Back to Journals » Drug Design, Development and Therapy » Volume 15
Immunoregulatory Effect of Acanthopanax trifoliatus (L.) Merr. Polysaccharide on T1DM Mice
Authors Li P, Chen Y, Luo L, Yang H, Pan Y
Received 6 March 2021
Accepted for publication 4 May 2021
Published 18 June 2021 Volume 2021:15 Pages 2629—2639
DOI https://doi.org/10.2147/DDDT.S309851
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Ping Li,1,2 Yanli Chen,1 Luxiang Luo,1 Huiwen Yang,1 Yufang Pan1
1School of Pharmacy, Guangdong Pharmaceutical University, Guangzhou, 510006, People’s Republic of China; 2Department of Pharmacy, Xiamen Children’s Hospital, Children’s Hospital of Fudan University at Xiamen, Xiamen, 361006, People’s Republic of China
Correspondence: Yufang Pan; Huiwen Yang
School of Pharmacy, Guangdong Pharmaceutical University, Guangzhou, 510006, People’s Republic of China
Email [email protected]; [email protected]
Background: Acanthopanax trifoliatus (L.) Merr. is a medicinal plant found in Southeast Asia, and its young leaves and shoots are consumed as a vegetable. The main bioactive components of this herb are polysaccharides that have significant anti-diabetic effects. The aim of this study was to evaluate the immunoregulatory effect of A. trifoliatus (L.) Merr. polysaccharide (ATMP) on a mouse model of type 1 diabetes mellitus (T1DM).
Methods: The monosaccharide composition and mean molecular mass of ATMP were determined by HPLC and HPGPC. T1DM was induced in mice using STZ, and 35, 70 and 140mg/kg ATMP was administered daily via the intragastric route for six weeks. Untreated and metformin-treated positive control groups were also included. The body weight of the mice, food and water intake and fasting glucose levels were monitored throughout the 6-week regimen. Histological changes in the pancreas and spleen were analyzed by H&E staining. Oral glucose tolerance was evaluated with the appropriate test. Peroxisome proliferator-activated receptor γ (PPARγ) mRNA and protein levels in the spleen were measured by quantitative real time PCR and Western blotting. IL-10, IFN-γ and insulin levels in the sera were determined by ELISA. The CD4+ and CD8+T cells in spleen tissues were detected by immunohistochemistry (IHC).
Results: ATMP and metformin significantly decreased fasting blood glucose, and the food and water intake after 6 weeks of treatment. In contrast, serum insulin levels, glucose tolerance and body weight improved considerably in the high and medium-dose ATMP and metformin groups. T1DM was associated with pancreatic and splenic tissue damage. The high dose (140mg/kg) of ATMP reduced infiltration of inflammatory cells into the pancreas and restored the structure of islet β-cells in the diabetic mice. Consistent with this, 35, 70 and 140mg/kg ATMP increased IL-10 levels and decreased that of IFN-γ, thereby restoring the CD4+/CD8+ and Th1/Th2 cytokine ratio. At the molecular level, high-dose ATMP up-regulated PPARγ in the splenic cells.
Conclusion: ATMP exerts a hypoglycemic effect in diabetic mice by restoring the immune balance in the spleen.
Keywords: Acanthopanax trifoliatus (L.) Merr, type 1 diabetes mellitus, PPARγ, CD4+ T cells, CD8+ T cells, immunoregulation
Introduction
Diabetes mellitus (DM) is a chronic metabolic disease and the third major cause of deaths worldwide. It is the result of either inadequate insulin production or insulin resistance.1 According to the International Diabetes Federation (IDF), the number of confirmed cases of diabetes increased from 151 million in 2000 to 425 million in 2017 globally, and is estimated to reach 629 million by 2045.2 Diabetes is associated with several life-threatening complications, such as diabetic nephropathy, retinopathy, and coronary heart disease.3 Exogenous insulin, α-glucosidase inhibitors and biguanides can control the symptoms of DM, but are associated with considerable side effects.4,5
Polysaccharides are the active component of various Chinese herbal medicine formulations.6,7 Recent studies show that polysaccharides derived from tea, lotus, litchi, Sargassum fusiforme etc. can mitigate diabetes with minimal side effects.8–11 The hypoglycemic effects of polysaccharides can be attributed to the inhibition of the mitochondrial apoptotic pathway in the pancreatic β-cells,8 activation of the PI3K/Akt survival pathway,10 modulation of plasma and intestinal GLP-1 levels by inhibiting alpha glucosidase,12 and anti-inflammatory and immunomodulation effects.8,9 In addition, polysaccharides have low toxicity, which significantly broaden their clinical applications.
