Back to Journals » Cancer Management and Research » Volume 14
Immunotherapy in Advanced Non-Small Cell Lung Cancers: Current Status and Updates
Authors Suraya R , Tachihara M , Nagano T , Nishimura Y, Kobayashi K
Received 16 March 2022
Accepted for publication 2 June 2022
Published 22 June 2022 Volume 2022:14 Pages 2079—2090
DOI https://doi.org/10.2147/CMAR.S366738
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Kattesh Katti
Ratoe Suraya, Motoko Tachihara, Tatsuya Nagano, Yoshihiro Nishimura, Kazuyuki Kobayashi
Division of Respiratory Medicine, Department of Internal Medicine, Kobe University Graduate School of Medicine, Kobe, Japan
Correspondence: Motoko Tachihara, Division of Respiratory Medicine, Department of Internal Medicine, Kobe University Graduate School of Medicine, 7-5-1 Kusunoki-cho, Chuo-ku, Kobe, Hyogo, 650-0017, Japan, Tel +81-78-382-5660, Fax +81-78-382-5661, Email [email protected]
Abstract: Non-small-cell lung cancer (NSCLC) is a major health burden, and novel therapeutic options are needed to help solve this problem. One such option is immunotherapy, which targets immune checkpoint molecules that inhibit cancer cells, decreasing immune system activation, for example, immunotherapies target PD-1, its ligand PD-L1, and CTLA-4. There have been major advances in the development of agents that inhibit these molecules, called immune checkpoint inhibitors, and several of them are already approved for usage in NSCLC patients, especially in advanced stages. In this review, the reasons why immune checkpoint inhibitors could be beneficial and the clinical results of studies using these drugs for advanced or recurrent NSCLC patients are discussed, as is the safety profile of the drugs.
Keywords: non-small cell lung cancer, immune checkpoint inhibitors, PD-1, PD-L1, CTLA-4
Introduction
Lung cancer is one of the most prominent causes of mortality and morbidity in the world; in 2018, there were estimated to be more than 2 million new cases and 1.7 million deaths caused by lung cancer alone, showing how much of a burden this terminal condition remains.1 One of the main forms of lung cancer is non-small-cell lung cancer (NSCLC), which accounts for approximately 85% of all lung cancer cases and is classified by the WHO into three different classes: adenocarcinoma, squamous cell carcinoma, and large-cell cancer.2,3 Current treatment options for NSCLC consist of resection with adjuvant/neoadjuvant chemotherapy in the early stages, while treatment options are more limited in the later stages.4 Importantly, novel therapeutic options for this condition have emerged in the past decade. For example, immunotherapy targets immune checkpoints that regulate proper immune responses to pathogenic agents such as cancer cells.5 In this review, we will discuss the molecular mechanisms of available immunotherapies and the rationale for their use in NSCLC. Furthermore, we will detail each of the immunotherapy drugs and discuss their efficacy and potential side effects in various clinical studies already conducted throughout the world. Finally, we will also mention other possible immune checkpoint inhibitors currently undergoing clinical development, as well as other potential therapeutic targets in the future.
Immune Checkpoints in Cancers
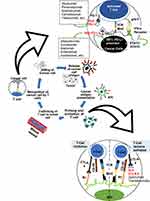
As an important system protecting the human body from pathogens and/or malignancies, the immune system plays an important role in preventing malignancies from spreading and affecting various locations.6,7 However, the ability of cancer cells to evade and/or limit immune responses, even though the immune system considers the cells foreign to the body, has been a major challenge in devising a treatment strategy.8 This immune evasion mechanism in cancer cells could be even more problematic, due to the fact that aberrant immune system activity can also lead to a direct increase in the growth capacity of cancer cells and modification of the tumor microenvironment, both of which can contribute to worse tumor malignancy.8,9 Several possible explanations that could cause this phenomenon include downregulation of antigen-presenting mechanisms, development of tumor tolerance in tumor-specific T cells, and immune checkpoint upregulation in the tumor microenvironment that enables the uncontrolled and exponential growth of cancer cells.9,10 The concept of immune checkpoints and its inhibitors are summarized in Figure 1.
