administration of low-dose radiation to as many lesions as possible. However, there is no research evidence or report on achieving the synergistic therapeutic effects of immunoradiotherapy by irradiating multiple STS lesions.
The use of predictive biomarker could be extremely helpful in stratifying patients for their risk and for their propensity to effectively responds to immunotherapy, thereby increasing therapeutic options for selected patients and reducing unnecessary side effects. A series of studies have demonstrated that higher tumor infiltrating immune cell infiltrates, sarcoma immune class E, tertiary lymphoid structure, neutrophil-to-lymphocyte ratio, immune-related adverse event are predictive biomarkers of survival in patients with STS who received immunotherapy.111–113 However, there are currently no reports of predictive biomarkers related to the immunoradiotherapy of STSs.
Ongoing Trials on Immunoradiotherapy for Sarcomas
Several clinical trials on the use of immunoradiotherapy for the treatment of STSs have been registered at clinicaltrials.gov and are currently ongoing (Table 2). Most of these trials are focused on the perioperative management of resectable STSs; only one study is focused on advanced STSs. The immunotherapeutic drugs used in these clinical trials include atezolizumab, durvalumab, ipilimumab, nivolumab, pembrolizumab, and tremelimumab. Conventional high-dose radiotherapy is the primary irradiation method used in these studies. The completion of these clinical trials will further validate the synergistic efficacy of different immunotherapeutic agents combined with radiotherapy for the treatment of sarcomas. This is a necessary step towards improving treatment outcomes for patients with STS. However, the research evidence obtained so far indicate that the designs of these clinical trials have obvious limitations. The major problems and drawbacks of these trials are as follows: 1) no specific sarcoma subtype was selected; 2) no solid theoretical and preclinical basis for the trial; 3) the effects of irradiation dose and fraction, as well as the number of irradiated lesions, were not considered; 4) evaluation was limited to perioperative treatment and did not include the verification of the abscopal effect in advanced disease; 5) the influence of the time of administration of immunotherapy agents was not considered; and 5) the influence of adjuvant drugs was not considered.
 |
Table 2 Ongoing Clinical Trials on Immunoradiotherapy for Soft Tissue Sarcomas |
Discussion
In this review, we discuss the synergistic mechanisms of immunoradiotherapy for the treatment of cancers. To our knowledge, this is the first review of the possible synergistic mechanism of immunoradiotherapy for cancer treatment, the effectiveness of the combined regimen for the treatment of several cancers, the evidence of efficacy of immunoradiotherapy for the treatment of STSs, and key ongoing clinical trials. Immunoradiotherapy has achieved positive results in the treatment of a variety of cancers. This may be an important breakthrough in the treatment of several cancers. However, application of immunoradiotherapy for STS is still in the preliminary validation stage. Existing research evidence indicate that immunoradiotherapy has a promising effect on some subtypes of STS, especially those subtypes sensitive to immunotherapy and/or radiotherapy. Such as UPS or synovial sarcoma. However, there is limited relevant evidence or ongoing clinical trials on this.
To improve the therapeutic effects on STS, the study of immunoradiotherapy for STS needs to be more in-depth and detailed (Figure 2). Several problems need to be addressed in future clinical studies. First, the sarcoma subtypes that are more responsive to combination therapy should be determined. The radiosensitivity of tumor cells, percentage of interstitial cells, structure of angiogenesis, and mode of metastasis for each type of sarcoma vary. This leads to a considerable difference in the responses of sarcoma subtypes to immunotherapy and radiotherapy. Therefore, it is necessary to study the therapeutic response of each sarcoma subtype to immunoradiotherapy.46 Second, the number of irradiated lesions is a key factor in immunoradiotherapy for STS. As previously mentioned, there are two irradiation methods for possibly achieving synergy between radiotherapy and immunotherapy. One strategy is to irradiate one lesion hoping to produce abscopal effects to shrink other non-irradiated lesions. Another strategy is to irradiate as many lesions as possible. According to the results of the studies discussed in this review, the latter approach is more feasible. Third, according to results of existing research, different radiation doses or fractions lead to completely different or even reverse immune responses. Therefore, it is necessary to study the effects of different total doses and fractions of radiation on immunoradiotherapy for STS. Fourth, attention should be paid to the timing of the administration of immunotherapy drugs, which has a significant impact on the effect of the therapy.73,114 If the timing of administration is inappropriate, the number of anti-tumor immune cells activated by the immunotherapeutic agent may be reduced by radiotherapy or the anti-tumor immune response activated by radiotherapy may not be synchronized with immunotherapy, resulting in poor anti-tumor efficacy. Fifth, a combination of additional treatments could be considered. As previously mentioned, radiotherapy results in a 100% abscopal effect.22 However, this effect is so weak that it rarely occurs in clinical practice. Therefore, immunoradiotherapy combined with other systemic treatments, such as chemotherapy, is worthy of exploration. Sixth, attention should be paid to the influence of concomitant use of medications. Many drugs have been preliminarily proven to inhibit the efficacy of immunotherapy.115 Therefore, the influence of concomitant use of drugs should be considered in detail when designing clinical trials on immunoradiotherapy for STS. Finally, therapeutic markers require further study.
