Back to Journals » Nature and Science of Sleep » Volume 13
Immunohistological Localization of Mel1a Melatonin Receptor in Pigeon Retina
Authors Sheng W, Weng S, Li F, Zhang Y, He Q, Sheng W, Fu Y, Yan H, Liu K
Received 6 November 2020
Accepted for publication 18 December 2020
Published 5 February 2021 Volume 2021:13 Pages 113—121
DOI https://doi.org/10.2147/NSS.S290757
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Steven A Shea
Wenlong Sheng,1 Shijun Weng,2 Fei Li,1 Yun Zhang,1 Qiuxia He,1 Wenxiang Sheng,1 Ying Fu,3 Haiyue Yan,4 Kechun Liu1
1Biology Institute, Qilu University of Technology (Shandong Academy of Sciences), Jinan, People’s Republic of China; 2State Key Laboratory of Medical Neurobiology, Institutes of Brain Science, Department of Ophthalmology, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China; 3Shandong Science and Technology Exchange Center, Jinan, People’s Republic of China; 4Shandong Institute of Scientific and Technical Information, Jinan, People’s Republic of China
Correspondence: Wenlong Sheng; Kechun Liu Email [email protected]; [email protected]
Background: Melatonin (N-acetyl-5-methoxytryptamine), a significant indoleamine neuromodulator implicated in circadian rhythms and sleep patterns, regulates diverse rhythmic functions via activating its high-affinity G-protein-coupled receptors. However, the detailed cellular expression of the Mel1a receptor in the retina is still a research gap.
Methods: The expression of the Mel1a receptor in pigeon retina was assessed using Western blot analysis and immunofluorescent staining. The cellular localization of the Mel1a receptor was studied using double immunofluorescent staining and laser-scanning confocal microscopy.
Results: Our data suggested that the Mel1a receptor was extensively expressed in the outer segment of Rho4D2-labeled rod and L/M-opsin-labeled red/green cone and in the somata of the CB-labeled horizontal cell, TH-labeled dopaminergic amacrine cell, ChAT-labeled cholinergic amacrine cell, PV-labeled AII amacrine cell, Brn3a-labeled conventional ganglion cell, melanopsin-containing ganglion cell and CRALBP-labeled Müller glial cell. In addition, the Mel1a receptor was diffusely distributed throughout the full thickness of the inner plexiform layer. However, the outer segment of S-opsin-labeled blue cone, the somata of ChX-10-labeled bipolar cell and outer plexiform layer seemed to lack immunoreactivity of the Mel1a receptor.
Conclusion: The finding that multiple types of retinal cells express the Mel1a receptor provides a new neurobiological basis for the participation of melatonin in the regulation of retinal functions through activating the Mel1a receptor.
Keywords: circadian rhythm, retinal Mel1a receptor, cellular localization, diurnal animal
Introduction
Neuromodulator melatonin, serving as a neurohormonal message of the photoperiod, is implicated in numerous biological activities, and in non-mammalian vertebrates it owns high-affinity G-protein-coupled receptors Mel1a (named MT1 in mammalian), Mel1b (named MT2 in mammalian), and Mel1c. Melatonin is produced and secreted in a daily rhythmic manner with the maximal concentration always occurring during the dark phase,1 and modulates numerous retinal processes, such as membrane conductance,2 resting potentials,3 multiple types of membrane channels4 and synaptic transmission.4 These actions of melatonin or melatonin receptors indicate the melatonin system is indeed implicated in many retinal functions. Functionally, retinal melatonin is involved in the modulation of various physiological responses, including rod disc shedding, dopamine release, visual sensitivity,5 retinal rhythmicity,6 and sleep.1