Immune checkpoints have recently gained considerable popularity as therapeutic targets in various form of cancers, including those in the lung.11,12 These immune checkpoints work to limit the duration and level of the responses of T cells that are dependent on T cell receptor (TCR) signaling during pathological conditions such as infection.13 However, this phenomenon can also paradoxically benefit cancer cell growth and development because it can limit the anticancer responses of T cells.13 Two major molecules have been identified as immune checkpoint regulators during T cell activation: PD-1 (programmed cell death protein 1), a type-I transmembrane receptor expressed in T cells; and CTLA-4 (cytotoxic T lymphocyte-associated protein 4), a receptor protein widely expressed in T cells and B cells.13 The activation of naïve T cells in lymphoid tissue requires two events to occur; the first is the presentation of major histocompatibility complex-bound antigenic peptide by antigen-presenting cells (APCs) to the TCRs of naïve T cells. The second event is a costimulatory signal in the form of the binding of CD28 on T cells with CD80/86 on APCs.14 To limit the overactivation of T cells, CTLA-4, also known as CD152, becomes upregulated on the activated T cell surface and binds to CD80/86, acting as a means to switch off the interaction and subsequent signaling of APCs and T cells while inhibiting the secretion of IL-2.15 This process is possible because CTLA-4 has homology with CD28 but greater affinity and avidity for CD80 and CD86, as such, it can compete with CD28 for binding CD80/86 and induce activation of a myriad of intrinsic and extrinsic pathways that lead to cell cycle arrest (related to the previously mentioned inhibition of activation and proliferation of T cells).15,16 CTLA-4 can also be found in regulatory T cells (Tregs), in which it exerts inhibitory effects that are similar to those it induced in other T cells.17
Whereas CTLA-4 inhibits the early activation and proliferation of T cells, PD-1, also known as CD279, is upregulated in the later phase of T cell activation. Similar to CTLA-4, PD-1 also has a homology with CD28, but it will instead bind to a specific ligand called PD-L1 (CD274), which is highly expressed on the surface of cancer cells and is cleverly utilized by these cells to evade and inhibit antitumor immune responses.18,19 Of note, the PD-L1 expression level has also been used as a biomarker in various cancers, including NSCLC.20 In addition, another ligand for PD-1, called PD-L2 (CD273), also exists in several types of tumor cells, although until now its exact role has not been studied as well as the role of PD-L1 has been.21 Stimulation of PD-1 inhibits further T cell responses via activation of protein tyrosine phosphatases, more specifically, SH2-containing phosphatase 2 (SHP2).22 This signaling is first mediated by the phosphorylation of PD-1 at the immune receptor tyrosine-based inhibitory motif (ITIM) and immune receptor tyrosine-based switch motif (ITSM), which subsequently bind to the Src homology 2 (SH2) domains of SHP2. This signaling cascade will result in the inactivation of T cells.22,23 Furthermore, since PD-1 is also expressed to a certain degree in various other immune cells, such as B cells and macrophages, it has been suggested that PD-1 has a broad negative effect on immune responses in general, and its effect might not be limited to T cells only.24 These important findings regarding the mechanisms surrounding immune checkpoints in cancer, as illustrated in Figure 1, led to the increased interest in targeting the aforementioned checkpoint molecules as a novel treatment strategy.
Immunotherapy Targeting Immune Checkpoints in Advanced NSCLC
Many major clinical trials have been conducted for immune checkpoint inhibitors (ICIs) that target major immune checkpoint molecules previously mentioned, such as PD-1, PD-L1, and CTLA-4. From these trials, there have fortunately been numerous encouraging results that have resulted in some of the ICIs already receiving approval for use in NSCLC by the FDA and EMA. The list of ICI agents that have been studied for use and their current status in NSCLC and/or other cancers are briefly summarized in Table 1, and the list of Phase III trials conducted for ICIs in advanced NSCLC patients is summarized in Table 2. The inhibitors studied will be explained in detail in the sections below. Of note, this review will specifically discuss inhibitor agents that have been approved for usage in advanced NSCLC or are currently undergoing clinical trials for NSCLC treatment.
 |
Table 1 Currently Available Immune Checkpoint Inhibitors and Its Current Clinical Status |
 |
Table 2 Major Phase III Clinical Trials Conducted for the First ICIs in Advanced NSCLC (Selected) |
PD-1 Inhibitor for Advanced NSCLC
The PD-1 protein is a very popular research topic, and there is currently an exponential growth in the number of studies geared at developing inhibitors of the PD-1 pathway.11,12 Several agents inhibiting either PD-1 or its known ligand PD-L1 have been approved as a therapeutic option for NSCLC treatment by the FDA or EMA. However, an important point to consider when analyzing the result of PD-1 inhibitor agents’ clinical trials is that many of these trials did not include patients with known EGFR or ALK alterations in their study besides IMpower-130 and IMpower-150. One prominent example of a widely studied ICI is the PD-1-targeting agent nivolumab. Nivolumab is a humanized immunoglobulin G4/IgG4 monoclonal antibody against PD-1 that inhibits its interaction with its ligands PD-L1 and PD-L2.25 Several clinical trials have assessed the efficacy and safety profile of nivolumab in NSCLC with varying degrees of success. One of the first phase III trials that assessed the efficacy of nivolumab in NSCLC was the CheckMate-026 phase III trial, which analyzed the efficacy of nivolumab administered as monotherapy in stage IV or recurrent NSCLC patients with PD-L1 levels ≥1%.26 Unfortunately, the initial results for nivolumab were not promising, where there was no difference in the progression-free survival rate and overall survival rate between the nivolumab-treated group and platinum-based chemotherapy group. Regardless, there was a considerably lower rate of treatment-related adverse events in the nivolumab-treated group than in the chemotherapy group, especially the rate of serious adverse events (17% in the nivolumab group compared to 50.6% in the chemotherapy group).26 Furthermore, the progression-free survival rate in the nivolumab-treated patient population with a high tumor mutation burden was higher than that in the chemotherapy group, indicating that there is an important place for nivolumab monotherapy in NSCLC treatment.26,27
Nivolumab alone has not been approved as a first-line therapy, but the combination of this drug and ipilimumab (an agent targeting another immune checkpoint, CTLA-4, which will be discussed later) has shown good effects and this combination therapy is currently being used in the clinical setting.28 An important trial that confirmed the efficacy of nivolumab combined with ipilimumab is the CheckMate-227, where NSCLC patients (notably including squamous NSCLC) nivolumab and ipilimumab combination was compared with nivolumab-only (in PD-L1 ≥1% group) or nivolumab + chemotherapy (in PD-L1 <1% group) and chemotherapy-only treatment regimens. Overall survival and duration of response was significantly improved in the nivolumab + ipilimumab treatment arm in the two different PD-L1 level groups that were analyzed, with comparable grade 3 or 4 adverse effect occurrence rate with the chemotherapy-only group.28 In addition, in another CheckMate trial, the CheckMate-9LA, patients were randomized to a nivolumab and ipilimumab treatment with additional two cycles of histology-based platinum doublet chemotherapy or four cycles of chemotherapy only, and this trial found significant improvements in overall survival, progression-free survival, and objective response rates.29 Further, 2-year follow-up data confirmed the effect of this combination therapy, with a 2-year overall survival rate of 38% in the nivolumab + ipilimumab + chemotherapy group compared to 26% in chemotherapy. Similar improvement could also be found in 2-year progression-free survival rate (20% in nivolumab + ipilimumab + chemotherapy vs 8.8% in chemotherapy only) and 2-year objective response rates (38% nivolumab + ipilimumab + chemotherapy vs 25% in chemotherapy only).30 The Nivolumab and ipilimumab combination will also be discussed further in the ipilimumab section.