 |
Figure 2 Problems need to be addressed in future mechanisms and clinical studies on immunoradiotherapy in soft-tissue sarcomas. |
In conclusion, immunoradiotherapy has shown synergistic effects in the treatment of some cancers (such as renal cell carcinoma, prostate cancer, colorectal cancer, pancreatic adenocarcinoma and lung cancer). However, there is still no overwhelmingly positive evidence of these effects in completed clinical trials. In addition, studies on immunoradiotherapy for the treatment of sarcomas are in the preliminary stages, and immunoradiotherapy may benefit patients with subtypes of sarcomas that are sensitive to immunotherapy and/or radiotherapy. Although there are problems that still need to be studied, there is no denying that immunoradiotherapy is a promising treatment for STSs.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Yang Z, Zheng R, Zhang S, et al. Incidence, distribution of histological subtypes and primary sites of soft tissue sarcoma in China. Cancer Biol Med. 2019;16(3):565–574. doi:10.20892/j.issn.2095-3941.2019.0041
2. Bessen T, Caughey GE, Shakib S, et al. A population-based study of soft tissue sarcoma incidence and survival in Australia: an analysis of 26,970 cases. Cancer Epidemiol. 2019;63:101590. doi:10.1016/j.canep.2019.101590
3. Kallen ME, Hornick JL. The 2020 WHO classification: what’s new in soft tissue tumor pathology? Am J Surg Pathol. 2021;45(1):e1–e23. doi:10.1097/PAS.0000000000001552
4. Nagar SP, Mytelka DS, Candrilli SD, et al. Treatment patterns and survival among adult patients with advanced soft tissue sarcoma: a retrospective medical record review in the United Kingdom, Spain, Germany, and France. Sarcoma. 2018;2018:5467057. doi:10.1155/2018/5467057
5. Ratan R, Patel SR. Chemotherapy for soft tissue sarcoma. Cancer. 2016;122(19):2952–2960. doi:10.1002/cncr.30191
6. Kunisada T, Nakata E, Fujiwara T, et al. Soft-tissue sarcoma in adolescents and young adults. Int j Clin Oncol. 2022;28(1):1–11. doi:10.1007/s10147-022-02119-7
7. Ardakani AHG, Woollard A, Ware H, Gikas P. Soft tissue sarcoma: recognizing a rare disease. Clev Clin j med. 2022;89(2):73–80. doi:10.3949/ccjm.89a.21078
8. Chen TW, Chiang RC, Le Cesne A, et al. Soft tissue sarcoma incidences and clinical characteristics are significantly different in France and Taiwan. Cancer Am Cancer Soc. 2022;128(18):3360–3369.