Acanthopanax trifoliatus (L.) Merr. is a herb of the family Araliaceae that is widely distributed in Asian subtropical countries, such as India, Japan, Philippines, Thailand, Vietnam and China.13 It is used in folk medicine in South-East Asia owing to ginseng-like effects.14,15 The roots and bark of A. trifoliatus (L.) Merr. are used as tonics and sedatives for treating rheumatism and diabetes,16 and the young leaves and shoots are often consumed as tea and vegetables.17 Studies show that A. trifoliatus (L.) Merr. extracts have anti-cancer,18 anti-inflammatory,19 and anti-depressant effects,20 although a hypoglycemic activity has not been reported so far.
Type 1 diabetes mellitus (T1DM) is the result of impaired insulin production due to autoreactive T cell-mediated destruction of the pancreatic islets of Langerhans,21 and the imbalance between the Th1 (type 1 helper) and Th2 (type 2 helper) cells. Polysaccharides can decrease the Th1/Th2 cytokine ratio in mice,22 which is suggestive of a potential anti-diabetic effect. The aim of this study was to analyze the therapeutic effects of A. trifoliatus (L.) Merr. polysaccharide (ATMP) in a streptozotocin (STZ)-induced mice model of TIDM, and explore the underlying molecular mechanisms.
Materials and Methods
Isolation and Purification of ATMP
A. trifoliatus (L.) Merr. were harvested from Enping (Guangdong Province, China) and identified by professor Liu Jizhu. The voucher specimen is deposited at the School of Traditional Chinese medicine, Guangdong Pharmaceutical University. ATMP was extracted as previously described.23 After removing proteins by the Sevag method, the crude polysaccharide fraction was purified using DEAE-52 Cellulose and Sephadex G-50 gel columns. ATMP (Mw=2.31KDa) was composed of glucose, galactose, mannose and rhamnose, and no nucleic acid or proteins were detected by HPLC and HPGPC.
Establishment of STZ Model and Treatment Regimen
Male C57BL/6 mice (6–8weeks, 20±2g) were purchased from Guangdong Medical Laboratory Animal Center, and housed in standard pathogen free conditions. The experiments were performed in accordance with the Guide for Care and Use of Laboratory Animals, and approved by the Institutional Animal Care and Use Committee of Guangdong Pharmaceutical University. Diabetes was induced by intraperitoneally injecting the mice with 60mg/kg STZ (sigma, United States) for five consecutive days, and healthy mice were given equal volume of the vehicle. Seven days after the last injection, fasting blood glucose levels were measured by glucose meter (Sinocare, China) and diabetes onset was diagnosed when it was >16.7mmol/L in 3 consecutive tests. The diabetic mice were randomly divided into the diabetes (water), metformin (285mg/kg·d−1), ATMP high-dose (ATMP-H, 140mg/kg·d−1), ATMP medium-dose (ATMP-M, 70mg/kg·d−1) and ATMP low-dose (ATMP-L, 35mg/kg·d−1) groups (n=8 each). Healthy mice were selected as normal controls (n=8) and given equal volume of vehicle. All drugs were administrated intragastrically at the dosage of 10mL/kg once a day. During the 6-weeks treatment regimen, the body weight, food and water intake and fasting glucose levels were monitored.
Oral Glucose Tolerance Test (OGTT)
At the end of the experiment, the mice were fasted for 8 h and OGTT was performed via intragastric administration of glucose solution (2g/kg). Blood glucose levels were respectively measured at 0, 30-, 60-, 90- and 120-min post intake.