Another PD-1 inhibitor already approved by the FDA and EMA for the treatment of advanced or metastatic NSCLC with positive PD-L1 expression is pembrolizumab. Mounting evidence stemming from the KEYNOTE family of clinical trials has successfully proven the efficacy of pembrolizumab. The first notable trial of the KEYNOTE trials, which is the phase Ib KEYNOTE-001 trial, showed that the overall survival after treatment with pembrolizumab in the initial follow-up period was 12.0 months, with progression-free survival of 3.7 months and objective response rate of 19.4%. A higher progression-free survival of 6.3 months and objective response rate of 45.2% could be seen in the PD-L1-high expression group (PD-L1 ≥50%).31 Encouragingly, there the 5-year overall survival rate in the previously untreated NSCLC patient group after treatment with pembrolizumab was 23%, with even higher result (29.6%) in the PD-L1-high expression arm.32 The subsequent phase III KEYNOTE-024 trial revealed that there was a significant improvement in the overall survival, progression-free survival, and overall response rates in the group of advanced NSCLC patients without any previous treatment with high PD-L1 expression treated with pembrolizumab in comparison to the platinum-based chemotherapy group.33 Of note, there were significantly fewer treatment-related adverse effects: the rate in the pembrolizumab-treated group (26.6%) was half that in the chemotherapy group (53.3%). The 5-year survival rate from this trial in a follow-up data presentation indicated a significantly prolonged median overall survival length, which doubled from 13.4 months to 26.3 months; there was also a higher 5-year overall survival rate (31.9% in the pembrolizumab group vs 16.3% in chemotherapy group) without notable increase in toxicity in the pembrolizumab group.34 This study, alongside other similar previous studies, led to the approval of pembrolizumab as a monotherapy for advanced NSCLC. Building from previous positive results found in earlier KEYNOTE studies, KEYNOTE-042 tried to explore whether it is possible to expand the population of patients that could benefit from pembrolizumab monotherapy.35 Whereas previous studies showed that pembrolizumab is especially effective in tumors with high PD-L1 expression, KEYNOTE-042 added exploratory ≥50%, ≥20%, ≥1%, and ≥1–49% PD-L1 expression groups; the study found that the overall survival rate was improved in all the groups except the ≥1–49% PD-L1 TPS group. It also found that there were fewer adverse effects occurring in the pembrolizumab monotherapy arm compared to the chemotherapy arm (18% vs 41%).35
In addition to its efficacy as a monotherapy in NSCLC, pembrolizumab has also shown efficacy when combined with chemotherapy. KEYNOTE-189 trial analyzed whether pembrolizumab in combination with pemetrexed and platinum-based drugs could be beneficial for non-squamous NSCLC in comparison to chemotherapy alone.36 The study found that the addition of pembrolizumab to the chemotherapy regimen significantly improved the overall 12-month survival rate (69.2% for pembrolizumab-combination group vs 49.4% for placebo-combination group), median progression-free survival (8.8 months for pembrolizumab-combination group vs 4.9 months for placebo-combination group), and overall survival across various PD-L1 categories analyzed, with comparable grade III adverse event occurrence rates. This study further confirms the overall benefit of pembrolizumab as a first-line treatment in NSCLC patients.36 The efficacy of pembrolizumab treatment in addition to chemotherapy is further shown in its 4-year follow-up updated analysis. There, a significant improvement in the overall survival could be seen for the pembrolizumab-combination group, where it was 22.0 months compared to 10.6 months for the placebo group.37 Progression-free survival was also improved in the pembrolizumab-combination group, similar to the initial analysis (9.0 months vs 4.9 months in the placebo-combination group).