9. Chen Y, Pei Y, Luo J, et al. Looking for the optimal PD-1/PD-L1 inhibitor in cancer treatment: a comparison in basic structure, function, and clinical practice. Front Immunol. 2020;11:1088. doi:10.3389/fimmu.2020.01088
10. Kornepati AVR, Vadlamudi RK, Curiel TJ. Programmed death ligand 1 signals in cancer cells. Nat Rev Cancer. 2022;22(3):174–189. doi:10.1038/s41568-021-00431-4
11. Seong G, D’Angelo SP. New therapeutics for soft tissue sarcomas: overview of current immunotherapy and future directions of soft tissue sarcomas. Front Oncol. 2023;13:1150765. doi:10.3389/fonc.2023.1150765
12. Husain M, Chen L, Liebner D, et al. Emerging Trends in Immunotherapy for Adult Sarcomas. Oncologist. 2023;28(5):e233–e241. doi:10.1093/oncolo/oyad052
13. Nakata E, Fujiwara T, Kunisada T, Ito T, Takihira S, Ozaki T. Immunotherapy for sarcomas. Jpn J Clin Oncol. 2021;51(4):523–537. doi:10.1093/jjco/hyab005
14. Clemente O, Ottaiano A, Di Lorenzo G, et al. Is immunotherapy in the future of therapeutic management of sarcomas? J Transl Med. 2021;19(1):173. doi:10.1186/s12967-021-02829-y
15. Birdi HK, Jirovec A, Cortes-Kaplan S, et al. Immunotherapy for sarcomas: new frontiers and unveiled opportunities. J Immunother Cancer. 2021;9(2):e001580. doi:10.1136/jitc-2020-001580
16. Robbins PF, Kassim SH, Tran TL, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res. 2015;21(5):1019–1027. doi:10.1158/1078-0432.CCR-14-2708
17. D’Angelo SP, Melchiori L, Merchant MS, et al. Antitumor activity associated with prolonged persistence of adoptively transferred NY-ESO-1 c259T cells in synovial sarcoma. Cancer Discov. 2018;8(8):944–957. doi:10.1158/2159-8290.CD-17-1417
18. Somaiah N, Chawla SP, Block MS, et al. A phase 1b study evaluating the safety, tolerability, and immunogenicity of CMB305, a lentiviral-based prime-boost vaccine regimen, in patients with locally advanced, relapsed, or metastatic cancer expressing NY-ESO-1. Oncoimmunology. 2020;9(1):1847846. doi:10.1080/2162402X.2020.1847846
19. Yi M, Zheng X, Niu M, et al. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. 2022;21(1):28. doi:10.1186/s12943-021-01489-2
20. Wu M, Huang Q, Xie Y, et al. Improvement of the anticancer efficacy of PD-1/PD-L1 blockade via combination therapy and PD-L1 regulation. J Hematol Oncol. 2022;15(1):24. doi:10.1186/s13045-022-01242-2
21. Tian Z, Yao W. PD-1/L1 inhibitor plus chemotherapy in the treatment of sarcomas. Front Immunol. 2022;13:898255. doi:10.3389/fimmu.2022.898255
22. Zhang Z, Liu X, Chen D, Yu J. Radiotherapy combined with immunotherapy: the Dawn of cancer treatment. Signal Transduct Target Ther. 2022;7(1):258. doi:10.1038/s41392-022-01102-y
23. Pointer KB, Pitroda SP, Weichselbaum RR. Radiotherapy and immunotherapy: open questions and future strategies. Trends Cancer. 2022;8(1):9–20. doi:10.1016/j.trecan.2021.10.003
24. Herrera FG, Romero P, Coukos G. Lighting up the tumor fire with low-dose irradiation. Trends Immunol. 2022;43(3):173–179. doi:10.1016/j.it.2022.01.006
25. Charpentier M, Spada S, Van Nest SJ, Demaria S. Radiation therapy-induced remodeling of the tumor immune microenvironment. Semin Cancer Biol. 2022;86(Pt 2):737–747. doi:10.1016/j.semcancer.2022.04.003
26. Balogun F, Raben D. When radiotherapy and immunotherapy dance-who leads so as not to step on toes? JAMA Oncol. 2022;8(2):240–241. doi:10.1001/jamaoncol.2021.6436