Histological Analysis
The spleen and pancreas were harvested and fixed in 4% paraformaldehyde for 24 hours at 25°C, and embedded in paraffin. The sections were stained with hematoxylin and eosin for histopathological examination.
Western Blotting
Total protein was extracted by homogenizing the spleen tissues in RIPA lysis buffer, and quantified using the BCA Assay kit (Thermo, China). Equal amounts of protein per sample (40μg) were denatured in the sample buffer at 95°C for 5 min, and separated by 10% SDS-PAGE. The protein bands were transferred to polyvinylidene fluoride membranes (PVDF), blocked for 1 hour, and incubated overnight with rabbit anti-PPARγ antibody (1:1000; C26H12; cell signaling technology; USA) and anti-β-actin monoclonal antibody (1:1000; 13E5; cell signaling technology; USA) at 4°C. After washing with tris-buffer saline with Tween-20 (TBST), the blots were incubated with goat anti-rabbit peroxidase-coupled secondary antibody (1:5000; GTX213110-01; Gene Tex; USA) for 1 hour. Positive bands were developed using an enhanced chemiluminescence (ECL) kit (Thermo, China), and detected by a chemiluminescence imaging system (Tanon 5200, China). The protein bands were normalized against β-actin and their relative densities were measured.
Real-Time Quantitative PCR
Spleens were removed and homogenized in Trizol, and the extracted RNA was quantified using a micro-spectrophotometer (Biotek epoch2, United States). One microgram RNA per sample was reverse transcribed to cDNA using the RNA reverse transcription kit (Takara, China). The cDNA template was diluted 1:20, and real-time PCR was performed on the Lightcycler 96 (Roche, United States) using Fast Start Universal SYBR Green Master kit (Takara, China). The cycling conditions were as follows: initial denaturation at 95°C for 120s, followed by 40 cycles of denaturation at 95°C for 5s, annealing at 55°C for 30s and extension at 72°C for 1 min. The relative expression level of PPARγ gene was normalized to β-actin, and calculated using the 2−ΔΔCt method. Each sample was analyzed thrice. The primers were used as follows: PPARγ forward: 5ʹ CGC CAA GGT GCT CCA GAA GATG 3ʹ, reverse: 5ʹ GGT GAA GGC TCA TGT CTG TCT CTG 3ʹ; β-actin forward: 5ʹ GTG CTA TGT TGC TCT AGA CTT CG 3ʹ, reverse: 5ʹ ATG CCA CAG GAT TCC ATA CC 3ʹ.
Enzyme Linked Immunosorbent Assay (ELISA)
At the end of the 6-week treatment, the mice were anesthetized with diethyl ether, and blood was collected by aortic puncture and left undisturbed at room temperature to separate the serum. IL-10 and IFN-γ levels in the spleen homogenates and insulin levels in the sera were determined using specific ELISA kits (CUSABIO, China) according to manufacturer’s instructions. Optical density at 450 nm was measured using a microplate reader (Thermo, United States).
Immunohistochemistry (IHC)
Spleen tissues sections were processed for IHC as per standard protocols,24 and incubated overnight with anti-CD4 antibody (1:500; ab183685; Abcam; United Kingdom) or anti-CD8 antibody (1:400; ab203035; Abcam; United Kingdom) at 4°C. Following incubation with HRP-conjugated secondary antibodies (1:200; G23303; Servicebio; China) for 1h at room temperature, the sections were stained with diaminobenzidine (DAB) and counterstained with hematoxylin. The staining intensity was assessed in terms of integrated optical density (IOD).
Statistical Analysis
Data were presented as the mean ± SEM, and compared between groups using one-way ANOVA followed by two-tailed Student’s t-test as appropriate. Real-time quantitative PCR results were analyzed by Wilcoxon matched-pairs signed rank test. P<0.05 was considered statistically significant.