KEYNOTE-407 trial provided important evidence for the use of pembrolizumab in combination with chemotherapy in squamous NSCLC condition. In this trial, previously untreated metastatic squamous NSCLC patients were randomized to either pembrolizumab or placebo for up to 35 cycles, with additional carboplatin and paclitaxel/nab-paclitaxel for the first four cycles. It was found that both overall survival (15.9 months in pembrolizumab group vs 11.3 in placebo group) and progression-free survival (6.4 months in pembrolizumab group vs 4.8 months in placebo group) were significantly improved in the pembrolizumab-treated group.38 It should be noted that discontinuation of treatment due to adverse effect was higher in the pembrolizumab group, although overall grade 3 or higher adverse effect occurrence rate was rather comparable between the two groups (69.8% in pembrolizumab group vs 68.2% in placebo group).38 Further, its 3-year follow-up updated analysis showed significant improvement in both overall survival (17.2 months vs 11.6 months) and progression-free survival (8.0 months vs 5.1 months), consistent with the initial analysis. It is notable that immune-mediated adverse event and infusion reaction rates were higher in pembrolizumab group compared to the placebo (35.6% vs 9.3%).39
Lastly, KEYNOTE-598 trial showed the results of combining pembrolizumab with ipilimumab in NSCLC patients expressing high level of PD-L1 (TPS ≥50%). However, compared to the pembrolizumab-only group, the investigators did not find significant benefit of additional ipilimumab in both overall survival (21.4 months in pembrolizumab + ipilimumab group vs 21.9 months in pembrolizumab-only group) and progression-free survival (8.2 months in pembrolizumab + ipilimumab group vs 8.4 months in pembrolizumab-only group).40 Furthermore, there is an increase in treatment-related adverse event rate in the combination group (76.2% vs 68.3%, with 35.1% vs 19.6% for grade 3–5 adverse effects).40
Given the results of the various clinical trials comparing nivolumab and pembrolizumab presented above, it is clear that there are differences in the efficacy of PD-1 inhibitors as monotherapies. Whereas nivolumab is not significantly effective in advanced NSCLC patients with high PD-L1 expression, inconsistent efficacy of pembrolizumab in treating NSCLC with high PD-L1 expression has been observed. An explanation for this unique phenomenon remains to be identified, but interestingly, several studies have not found any significant difference in progression-free survival between nivolumab and pembrolizumab, although in one study, there was a higher overall response rate in the pembrolizumab group.41 However, in the combination therapy setting, when comparing between the results of KEYNOTE-598 and CheckMate 9LA which both in similar PD-L1 TPS ≥50% but with different combination (nivolumab + ipilimumab for CheckMate 9LA and pembrolizumab + ipilimumab for KEYNOTE-598) showed differing effects, where the nivolumab + ipilimumab combination showed efficacy in treating the high PD-L1 NSCLC patient population. This result was not achieved by the pembrolizumab + ipilimumab combination in KEYNOTE-598, thus furthering the controversy in the choice between the two PD-1 inhibitors.29,40 Thus, it is still unclear whether anti-CTLA4 antibody should be used in combination with PD-1 inhibitor for patients with high levels of PD-L1 considering the risks and benefits. The use of an approved predictive biomarker such as the widely used PD-L1 level is important in determining which patients could benefit the most from the addition of immunotherapy and which patients might not achieve desirable responses to therapy.20,42
Agents that are currently in clinical trials as PD-1 inhibitors for advanced NSCLC treatment include pidilizumab/CT-011 (anti-PD-1 IgG1 monoclonal antibody), the Phase II trial of which has been completed; spartalizumab (humanized anti-PD-1 IgG4 monoclonal antibody), the phase II trial in NSCLC of which has been completed; dostarlimab (anti-PD-1 IgG4 monoclonal antibody), which is being tested in an ongoing phase II trial; camrelizumab (a high-affinity, humanized, anti-PD-1 IgG4-κ mAb), the phase III trial in NSCLC of which has been completed; sintilimab (human IgG4 monoclonal antibody against PD-1), which has finished its phase III trial; and tislelizumab, which also have been tested in similar phase III trials, among other agents.43–45 It is hoped that with the rapid development of various agents to inhibit PD-1, there will be a wide range of patient populations that can receive beneficial effects from this treatment option.
PD-L1 Inhibitors in Advanced NSCLC
In addition to targeting PD-1, agents that target the ligand PD-L1 have also been approved for treatment in advanced NSCLC. An example of a PD-L1 inhibitor that has been approved for use in NSCLC is atezolizumab, which was supported by the success of the phase II BIRCH and POPLAR trials, in addition to the phase III OAK trial results in previously treated advanced NSCLC and IMpower series of trials that established atezolizumab as a first-line treatment for advanced NSCLC. The results of these trials showed varying degrees of improvement in progression-free survival and overall survival rate, among other factors, with favorable serious adverse event occurrence rates between the atezolizumab-treated and nontreated groups.46–49 Adverse effects of atezolizumab include anemia, hyponatremia and hyperkalemia. As with other ICIs, atezolizumab can be used not only as a monotherapy but also in combination with chemotherapy.50 IMpower130 trial, among other trials, has shown that when combined with carboplatin and nab-paclitaxel chemotherapy treatment regimens, atezolizumab shows a superior overall survival rate (18.6 months with atezolizumab compared to 13.9 months without) and median progression-free survival (7 months with atezolizumab vs 5.5 months without).50 However, there was an increase in treatment-related adverse effects in the atezolizumab-treated group (24% vs 13% without atezolizumab), with the most common high-grade adverse effect being neutropenia. Another important atezolizumab trial to mention is the IMpower-150, which in its final analysis showed a significant improvement of overall survival when additional atezolizumab treatment was administered in addition to bevacizumab and chemotherapy in metastatic non-squamous NSCLC.51 IMpower 150 is a trial that incorporated sensitizing EGFR mutation patients, and in this exploratory group, this trial revealed that improved overall survival in the atezolizumab + bevacizumab + chemotherapy arm compared to bevacizumab + chemotherapy group (29.4 months vs 18.1 months). However, a similar improvement could not be observed when patients were treated with atezolizumab alone (19.0 months in atezolizumab + chemotherapy group vs 18.1 months in bevacizumab + chemotherapy group).52 These findings show that atezolizumab has efficacy in treating advanced NSCLC in a combination setting with bevacizumab + chemotherapy.