27. Yenyuwadee S, Aliazis K, Wang Q, et al. Immune cellular components and signaling pathways in the tumor microenvironment. Semin Cancer Biol. 2022;86(Pt 2):187–201. doi:10.1016/j.semcancer.2022.08.004
28. Lv B, Wang Y, Ma D, et al. Immunotherapy: reshape the tumor immune microenvironment. Front Immunol. 2022;13:844142. doi:10.3389/fimmu.2022.844142
29. Xiang X, Niu Y-R, Wang Z-H, et al. Cancer-associated fibroblasts: vital suppressors of the immune response in the tumor microenvironment. Cytokine Growth Factor Rev. 2022;67:35–48. doi:10.1016/j.cytogfr.2022.07.006
30. Yan Y, Huang L, Liu Y, et al. Metabolic profiles of regulatory T cells and their adaptations to the tumor microenvironment: implications for antitumor immunity. J Hematol Oncol. 2022;15(1):104. doi:10.1186/s13045-022-01322-3
31. Wang Q, Xie B, Liu S, et al. What happens to the immune microenvironment after PD-1 inhibitor therapy? Front Immunol. 2021;12:773168. doi:10.3389/fimmu.2021.773168
32. Bozyk A, Wojas-Krawczyk K, Krawczyk P, Milanowski J. Tumor microenvironment-A short review of cellular and interaction diversity. Biology. 2022;11(6):929. doi:10.3390/biology11060929
33. Bejarano L, Jordao MJC, Joyce JA. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov. 2021;11(4):933–959. doi:10.1158/2159-8290.CD-20-1808
34. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi:10.1016/j.immuni.2013.07.012
35. Wang F, Lau JKC, Yu J. The role of natural killer cell in gastrointestinal cancer: killer or helper. Oncogene. 2021;40(4):717–730. doi:10.1038/s41388-020-01561-z
36. Mikelez-Alonso I, Magadan S, Gonzalez-Fernandez A, Borrego F. Natural killer (NK) cell-based immunotherapies and the many faces of NK cell memory: a look into how nanoparticles enhance NK cell activity. Adv Drug Deliv Rev. 2021;176:113860. doi:10.1016/j.addr.2021.113860
37. Murphy TL, Murphy KM. Dendritic cells in cancer immunology. Cell Mol Immunol. 2022;19(1):3–13. doi:10.1038/s41423-021-00741-5
38. Kvedaraite E, Ginhoux F. Human dendritic cells in cancer. Sci Immunol. 2022;7(70):eabm9409. doi:10.1126/sciimmunol.abm9409
39. Ben Khelil M, Godet Y, Abdeljaoued S, et al. Harnessing antitumor CD4+ T cells for cancer immunotherapy. Cancers. 2022;14(1):260. doi:10.3390/cancers14010260
40. Raskov H, Orhan A, Christensen JP, Gogenur I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br J Cancer. 2021;124(2):359–367. doi:10.1038/s41416-020-01048-4
41. Zeng Z, Chew HY, Cruz JG, et al. Investigating T cell immunity in cancer: achievements and prospects. Int J Mol Sci. 2021;22(6):2907. doi:10.3390/ijms22062907
42. Tuccitto A, Shahaj E, Vergani E, et al. Immunosuppressive circuits in tumor microenvironment and their influence on cancer treatment efficacy. Virchows Arch. 2019;474(4):407–420. doi:10.1007/s00428-018-2477-z
43. Rückert M, Flohr AS, Hecht M, Gaipl US. Radiotherapy and the immune system: more than just immune suppression. Stem Cells. 2021;39(9):1155–1165. doi:10.1002/stem.3391
44. Park B, Yee C, Lee KM. The effect of radiation on the immune response to cancers. Int J Mol Sci. 2014;15(1):927–943. doi:10.3390/ijms15010927
45. Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15(7):409–425. doi:10.1038/nrc3958
46. Mortezaee K, Najafi M. Immune system in cancer radiotherapy: resistance mechanisms and therapy perspectives. Crit Rev Oncol Hematol. 2021;157:103180. doi:10.1016/j.critrevonc.2020.103180