Results
ATMP Restored Body Weight, Food Intake and Water Intake of Diabetic Mice
Compared to the normal controls, the diabetic mice presented the typical symptoms of T1DM, such as decreased body weight, and increased food and water intake. Six weeks of metformin and ATMP treatment significantly increased the body weight of the diabetic mice in a dose-dependent manner (P<0.05), with 140mg/kg ATMP (high dose) showing similar ameliorative effects as 185mg/kg metformin (Table 1). As shown in Figure 1, the intake of food and water was significantly higher in the STZ-induced diabetic mice compared to that of the normal group before the treatment regimen (P<0.05), and was restored to normal levels by both metformin and high dose ATMP. Taken together, ATMP can normalize the food and water intake in diabetic mice.
 |
Table 1 Effect of ATMP on Body Weight in All Groups |
ATMP Restored Fasting Blood-Glucose and Serum Insulin (INS) Levels
Since T1DM is characterized by abnormal blood glucose and insulin levels, we next estimated these indices in the different treatment groups. As shown in Figure 2A, the fasting blood glucose levels in the diabetic mice were significantly higher compared to that in normal mice, and were decreased by metformin and ATMP in a dose-dependent manner (P<0.05). However, even 140mg/kg ATMP showed lower hypoglycemic effect than 285mg/kg·d−1 metformin. In addition, the low insulin levels in the diabetic mice were restored by both metformin and ATMP, with stronger effect of 140mg/kg ATMP compared to metformin (Figure 2B). Taken together, ATMP can effectively regulate the fasting blood-glucose levels and improve serum insulin in diabetes.
ATMP Improved Glucose Tolerance in the Diabetic Mice
The effect of ATMP on glucose metabolism was analyzed during the last week of treatment by OGTT. As shown in Figure 3A, the blood glucose level increased significantly in the diabetic mice within 30 min of the oral glucose challenge (P<0.05), and the hyperglycemic state persisted in the absence of any treatment. In contrast, the metformin and ATMP-treated mice showed a decrease in blood glucose levels after 60 min, which dropped significantly after 120 min of the glucose challenge (P<0.05). Further analysis of the area under the glucose-responding curve (AUC) over 120 min showed disrupted glucose tolerance in the STZ-induced diabetic mice, which was improved by metformin and ATMP (Figure 3B).
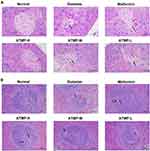
ATMP Alleviated Diabetes-Induced Tissue and Cellular Damage in the Pancreas and Spleen
To determine the cellular basis of the anti-diabetic effects of ATMP, the pancreas and spleen tissues of different groups were examined histologically. As shown in Figure 4A, the untreated normal mice had intact pancreatic islets with abundant and regularly-arranged β-cells, while STZ-induced diabetes resulted in the infiltration of inflammatory cells, severe degeneration of the islet cells and cellular swelling in the internal islets. ATMP treatment significantly alleviated pancreatic injury, indicated by decreased infiltration and more organized tissue structure in the islets of Langerhans, in a dose-dependent manner, and the structure of pancreatic islets was restored to a greater extent in the high dose group.
The spleens of the diabetic mice showed a reduction in white pulp volume and expansion in the range of germinal centers, and activation of red pulp tissue compared to the normal group (Figure 4B). In the spleen tissues of the diabetes group, lymphoid follicles were irregularly shaped, and germinal centers were visible. In addition, some cells in the germinal centers were necrotic and apoptotic, with pyknotic or fragmented nuclei. Treatment with ATMP partly reversed these alterations in the white pulp and red pulp, and reduced cell death and apoptosis. In addition, the splenocytes of the normal group were tightly and uniformly arranged, and showed clear nucleoli and small stroma space. The splenocytes of the ATMP-H group was the closest to that of healthy mice. Taken together, ATMP can significantly mitigate the diabetes-induced tissue damage in the pancreas and spleen.
ATMP Upregulated the Expression of PPARγ of Diabetic Mice
The immunoregulatory transcription factor PPARγ was significantly downregulated in the diabetic animals, and partly restored by medium- and high-dose ATMP (P<0.05), whereas the lower dose of ATMP and metformin had no significant effects (Figure 5).