Another example is durvalumab, a human IgG1 monoclonal antibody targeting PD-L1.53 Durvalumab is currently approved by the FDA and EMA in stage III NSCLC at a dose of 10 mg/kg every 2 weeks or 1500 mg every 4 weeks. The PACIFIC trial confirmed the efficacy of durvalumab in NSCLC, showing a significantly increased survival rate in the treatment arm with comparable stage 3 or 4 adverse effect rates in the treatment and placebo groups.53 Durvalumab has also been studied in combination with the CTLA-4 inhibitor tremelimumab, which has already received approval by the FDA and EMA. This combination will also be discussed later in the CTLA-4 inhibitor section.54 An important trial that analyzed the importance of durvalumab is the POSEIDON trial, which examined the effect of Durvalumab in combination with chemotherapy compared to chemotherapy alone or when durvalumab + chemotherapy were additionally combined with tremelimumab. In the trial that enrolled both squamous and non-squamous NSCLC patients, it was found that the durvalumab + tremelimumab combination in addition to chemotherapy had superior overall survival (14.0 months vs 11.7 months in chemotherapy-only) and progression-free survival (6.2 months vs 4.8 months in chemotherapy-only) compared to chemotherapy alone. This trial showed the efficacy of durvalumab when combined with tremelimumab in advanced NSCLC.
Another agent inhibiting PD-L1 is avelumab. Avelumab is a human monoclonal antibody against PD-L1 already approved for the treatment of Merkel cell carcinoma, renal cell carcinoma and urothelial carcinoma, among other cancers.55 In the treatment of NSCLC, avelumab has shown its efficacy in the phase III JAVELIN Lung 200 trial, an open-label, randomized trial involving 792 advanced-stage NSCLC patients already receiving platinum-based therapy who were evenly divided into avelumab treatment and docetaxel treatment groups.56 The mean overall survival rate in the group that received a 10 mg/kg dose of avelumab every 2 weeks was not significantly improved compared with that in the docetaxel group (11.4 months in avelumab group vs 10.3 in docetaxel group); however, favorable results were obtained with avelumab in terms of its favorable safety profile in comparison to docetaxel: serious treatment-related adverse events only occurred in approximately 9% of the avelumab-treated patients in comparison to 21% of the docetaxel-treated patients.56 Adverse events of avelumab treatment include infusion-related reactions and increased lipase. Overall, more studies are warranted to confirm the efficacy of avelumab in NSCLC and to establish its usefulness as a treatment option.
Similar to the wealth of PD-1 inhibitors currently undergoing clinical trials, several PD-L1 inhibitors are currently being studied in clinical trials for use in NSCLC treatment. These agents include cosibelimab, which is currently being tested in phase III trials for metastatic NSCLC and LY3300054, a fully human IgG1λ monoclonal anti-PD-L1 antibody currently being tested in Phase I trials for solid tumors. Future advances in PD-L1 inhibitor development are eagerly awaited to enrich the treatment options for NSCLC. One last important point to note is that there is a striking difference in the incidence of severe adverse events between PD-1 and PD-L1 inhibitors: several PD-L1 inhibitors have shown significantly lower rates of severe adverse events, most notably immune-related adverse events such as pneumonitis, in comparison to PD-1 inhibitors. This phenomenon is yet another important aspect to consider when choosing between these two types of ICIs in the clinical setting.57,58
CTLA-4 Inhibitors in Advanced NSCLC
As mentioned previously, targeting CTLA-4 can prevent the activation and proliferation of T cells in the early stages of their activation via its prevention of CD28 binding to the CD80/CD86 ligands in addition to preventing IL-2 secretion; this is theoretically a very promising strategy to ensure the availability of active immune cells for fighting cancer cells and preventing cancer progression.16 However, in comparison to the high numbers of PD-1 and PD-L1 inhibitors, the number of agents targeting CTLA-4 and its signaling pathway is limited. Two drugs that are known to act on CTLA-4, ipilimumab and tremelimumab, are currently being tested in various clinical trials to confirm its efficacy in NSCLC patients.57 These two drugs have previously been approved by the FDA for the treatment of metastatic melanoma and, in the case of ipilimumab, are also approved by the FDA and EMA for the treatment of NSCLC in combination with the aforementioned nivolumab. In general, it is thought that by inhibiting CTLA-4, it could act as a brake that prevents the activation of Tregs, and as a consequence, conventional T cells will be released via blockade of the CTLA-4−B7 interaction. Additionally, it could also induce the depletion of target CTLA-4-expressing cells (such as the aforementioned Tregs) via antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity.17 It is widely thought that ipilimumab and tremelimumab exhibit their clinical effects via these signaling pathways.59