47. Seiwert TY, Kiess AP. Time to debunk an urban Myth? The “Abscopal Effect” With radiation and anti-PD-1. J Clin Oncol. 2021;39(1):1–3. doi:10.1200/JCO.20.02046
48. Yang Y, Wu M, Cao D, et al. ZBP1-MLKL necroptotic signaling potentiates radiation-induced antitumor immunity via intratumoral STING pathway activation. Sci Adv. 2021;7(41):eabf6290. doi:10.1126/sciadv.abf6290
49. Patel RB, Hernandez R, Carlson P, et al. Low-dose targeted radionuclide therapy renders immunologically cold tumors responsive to immune checkpoint blockade. Sci transl med. 2021;13(602):eabb3631. doi:10.1126/scitranslmed.abb3631
50. Lussier DM, Alspach E, Ward JP, et al. Radiation-induced neoantigens broaden the immunotherapeutic window of cancers with low mutational loads. Proc Natl Acad Sci U S A. 2021;118(24):e2102611118. doi:10.1073/pnas.2102611118
51. McLaughlin M, Patin EC, Pedersen M, et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer. 2020;20(4):203–217. doi:10.1038/s41568-020-0246-1
52. Hou Y, Liang HL, Yu X, et al. Radiotherapy and immunotherapy converge on elimination of tumor-promoting erythroid progenitor cells through adaptive immunity. Sci transl med. 2021;13(582):eabb0130. doi:10.1126/scitranslmed.abb0130
53. Kho VM, Mekers VE, Span PN, et al. Radiotherapy and cGAS/STING signaling: impact on MDSCs in the tumor microenvironment. Cell Immunol. 2021;362:104298. doi:10.1016/j.cellimm.2021.104298
54. Rodriguez-Ruiz ME, Vanpouille-Box C, Melero I, et al. Immunological mechanisms responsible for radiation-induced abscopal effect. Trends Immunol. 2018;39(8):644–655. doi:10.1016/j.it.2018.06.001
55. Sato H, Niimi A, Yasuhara T, et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat Commun. 2017;8(1):1751. doi:10.1038/s41467-017-01883-9
56. Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74(19):5458–5468. doi:10.1158/0008-5472.CAN-14-1258
57. Ashrafizadeh M, Farhood B, Eleojo Musa A, et al. Abscopal effect in radioimmunotherapy. Int Immunopharmacol. 2020;85:106663. doi:10.1016/j.intimp.2020.106663
58. Craig DJ, Ambrose S, Stanbery L, et al. Systemic benefit of radiation therapy via abscopal effect. Front Oncol. 2022;12:987142. doi:10.3389/fonc.2022.987142
59. Lippert TP, Greenberg RA. The abscopal effect: a sense of DNA damage is in the air. J Clin Invest. 2021;131(9):e148274. doi:10.1172/JCI148274
60. Craig DJ, Nanavaty NS, Devanaboyina M, et al. The abscopal effect of radiation therapy. Future Oncol. 2021;17(13):1683–1694. doi:10.2217/fon-2020-0994
61. Meng L, Xu J, Ye Y, et al. The combination of radiotherapy with immunotherapy and potential predictive biomarkers for treatment of non-small cell lung cancer patients. Front Immunol. 2021;12:723609. doi:10.3389/fimmu.2021.723609
62. Pevzner AM, Tsyganov MM, Ibragimova MK, et al. Abscopal effect in the radio and immunotherapy. Radiat Oncol J. 2021;39(4):247–253. doi:10.3857/roj.2021.00115
63. Liu Z, Yu Z, Chen D, et al. Pivotal roles of tumor-draining lymph nodes in the abscopal effects from combined immunotherapy and radiotherapy. Cancer Commun. 2022;42(10):971–986. doi:10.1002/cac2.12348
64. Kodet O, Němejcova K, Strnadová K, et al. The abscopal effect in the era of checkpoint inhibitors. Int J Mol Sci. 2021;22(13):7204. doi:10.3390/ijms22137204
65. Abbasi AN, Hammad Khan AM, Mansha MA, et al. Abscopal effect: an old concept with a new horizon. Gulf J Oncolog. 2021;1(36):60–66.