ATMP Regulated IFN-γ and IL-10 Levels to Restore IFN-γ/IL-10 Ratio
As shown in Figure 6, STZ-induced diabetes was accompanied by a significant decrease in IL-10 and increase in IFN-γ levels (P<0.05), which were reversed by ATMP, resulting in a lower IFN-γ/IL-10 ratio. However, metformin showed no significant effects on the cytokine levels.
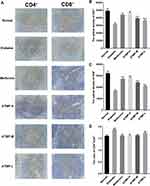
ATMP Improved CD4+ and CD8+ Expression in Spleen
To further elucidate the immunoregulatory effects of ATMP, we analyzed the proportion of CD4+ and CD8+ cells in the spleens. As shown in Figure 7, the diabetic mice showed lower CD4+ and CD8+ expression compared to the normal mice, and ATMP treatment markedly increased both populations (P<0.05). However, the ratio of CD4+/CD8+ cells was decreased in the metformin and ATMP-treated mice compared to the untreated diabetic mice. Taken together, ATMP can restore immune function in the STZ-induced diabetic mice.
Discussion
STZ-induced diabetes in rodents simulates the immunological and clinical signs of T1DM in humans,25 and is a suitable model for studying the molecular and immunological mechanisms underlying autoimmune insulitis.26 Accordingly, we established the STZ-induced T1DM model in C57BL/6 mice to investigate the immunotherapeutic action of ATMP.
Polysaccharides are macromolecular compounds formed by more than 10 monosaccharide molecules linked by glycosidic bonds, and exhibit anti-tumor, anti-viral, immunoregulatory, hypoglycemic and hypolipidemic effects. The drugs currently used to treat diabetes include metformin, acarbose etc., which are associated with multiple side effects and toxicity.4,5 In contrast, several plant-derived polysaccharides can reduce blood glucose levels by regulating glucose metabolism, oxidative stress, inflammatory response and the intestinal flora, with little to no toxicity.8–12 The composition and structure of polysaccharides are closely related to their functions. Glucose-rich polysaccharides are usually more bioactive due to higher heteroglucan content.27 The proportion of glucans and galactose in ATMP is 72%, which correlated to a potent anti-diabetic effect on the STZ-induced model, as characterized by increased body weight, decreased food and water intake, and improved insulin secretion and glycemic control. T1DM is an autoimmune disorder caused by the loss of pancreatic islet β-cells due to autoreactive CD4+T-cells that target β cell-specific antigens, which eventually disrupts blood glucose control.28–30 Polysaccharides decelerate β-cell death by suppressing apoptosis and mitigating pancreatic inflammation, which in turn alleviates the symptoms of T1DM. Histopathological examination showed that ATMP decreased tissue damage in the pancreas and spleen of the diabetic mice, thereby preserving pancreas function and blocking T1DM development.
Peroxisome proliferator-activated receptor γ (PPARγ) is a ligand-activated nuclear receptor that regulates glycolipid metabolism, and is a potential therapeutic target against diabetes.31,32 It is expressed in various immune cell types, especially the T and B cells. Studies show that PPARγ can alter the CD4+/CD8+ ratio and Treg cells numbers to regulate T cell responses.33,34 Consistent with this, the diabetic mice were deficient in PPARγ, which corresponded to a higher CD4+/CD8+ ratio in the spleens. In addition, PPARγ also decreases the levels of Th1 cytokines and increases that of Th2 cytokines, which results in a Th1/Th2 imbalance that is known to drive autoimmune diseases including T1DM.35,36 Reversing this Th1/Th2 imbalance can potential prevent and even reverse T1DM.37 The diabetic mice also had increased circulating levels of IFN-γ and lower IL-10, indicating an immune imbalance. Consistent with previous reports,38–40 ATMP not only restored the CD4+/CD8+ ratio by up-regulating PPARγ, but also decreased the Th1/Th2 cytokine ratio.
Taken together, ATMP alleviates the immune imbalance in the diabetic and reverses β-cell destruction by up-regulating PPARγ, indicating its therapeutic potential in T1DM.