A notable CTLA-4 inhibiting agent currently approved for clinical use is ipilimumab. Ipilimumab is a well-known inhibitor of CTLA-4 and has been approved for use in combination with the anti-PD-1 agent nivolumab in NSCLC.28 Ipilimumab is a recombinant human IgG1κ monoclonal antibody capable of binding to CTLA-4 to prevent its interaction with CD80 and CD86 in inhibiting T cell activation and proliferation.60 Ipilimumab was originally analyzed in unresectable or metastatic melanoma with progression after treatment with standard therapy, in which 3 mg/kg intravenous infusion administered every 3 weeks for four doses showed efficacy in increasing the overall survival rate.60 Ipilimumab has been approved by the FDA and EMA for the treatment of NSCLC in combination with the PD-1 inhibitor nivolumab at a 1 mg/kg dose every 6 weeks. As previously mentioned, CheckMate-227 compared the ipilimumab plus nivolumab dual ICI treatment combination, nivolumab monotherapy, and chemotherapy (with patients divided evenly into subgroups with PD-L1 expression >1% and <1%) found that the dual ICI treatment combination with ipilimumab and nivolumab effectively improved the mean overall survival duration of the patients (17.1 months in the ipilimumab and nivolumab-treated group vs 14.9 months in the chemotherapy group in the PD-L1-high subgroup; 17.2 months in the ipilimumab and nivolumab-treated group vs 12.2 months in chemotherapy group in the PD-L1-low subgroup).28 Of note, this efficacy is found regardless of the PD-L1 expression level. In updated data, the 4-year overall survival rate was also improved in the dual ICI group compared with the chemotherapy group (37.0% vs 20.0%).28 In CheckMate-9LA study, among patients with brain metastasis, intracranial efficacy was improved with ipilimumab + nivolumab + chemotherapy vs chemotherapy, consistent with systemic efficacy. Overall, there is a real hope that targeting both major immune checkpoint pathways (the PD-1 and CTLA-4 pathways) could serve as a promising strategy for future therapies, although an increase in the risk of pneumonitis, skin rash, hypothyroidism and colitis in the ipilimumab combined with the PD-1 inhibitor group compared to the PD-1 inhibitor monotherapy was also found.28,40
Another recently developed CTLA-4 inhibitors is the novel agent tremelimumab. Similar to ipilimumab, which is also an IgG2 monoclonal anti-CTLA-4 antibody that binds to CTLA-4 to prevent its binding to CD86 and CD80, it is currently being tested in a Phase 3 trial for use in NSCLC, and it has also been studied in combination with the anti-PD-L1 antibody durvalumab, as mentioned previously.54 With linear clearance and no reported pharmacokinetic interaction between the two inhibitors, tremelimumab is an ideal dual-checkpoint inhibitor combination agent that can be used in a similar way to nivolumab/ipilimumab combination treatment. With that advantage in mind, the investigators of the MYSTIC phase III trial tried to evaluate the safety and efficacy of the combination of durvalumab and tremelimumab and compared the values with those of durvalumab or chemotherapy alone in their clinical study.54 As a result, although the overall survival rate was not significantly improved in the general analysis of the trial, a further exploratory analysis showed that there was a benefit of the dual-inhibitor therapy combination in patients with blood tumor mutational burden or bMTB of ≥20 mutations per megabase, with an improvement in the overall survival rate compared to that in the chemotherapy-only group. The most frequent adverse effects of the combination therapy included nausea, fatigue, diarrhea, pruritus, and rash. In conclusion, although there is promise, more trials are needed to ascertain the efficacy of tremelimumab treatment in NSCLC patients.
In addition to the obvious difference in isotype between ipilimumab (IgG1) and tremelimumab (IgG2), there are reported differences in efficacy between ipilimumab and tremelimumab in several different types of tumors, not only in NSCLC but also in melanoma and others. One study compared the binding properties of the two agents and found that although the two agents have similar binding affinities, their paratopes are distinct, indicating the possible utility of monoclonal antibodies targeting CTLA-4 in regard to its therapeutic efficacy, although more studies are needed to confirm this finding.57 Due to the current relative dearth of anti-CTLA-4 agents in comparison to PD-1 and PD-L1 inhibitors, the development of drugs targeting this promising pathway is sorely needed to further add to the clinical variety of immunotherapies for NSCLC treatment.
Conclusion
Immunotherapy for non-small cell lung cancer has a central role as a therapeutic option with two clear target immune checkpoint pathways (PD-1 and CTLA-4) that can affect the activity of the immune system in fighting cancer cells. The strategy of inhibiting immune checkpoints using immune checkpoint inhibitors has been tested in various clinical trials and has been shown to produce major benefits in NSCLC patients, especially those with advanced stages of disease. The currently mentioned drugs are only the beginning for the field of ICI development. As listed in Table 1, there are many more novel targets that are being developed with promise, such as chimeric antigen receptor (CAR) T cell therapy and agents targeting LAG-3, TIM-3, TIGIT, B7-H3, VISTA, a PD-1 homolog, BTLA, and so on. Further basic and clinical studies of ICIs are warranted to confirm the efficacy of the newer ICI agents as well as novel combinations of ICIs and other therapeutic options, such as chemotherapies, in addition to confirming their safety profile in patients. The hope is that future studies can also reveal targets for inhibiting immune checkpoints.