66. Wang D, Zhang X, Gao Y, et al. Research progress and existing problems for abscopal effect. Cancer Manag Res. 2020;12:6695–6706. doi:10.2147/CMAR.S245426
67. Barsoumian HB, Ramapriyan R, Younes AI, et al. Low-dose radiation treatment enhances systemic antitumor immune responses by overcoming the inhibitory stroma. J Immunother Cancer. 2020;8(2):e000537. doi:10.1136/jitc-2020-000537
68. Zhao X, Shao C. Radiotherapy-mediated immunomodulation and anti-tumor abscopal effect combining immune checkpoint blockade. Cancers. 2020;12(10):2762. doi:10.3390/cancers12102762
69. Oweida A, Lennon S, Calame D, et al. Ionizing radiation sensitizes tumors to PD-L1 immune checkpoint blockade in orthotopic murine head and neck squamous cell carcinoma. Oncoimmunology. 2017;6(10):e1356153. doi:10.1080/2162402X.2017.1356153
70. Sharabi AB, Nirschl CJ, Kochel CM, et al. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res. 2015;3(4):345–355. doi:10.1158/2326-6066.CIR-14-0196
71. Deng L, Liang H, Burnette B, et al. Radiation and anti-PD-L1 antibody combinatorial therapy induces T cell-mediated depletion of myeloid-derived suppressor cells and tumor regression. Oncoimmunology. 2014;3:e28499. doi:10.4161/onci.28499
72. Demaria S, Guha C, Schoenfeld J, et al. Radiation dose and fraction in immunotherapy: one-size regimen does not fit all settings, so how does one choose? J Immunother Cancer. 2021;9(4):e002038. doi:10.1136/jitc-2020-002038
73. Zhao X, Li J, Zheng L, et al. Immune response on optimal timing and fractionation dose for hypofractionated radiotherapy in non-small-cell lung cancer. Front Mol Biosci. 2022;9:786864. doi:10.3389/fmolb.2022.786864
74. Siva S, Bressel M, Wood ST, et al. Stereotactic radiotherapy and short-course pembrolizumab for oligometastatic renal cell carcinoma-the RAPPORT trial. Eur Urol. 2022;81(4):364–372. doi:10.1016/j.eururo.2021.12.006
75. Kwan EM, Spain L, Anton A, et al. Avelumab combined with stereotactic ablative body radiotherapy in metastatic castration-resistant prostate cancer: the phase 2 ICE-PAC clinical trial. Eur Urol. 2022;81(3):253–262. doi:10.1016/j.eururo.2021.08.011
76. Theelen W, Chen D, Verma V, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet resp med. 2021;9(5):467–475. doi:10.1016/S2213-2600(20)30391-X
77. Parikh AR, Szabolcs A, Allen JN, et al. Radiation therapy enhances immunotherapy response in microsatellite stable colorectal and pancreatic adenocarcinoma in a Phase II trial. Nat Cancer. 2021;2(11):1124–1135. doi:10.1038/s43018-021-00269-7
78. Luke JJ, Lemons JM, Karrison TG, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol. 2018;36(16):1611–1618. doi:10.1200/JCO.2017.76.2229
79. Schoenfeld JD, Giobbie-Hurder A, Ranasinghe S, et al. Durvalumab plus tremelimumab alone or in combination with low-dose or hypofractionated radiotherapy in metastatic non-small-cell lung cancer refractory to previous PD(L)-1 therapy: an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2022;23(2):279–291. doi:10.1016/S1470-2045(21)00658-6
80. Masini C, Iotti C, De Giorgi U, et al. Nivolumab in combination with stereotactic body radiotherapy in pretreated patients with metastatic renal cell carcinoma. results of the phase II NIVES study. Eur Urol. 2022;81(3):274–282. doi:10.1016/j.eururo.2021.09.016
81. Kim S, Wuthrick E, Blakaj D, et al. Combined nivolumab and ipilimumab with or without stereotactic body radiation therapy for advanced Merkel cell carcinoma: a randomised, open label, phase 2 trial. Lancet. 2022;400(10357):1008–1019. doi:10.1016/S0140-6736(22)01659-2