Conclusions
This study demonstrates the therapeutic effect of ATMP against T1DM for the first time. ATMP relieved the symptoms of STZ-induced T1DM in mice, and attenuated hyperglycemia by restoring the CD4+/CD8+ T cells and Th1/Th2 balance via PPARγ upregulation in the spleen. ATMP is a promising drug for treating T1DM and warrants clinical investigation.
Abbreviations
ATMP, Acanthopanax trifoliatus (L.) Merr. polysaccharide; T1DM, type 1 diabetes mellitus; HPLC, high performance liquid chromatography; HPGPC, high performance gel permeation chromatography; OGTT, oral glucose tolerance test; PPARγ, peroxisome proliferator-activated receptor gamma; H&E, hematoxylin and eosin; STZ, streptozotocin; IL-10, Interleukin-10; IFN-γ, Interferon-γ; INS, insulin; ELISA, enzyme-linked immunosorbent assay; TBST, tris-buffer saline with Tween-20; IHC, immunohistochemistry; DAB, diaminobenzidine; IOD, integrated optical density; PVDF, polyvinylidene fluoride; Normal, normal group; Diabetes, untreated diabetic group; Metformin, diabetic group treated with metformin; ATMP-H, diabetic group treated with high dose ATMP; ATMP-M, diabetic group treated with medium dose ATMP; ATMP-L, diabetic group treated with low dose ATMP.
Acknowledgments
This study was supported by grants from the Natural Science Foundation of Guangdong Province (2017A030313623), Science and Technology Planning Project of Guangdong Province, China (2016ZC0172), the projects of Guangzhou key laboratory of construction and application of new drug screening model systems (No.201805010006) and Key Laboratory of New Drug Discovery and Evaluation of ordinary universities of Guangdong province (No. 2017KSYS002).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Steck AK, Winter WE. Review on monogenic diabetes. Curr Opin Endocrinol Diabetes Obes. 2011;18(4):252–258. doi:10.1097/MED.0b013e3283488275
2. International Diabetes Federation. IDF Diabetes Atlas.
3. Chen X, Tang J, Xie W, et al. Protective effect of the polysaccharide from ophiopogon japonicus on streptozotocin-induced diabetic rats. Carbohydr Polym. 2013;94(1):378–385. doi:10.1016/j.carbpol.2013.01.037
4. Sæther T, Paulsen SM, Tungen JE, et al. Synthesis and biological evaluations of marine oxohexadecenoic acids: pPARα/γ dual agonism and anti-diabetic target gene effects. Eur J Med Chem. 2018;155:736–753. doi:10.1016/j.ejmech.2018.06.034
5. Samed N, Sharma V, Sundaramurthy A. Hydrogen bonded niosomes for encapsulation and release of hydrophilic and hydrophobic anti-diabetic drugs: an efficient system for oral anti-diabetic formulation. Appl Surf Sci. 2018;449:567–573. doi:10.1016/j.apsusc.2017.11.055
6. Wang D, Zhao Y, Jiao Y, Yu L, Yang S, Yang X. Antioxidative and hepatoprotective effects of the polysaccharides from Zizyphus jujube cv. Shaanbeitanzao. Carbohydr Polym. 2012;88(4):1453–1459. doi:10.1016/j.carbpol.2012.02.046
7. Wang D, Zhao Y, Sun Y, Yang X. Protective effects of Ziyang tea polysaccharides on CCl4-induced oxidative liver damage in mice. Food Chem. 2014;143:371–378. doi:10.1016/j.foodchem.2013.08.005
8. Fu QY, Li QS, Lin XM, et al. Antidiabetic effects of tea. Molecules. 2017;22(5):849. doi:10.3390/molecules22050849
9. Liao CH, Lin JY. Lotus (Nelumbo nucifera Gaertn) plumule polysaccharide ameliorates pancreatic islets loss and serum lipid profiles in non-obese diabetic mice. Food Chem Toxicol. 2013;58:416–422. doi:10.1016/j.fct.2013.05.018
10. Zhao L, Wang K, Wang K, Zhu J, Hu ZY. Nutrient components, health benefits, and safety of litchi (Litchi chinensis Sonn.): a review. Compr Rev Food Sci Food Saf. 2020;19(4):2139–2163. doi:10.1111/1541-4337.12590
11. Jia RB, Li ZR, Wu J, et al. Antidiabetic effects and underlying mechanisms of anti-digestive dietary polysaccharides from Sargassum fusiforme in rats. Food Funct. 2020;11(8):7023–7036. doi:10.1039/d0fo01166e
12. Bhateja PK, Kajal A, Singh R. Amelioration of Diabetes mellitus by modulation of GLP-1 via targeting alpha-glucosidase using Acacia tortilis polysaccharide in Streptozotocin-Nicotinamide induced diabetes in rats. J Ayurveda Integr Med. 2020;11(4):405–413. doi:10.1016/j.jaim.2019.06.003
13. eFloras. Flora of China, Missouri Botanical Garde. MA: St. Louis, MO & Harvard University Herbaria; 2014.