Disclosure
Dr. Motoko Tachihara reports grants and personal fees from AstraZeneca K.K; personal fees from Eli Lilly Japan K.K., Chugai Pharmaceutical Co. Ltd., Ono Pharmaceutical Co. Ltd., MSD K.K., Nippon Boehringer Ingelheim Co. Ltd., Bristol-Myers Squibb Co. Ltd., and Novartis pharmaceuticals K.K, outside the submitted work. Prof. Dr. Yoshihiro Nishimura reports personal fees from AstraZeneca and grants from Chugai Pharmaceutical Co., Ltd., outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Bade BC, Dela Cruz CS. Lung Cancer 2020. Clin Chest Med. 2020;41(1):1–24. doi:10.1016/j.ccm.2019.10.001
3. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors. J Thorac Oncol. 2015;10(9):1243–1260. doi:10.1097/JTO.0000000000000630
4. Bironzo P, Di Maio M. A review of guidelines for lung cancer. J Thorac Dis. 2018;10(S13):S1556–S1563. doi:10.21037/jtd.2018.03.54
5. Lim SM, Hong MH, Kim HR. Immunotherapy for non-small cell lung cancer: current landscape and future perspectives. Immune Netw. 2020;20:e10. doi:10.4110/in.2020.20.e10
6. Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32(19–20):1267–1284. doi:10.1101/gad.314617.118
7. Gaissmaier L, Christopoulos P. Immune modulation in lung cancer: current concepts and future strategies. Respiration. 2020;99(11):903–929. doi:10.1159/000510385
8. Vinay DS, Ryan EP, Pawelec G, et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35:S185–S198. doi:10.1016/j.semcancer.2015.03.004
9. Spranger S, Gajewski TF. Mechanisms of tumor cell–intrinsic immune evasion. Annu Rev Cancer Biol. 2018;2(1):213–228. doi:10.1146/annurev-cancerbio-030617-050606
10. Muenst S, Läubli H, Soysal SD, Zippelius A, Tzankov A, Hoeller S. The immune system and cancer evasion strategies: therapeutic concepts. J Intern Med. 2016;279(6):541–562. doi:10.1111/joim.12470
11. Huang Z, Su W, Lu T, et al. First-line immune-checkpoint inhibitors in non-small cell lung cancer: current landscape and future progress. Front Pharmacol. 2020;11. doi:10.3389/fphar.2020.578091
12. Herzberg B, Campo MJ, Gainor JF. Immune checkpoint inhibitors in non‐small cell lung cancer. Oncologist. 2017;22(1):81–88. doi:10.1634/theoncologist.2016-0189
13. He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30(8):660–669. doi:10.1038/s41422-020-0343-4
14. Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27(1):591–619. doi:10.1146/annurev.immunol.021908.132706
15. Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways. Am J Clin Oncol. 2016;39(1):98–106. doi:10.1097/COC.0000000000000239
16. Brunner-Weinzierl MC, Rudd CE. CTLA-4 and PD-1 control of T-cell motility and migration: implications for tumor immunotherapy. Front Immunol. 2018;9. doi:10.3389/fimmu.2018.02737
17. Walker LSK. Treg and CTLA-4: two intertwining pathways to immune tolerance. J Autoimmun. 2013;45:49–57. doi:10.1016/j.jaut.2013.06.006
18. Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the Pd-1 immunoinhibitory receptor by a Novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi:10.1084/jem.192.7.1027
19. Fife BT, Pauken KE. The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann N Y Acad Sci. 2011;1217(1):45–59. doi:10.1111/j.1749-6632.2010.05919.x
20. Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):278. doi:10.1186/s40425-019-0768-9
21. Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–268. doi:10.1038/85330
22. Marasco M, Berteotti A, Weyershaeuser J, et al. Molecular mechanism of SHP2 activation by PD-1 stimulation. Sci Adv. 2020;6(5):eaay4458. doi:10.1126/sciadv.aay4458
23. Patsoukis N, Duke-Cohan JS, Chaudhri A, et al. Interaction of SHP-2 SH2 domains with PD-1 ITSM induces PD-1 dimerization and SHP-2 activation. Commun Biol. 2020;3(1):128. doi:10.1038/s42003-020-0845-0
24. Thibult M-L, Mamessier E, Gertner-Dardenne J, et al. PD-1 is a novel regulator of human B-cell activation. Int Immunol. 2013;25(2):129–137. doi:10.1093/intimm/dxs098
25. Sekhon N, Kumbla RA, Mita M. Current trends in cancer therapy. In: Cardio-Oncology. Elsevier; 2017:1–24. doi:10.1016/B978-0-12-803547-4.00001-X
26. Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage iv or recurrent non–small-cell lung cancer. N Engl J Med. 2017;376(25):2415–2426. doi:10.1056/NEJMoa1613493
27. Antonia SJ, Borghaei H, Ramalingam SS, et al. Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis. Lancet Oncol. 2019;20(10):1395–1408. doi:10.1016/S1470-2045(19)30407-3
28. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020–2031. doi:10.1056/NEJMoa1910231
29. Paz-Ares L, Ciuleanu T-E, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198–211. doi:10.1016/S1470-2045(20)30641-0
30. Reck M, Ciuleanu T-E, Cobo M, et al. First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small-cell lung cancer: checkMate 9LA 2-year update. ESMO Open. 2021;6(5):100273. doi:10.1016/j.esmoop.2021.100273
31. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi:10.1056/NEJMoa1501824
32. Garon EB, Hellmann MD, Rizvi NA, et al. Five-year overall survival for patients with advanced non‒small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol. 2019;37(28):2518–2527. doi:10.1200/JCO.19.00934
33. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi:10.1056/NEJMoa1606774
34. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non–small-cell lung cancer with PD-L1 tumor proportion score ≥ 50%. J Clin Oncol. 2021;39(21):2339–2349. doi:10.1200/JCO.21.00174
35. Mok TSK, Wu Y-L, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830. doi:10.1016/S0140-6736(18)32409-7
36. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi:10.1056/NEJMoa1801005
37. Gray J, Rodríguez-Abreu D, Powell SF, et al. FP13.02 Pembrolizumab + Pemetrexed-platinum vs Pemetrexed-platinum for metastatic NSCLC: 4-year follow-up from KEYNOTE-189. J Thorac Oncol. 2021;16(3):S224. doi:10.1016/j.jtho.2021.01.141
38. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi:10.1056/NEJMoa1810865
39. Robinson A, Vicente Baz D, Tafreshi A, et al. First-line pembrolizumab plus chemotherapy for patients with advanced squamous NSCLC: 3-year follow-up from KEYNOTE-407. J Thorac Oncol. 2021;16(4):S748–S802. doi:10.1016/S1556-0864(21)01939-0
40. Boyer M, Şendur MAN, Rodríguez-Abreu D, et al. Pembrolizumab plus ipilimumab or placebo for metastatic non–small-cell lung cancer with PD-L1 tumor proportion score ≥ 50%: randomized, double-blind phase III KEYNOTE-598 study. J Clin Oncol. 2021;39(21):2327–2338. doi:10.1200/JCO.20.03579
41. Cui P, Li R, Huang Z, et al. Comparative effectiveness of pembrolizumab vs. nivolumab in patients with recurrent or advanced NSCLC. Sci Rep. 2020;10(1):13160. doi:10.1038/s41598-020-70207-7
42. Zhang J, Yan Y, Li J, Adhikari R, Fu L. PD-1/PD-L1 based combinational cancer therapy: icing on the cake. Front Pharmacol. 2020;11. doi:10.3389/fphar.2020.00722
43. Lin -C-C, Taylor M, Boni V, et al. Phase I/II study of spartalizumab (PDR001), an anti-PD1 mAb, in patients with advanced melanoma or non-small cell lung cancer. Ann Oncol. 2018;29:viii413. doi:10.1093/annonc/mdy288.032
44. Xu Y, Wan B, Chen X, et al. The association of PD-L1 expression with the efficacy of anti-PD-1/PD-L1 immunotherapy and survival of non-small cell lung cancer patients: a meta-analysis of randomized controlled trials. Transl Lung Cancer Res. 2019;8(4):413–428. doi:10.21037/tlcr.2019.08.09
45. Zhou C, Chen G, Huang Y, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med. 2021;9(3):305–314. doi:10.1016/S2213-2600(20)30365-9
46. Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for first-line treatment of PD-L1–selected patients with NSCLC. N Engl J Med. 2020;383(14):1328–1339. doi:10.1056/NEJMoa1917346
47. Peters S, Gettinger S, Johnson ML, et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1–selected advanced non–small-cell lung cancer (BIRCH). J Clin Oncol. 2017;35(24):2781–2789. doi:10.1200/JCO.2016.71.9476
48. Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, Phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846. doi:10.1016/S0140-6736(16)00587-0
49. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi:10.1016/S0140-6736(16)32517-X
50. West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 tria. Lancet Oncol. 2019;20(7):924–937. doi:10.1016/S1470-2045(19)30167-6
51. Socinski MA, Nishio M, Jotte RM, et al. IMpower150 final overall survival analyses for atezolizumab plus bevacizumab and chemotherapy in first-line metastatic nonsquamous NSCLC. J Thorac Oncol. 2021;16(11):1909–1924. doi:10.1016/j.jtho.2021.07.009
52. Nogami N, Barlesi F, Socinski MA, et al. IMpower150 final exploratory analyses for atezolizumab plus bevacizumab and chemotherapy in key NSCLC patient subgroups with EGFR mutations or metastases in the liver or brain. J Thorac Oncol. 2021. doi:10.1016/j.jtho.2021.09.014
53. Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–2350. doi:10.1056/NEJMoa1809697
54. Rizvi NA, Cho BC, Reinmuth N, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non–small cell lung cancer. JAMA Oncol. 2020;6(5):661. doi:10.1001/jamaoncol.2020.0237
55. Barlesi F, Vansteenkiste J, Spigel D, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol. 2018;19(11):1468–1479. doi:10.1016/S1470-2045(18)30673-9
56. Verschraegen CF, Jerusalem G, McClay EF, et al. Efficacy and safety of first-line avelumab in patients with advanced non-small cell lung cancer: results from a phase Ib cohort of the JAVELIN Solid Tumor study. J Immunother Cancer. 2020;8(2):e001064. doi:10.1136/jitc-2020-001064
57. Pillai RN, Behera M, Owonikoko TK, et al. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: a systematic analysis of the literature. Cancer. 2018;124(2):271–277. doi:10.1002/cncr.31043
58. Banna GL, Cantale O, Bersanelli M, et al. Are anti-PD1 and anti-PD-L1 alike? The non-small-cell lung cancer paradigm. Oncol Rev. 2020;14:490. doi:10.4081/oncol.2020.490
59. He M, Chai Y, Qi J, et al. Remarkably similar CTLA-4 binding properties of therapeutic ipilimumab and tremelimumab antibodies. Oncotarget. 2017;8(40):67129–67139. doi:10.18632/oncotarget.18004
60. Sondak VK, Smalley KSM, Kudchadkar R, Grippon S, Kirkpatrick P. Ipilimumab. Nat Rev Drug Discov. 2011;10(6):411–412. doi:10.1038/nrd3463
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.