82. Chen IM, Johansen JS, Theile S, et al. Randomized phase II study of nivolumab with or without ipilimumab combined with stereotactic body radiotherapy for refractory metastatic pancreatic cancer (CheckPAC). J Clin Oncol. 2022;40(27):3180–3189. doi:10.1200/JCO.21.02511
83. McBride S, Sherman E, Tsai CJ, et al. Randomized phase II trial of nivolumab with stereotactic body radiotherapy versus nivolumab alone in metastatic head and neck squamous cell carcinoma. J Clin Oncol. 2021;39(1):30–37. doi:10.1200/JCO.20.00290
84. Monjazeb AM, Giobbie-Hurder A, Lako A, et al. A randomized trial of combined PD-L1 and CTLA-4 inhibition with targeted low-dose or hypofractionated radiation for patients with metastatic colorectal cancer. Clin Cancer Res. 2021;27(9):2470–2480. doi:10.1158/1078-0432.CCR-20-4632
85. Salerno KE. Radiation therapy for soft tissue sarcoma: indications, timing, benefits, and consequences. Surg Clin North Am. 2022;102(4):567–582. doi:10.1016/j.suc.2022.04.001
86. Spraker MB. Considerations for optimal implementation of radiation therapy into multidisciplinary care for patients with sarcoma. Pract Radiat Oncol. 2022;12(2):84–86. doi:10.1016/j.prro.2021.08.015
87. von Mehren M, Kane JM, Agulnik M, et al. Soft tissue sarcoma, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(7):815–833. doi:10.6004/jnccn.2022.0035
88. Shah NK, Yegya-Raman N, Jones JA, et al. Radiation therapy in metastatic soft tissue sarcoma: from palliation to ablation. Cancers. 2021;13(19):4775. doi:10.3390/cancers13194775
89. Panagi M, Pilavaki P, Constantinidou A, et al. Immunotherapy in soft tissue and bone sarcoma: unraveling the barriers to effectiveness. Theranostics. 2022;12(14):6106–6129. doi:10.7150/thno.72800
90. Weng W, Yu L, Li Z, et al. The immune subtypes and landscape of sarcomas. BMC Immunol. 2022;23(1):46. doi:10.1186/s12865-022-00522-3
91. Keung EZ, Tsai JW, Ali AM, et al. Analysis of the immune infiltrate in undifferentiated pleomorphic sarcoma of the extremity and trunk in response to radiotherapy: rationale for combination neoadjuvant immune checkpoint inhibition and radiotherapy. Oncoimmunology. 2018;7(2):e1385689. doi:10.1080/2162402X.2017.1385689
92. Patel KR, Martinez A, Stahl JM, et al. Increase in PD-L1 expression after pre-operative radiotherapy for soft tissue sarcoma. Oncoimmunology. 2018;7(7):e1442168. doi:10.1080/2162402X.2018.1442168
93. Wisdom AJ, Mowery YM, Hong CS, et al. Single cell analysis reveals distinct immune landscapes in transplant and primary sarcomas that determine response or resistance to immunotherapy. Nat Commun. 2020;11(1):6410. doi:10.1038/s41467-020-19917-0
94. Goff PH, Riolobos L, LaFleur BJ, et al. Neoadjuvant therapy induces a potent immune response to sarcoma, dominated by myeloid and B cells. Clin Cancer Res. 2022;28(8):1701–1711. doi:10.1158/1078-0432.CCR-21-4239
95. Snow H, Mitchell C, Hendry S, et al. Characterising the immune microenvironment in liposarcoma, its impact on prognosis and the impact of radiotherapy. J Surg Oncol. 2021;123(1):117–126. doi:10.1002/jso.26261
96. Kim SS, Shen S, Miyauchi S, et al. B cells improve overall survival in HPV-associated squamous cell carcinomas and are activated by radiation and PD-1 blockade. Clin Cancer Res. 2020;26(13):3345–3359. doi:10.1158/1078-0432.CCR-19-3211
97. Kendal JK, Shehata MS, Lofftus SY, et al. Cancer-associated B cells in sarcoma. Cancers. 2023;15(3):622. doi:10.3390/cancers15030622
98. Italiano A, Bessede A, Pulido M, et al. Pembrolizumab in soft-tissue sarcomas with tertiary lymphoid structures: a phase 2 PEMBROSARC trial cohort. Nat Med. 2022;28(6):1199–1206. doi:10.1038/s41591-022-01821-3