14. Huang KC, Michael Williams W. The Pharmacology of Chinese Herbs. Vol. 874. New York: CRC Press LLC; 1999.
15. Bucci LR. Selected herbals and human exercise performance. Am J Clin Nutr. 2000;72(2):624S–636S. doi:10.1093/ajcn/72.2.624S
16. Schultes RE. Medicinal plants of east and southeast Asia. MIT Press, Cambridge. J Ethnopharmacol. 1981;4(1):124. doi:10.1016/0378-8741(81)90028-3
17. Li D-L, Zheng X, Chen Y-C, et al. Terpenoid composition and the anticancer activity of Acanthopanax trifoliatus. Arch Pharm Res. 2016;39(1):51–58. doi:10.1007/s12272-015-0655-y
18. Wang HQ, Li DL, Lu YJ, et al. Anticancer activity of Acanthopanax trifoliatus (L) Merr extracts is associated with inhibition of NF-kB activity and decreased Erk1/2 and Akt phosphorylation. Asian Pac J Cancer Prev. 2014;15(21):9341–9346. doi:10.7314/apjcp.2014.15.21.9341
19. Hamid R, Kee T, Othman F. Anti-inflammatory and anti-hyperalgesic activities of Acanthopanax trifoliatus (L) Merr leaves. Pharmacognosy Res. 2013;5(2):129–133. doi:10.4103/0974-8490.110544
20. Sithisarn P, Rojsanga P, Jarikasem S, Tanaka K, Matsumoto K. Ameliorative effects of acanthopanax trifoliatus on cognitive and emotional deficits in olfactory bulbectomized mice: an animal model of depression and cognitive deficits. Evid Based Complement Alternat Med. 2013;2013:701956. doi:10.1155/2013/701956
21. Lo J, Xia C-Q, Peng R, Clare-Salzler MJ. Immature dendritic cell therapy confers durable immune modulation in an antigen-dependent and antigen-independent manner in nonobese diabetic mice. J Immunol Res. 2018;2018:5463879. doi:10.1155/2018/5463879.