99. Petitprez F, de Reynies A, Keung EZ, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577(7791):556–560. doi:10.1038/s41586-019-1906-8
100. Orton A, Wright J, Buchmann L, et al. A case of complete abscopal response in high-grade pleiomorphic sarcoma treated with radiotherapy alone. Cureus. 2016;8(10):e821. doi:10.7759/cureus.821
101. Brenneman RJ, Sharifai N, Fischer-Valuck B, et al. Abscopal effect following proton beam radiotherapy in a patient with inoperable metastatic retroperitoneal sarcoma. Front Oncol. 2019;9:922. doi:10.3389/fonc.2019.00922
102. Marcrom S, De Los Santos JF, Conry RM. Complete response of mediastinal clear cell sarcoma to pembrolizumab with radiotherapy. Clin Sarcoma Res. 2017;7(1):14. doi:10.1186/s13569-017-0079-1
103. Tolay S, Nair R, McIntosh AF, et al. Dramatic response to concurrent anti-PD-1 therapy and radiation in resistant tumors with sarcomatoid differentiation. Oncologist. 2019;24(1):e49–e52. doi:10.1634/theoncologist.2018-0205
104. Okamoto M, Sato H, Gao X, et al. Pembrolizumab after carbon ion radiation therapy for alveolar soft part sarcoma shows a remarkable abscopal effect: a case report. Adv Radiat Oncol. 2022;7(3):100893. doi:10.1016/j.adro.2021.100893
105. Callaghan CM, Seyedin SN, Mohiuddin IH, et al. The effect of concurrent stereotactic body radiation and anti-PD-1 therapy for recurrent metastatic sarcoma. Radiat Res. 2020;194(2):124–132. doi:10.1667/RADE-20-00017
106. Baldi GG, Gronchi A, Tazzari M, et al. Immunotherapy in soft tissue sarcoma: current evidence and future perspectives in a variegated family of different tumor. Expert Rev Anticanc. 2022;22(5):491–503. doi:10.1080/14737140.2022.2065986
107. O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235–2241. doi:10.1016/S0140-6736(02)09292-9
108. Ngo C, Postel-Vinay S. Immunotherapy for SMARCB1-deficient sarcomas: current evidence and future developments. Biomedicines. 2022;10(3):650. doi:10.3390/biomedicines10030650
109. Li J, Mulvihill TS, Li L, et al. A role for SMARCB1 in synovial sarcomagenesis reveals that SS18-SSX induces canonical BAF destruction. Cancer Discov. 2021;11(10):2620–2637. doi:10.1158/2159-8290.CD-20-1219
110. Ramachandran I, Lowther DE, Dryer-Minnerly R, et al. Systemic and local immunity following adoptive transfer of NY-ESO-1 SPEAR T cells in synovial sarcoma. J Immunother Cancer. 2019;7(1):276. doi:10.1186/s40425-019-0762-2
111. Husain M, Quiroga D, Kim HG, et al. Clinical markers of immunotherapy outcomes in advanced sarcoma. BMC Cancer. 2023;23(1):326. doi:10.1186/s12885-023-10758-w
112. Haddox CL, Riedel RF. Emerging predictive biomarkers in the management of bone and soft tissue sarcomas. Expert Rev Anticancer Ther. 2023;23(5):495–502. doi:10.1080/14737140.2023.2200169
113. Cheng Y, Mo F, Pu L, et al. Pretreatment inflammatory indexes as prognostic predictors of survival in patients suffering from synovial sarcoma. Front Oncol. 2019;9:955. doi:10.3389/fonc.2019.00955
114. Zhu C, Shi Y, Li Q, et al. Rational administration sequencing of immunochemotherapy elicits powerful anti-tumor effect. J Control Release. 2022;341:769–781. doi:10.1016/j.jconrel.2021.12.022
115. Cortellini A, Tucci M, Adamo V, et al. Integrated analysis of concomitant medications and oncological outcomes from PD-1/PD-L1 checkpoint inhibitors in clinical practice. J Immunother Cancer. 2020;8(2):e001361. doi:10.1136/jitc-2020-001361
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.