22. Kang H, Ahn K-S, Cho C, Bae H-S. Immunomodulatory effect of astragali radix extract on murine Th1/Th2 cell lineage development. Biol Pharm Bull. 2004;27(12):1946–1950. doi:10.1248/bpb.27.1946
23. Cheng XX, Zhang XH, Yang HW. Isolation, purification and anti-oxidant activity of polysaccharides from Acanthopanax trifoliatus. Chin Tradit Herb Drugs. 2017;48:4219–4223. doi:10.7501/j.issn.0253-2670.2017.20.015
24. Yaochite JNU, Caliari-Oliveira C, Davanso MR, et al. Dynamic changes of the Th17/Tc17 and regulatory T cell populations interfere in the experimental autoimmune diabetes pathogenesis. Immunobiology. 2013;218(3):338–352. doi:10.1016/j.imbio.2012.05.010
25. Müller A, Schott-Ohly P, Dohle C, Gleichmann H. Differential regulation of Th1-Type and Th2-type cytokine profiles in pancreatic islets of C57BL/6 and BALB/c mice by multiple low doses of streptozotocin. Immunobiology. 2002;205(1):35–50. doi:10.1078/0171-2985-00109
26. Mensah-Brown EPK, Stosic Grujicic S, Maksimovic D, Jasima A, Shahin A, Lukic ML. Downregulation of apoptosis in the target tissue prevents low-dose streptozotocin-induced autoimmune diabetes. Mol Immunol. 2002;38(12):941–946. doi:10.1016/S0161-5890(02)00021-4
27. Yang SW, Qu YH, Zhang H, et al. Hypoglycemic effects of polysaccharides from Gomphidiaceae rutilus fruiting bodies and their mechanisms. Food Funct. 2020;11(1):424–434. doi:10.1039/c9fo02283j
28. Nakayama M, Beilke JN, Jasinski JM, et al. Priming and effector dependence on insulin B:9-23 peptide in NOD islet autoimmunity. J Clin Invest. 2007;117(7):1835–1843. doi:10.1172/JCI31368
29. Roep BO, Arden SD, de Vries RRP, Hutton JC. T-cell clones from a type-1 diabetes patient respond to insulin secretory granule proteins. Nature. 1990;345(6276):632–634. doi:10.1038/345632a0
30. Yoon JW, Yoon CS, Lim HW, et al. Control of autoimmune diabetes in NOD Mice by GAD expression or suppression in cells. Science. 1999;284(5417):1183–1187. doi:10.1126/science.284.5417.1183
31. Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol Metab. 2014;25(6):293–302. doi:10.1016/j.tem.2014.04.001
32. Wang S, Dougherty EJ, Danner RL. PPARγ signaling and emerging opportunities for improved therapeutics. Pharmacol Res. 2016;111:76–85. doi:10.1016/j.phrs.2016.02.028
33. Malur A, McCoy AJ, Arce S, et al. Deletion of PPAR gamma in alveolar macrophages is associated with a Th-1 pulmonary inflammatory response. J Immunol. 2009;182(9):5816–5822. doi:10.4049/jimmunol.0803504
34. Wohlfert EA, Nichols FC, Nevius E, Clark RB. Peroxisome proliferator-activated receptor gamma (PPARgamma) and immunoregulation: enhancement of regulatory T cells through PPARgamma-dependent and -independent mechanisms. J Immunol. 2007;178(7):4129–4135. doi:10.4049/jimmunol.178.7.4129
35. Li R-J, Qiu S-D, Chen H-X, Tian H, Wang H-X. The immunotherapeutic effects of Astragalus polysaccharide in type 1 diabetic mice. Biol Pharm Bull. 2007;30(3):470–476. doi:10.1248/bpb.30.470
36. Saubermann LJ, Nakajima A, Wada K, et al. Peroxisome proliferator-activated receptor gamma agonist ligands stimulate a Th2 cytokine response and prevent acute colitis. Inflamm Bowel Dis. 2002;8(5):330–339. doi:10.1097/00054725-200209000-00004
37. Lenschow DJ, Herold KC, Rhee L, et al. CD28/B7 regulation of Th1 and Th2 subsets in the development of autoimmune diabetes. Immunity. 1996;5(3):285–293. doi:10.1016/S1074-7613(00)80323-4
38. Welch JS, Ricote M, Akiyama TE, Gonzalez FJ, Glass CK. PPARγ and PPARδ negatively regulate specific subsets of lipopolysaccharide and IFN-γ target genes in macrophages. Proc Natl Acad Sci U S A. 2003;100(11):6712. doi:10.1073/pnas.1031789100
39. Cunard R, Ricote M, DiCampli D, et al. Regulation of cytokine expression by ligands of peroxisome proliferator activated receptors. J Immunol. 2002;168(6):2795. doi:10.4049/jimmunol.168.6.2795
40. Gor DO, Rose NR, Greenspan NS. TH1-TH2: a Procrustean paradigm. Nat Immunol. 2003;4(6):503–505. doi:10.1038/ni0603-503
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.