Previous studies show that the localization of melatonin receptors appears enormously species-dependent and/or cell subtype-dependent. This might help to explain why the biological rhythm is abundant and various in diurnal animals and nocturnal animals. A number of studies related to the expression of retinal melatonin receptors have been carried out in nocturnal animals, such as the mouse and rat.6–9 However, the localization of the melatonin receptor in diurnal animals is less investigated. In humans, melatonin exerts its neuromodulation via its specific receptors.10,11 In a description of morphology on mRNA transcription these receptors are widely located in chick retina.12 In fact, they do not fully identify the type of neuron or glial cell which expresses Mel1a, Mel1b, and Mel1c receptors in the retina. Our recent work shows that multiple types of retinal cell, including Rho4D2-labeled rod, L/M-opsin-labeled red/green cone, S-opsin-labeled blue cone, TH-labeled dopaminergic amacrine cell (AC), ChAT-labeled cholinergic AC, PV-labeled glycinergic AII AC, Brn3a-labeled conventional ganglion cell (GC), melanopsin-containing GC express Mel1b receptor in pigeon retina, while CB-labeled horizontal cell (HC), ChX-10-labeled bipolar cell (BC), and CRALBP-labeled Müller glial cell seem to lack Mel1b receptor immunoreactivity.8 However, the detailed cellular expression of the Mel1a receptor in the retina is still a research gap. Here, we investigate the cellular localization of melatonin receptor Mel1a in pigeon retina. Our findings indicate that the Mel1a receptor is widely and selectively expressed in retinal neurons and glial cells as well as inner plexiform layer (IPL). These results suggest melatonin in the retina may directly modulate the diversified activities of retinal neurons and glial cells by modulating melatonin receptor Mel1a.
Materials and Methods
Animals and Ethics Statement
A total of nine adult pigeons (Columba livia domestica) were recruited in the present study. Adult pigeons were housed for at least 3 weeks in a 14 hour light (~1,200 lux): 10 hour dark (LD) cycle before sacrifice. Additionally, to reduce possible diurnal factors on the level of the Mel1a receptor expression, all tissues were collected at zeitgeber time 6–8 (ZT6-8). Use and handling of animals were strictly in accordance with the Institutional Animal Care and Use Committees of Qilu University of Technology whose guidelines were based on the US National Institutes of Health (NIH). Ethical approval was granted by the Institutional Animal Care and Use Committees of Qilu University of Technology.
Tissue Preparation
Experimental birds were euthanized with urethane overdose and enucleated. Eyecups were rapidly isolated in Hank’s balanced salt solution and transferred into freshly prepared 4% PFA in ice-cold 0.01 M PBS (pH 7.5) for 0.5 hour. Then tissues were emerged in 30% sucrose in PBS in a refrigerator for dehydration, and eyecups were embedded in embedding agent. A CM1950 cryostat was selected to cut 20 μm slices, and slices were finally fixed to microscope slides.
Antibodies
A new but widely-used polyclonal antibody6,7 designed against a peptide corresponding to a region of the 3rd intracellular loop of the mouse MT1 receptor (residues 223–236 (C) RVKPDNKPKLKPQD) was generated in rabbits (AMR-031, 1:200 dilution, Alomone laboratories, Jerusalem, Israel) for immunohistochemical staining of the Mel1a receptor. For double labeling, the sections were incubated sequentially in a mixture of two kinds of primary or secondary antibody mentioned in our previous study.8 Mouse anti-Rho4D2 antibody (1:1,500 dilution, ab98887, Abcam plc., UK), goat anti-S-opsin antibody (1:500 dilution, sc-14363, Santa Cruz Bio-technology, USA) and goat anti-L/M-opsin antibody (1:800 dilution, sc-22117, Santa Cruz Bio-technology, USA) were separately used for labeling rods, blue cones, and red/green cones. Mouse anti-calbindin D-28k (CB) monoclonal antibody (1:2,000 dilution, 300, Swant, Switzerland) and sheep anti-homeobox protein ChX10 polyclonal antibody (1:400 dilution, ab16141, Abcam plc., UK) were respectively used for labeling HCs and BCs. Antibodies used to mark different subtypes of ACs were as follows: goat anti-choline acetyltransferase (ChAT) polyclonal antibody (1:800 dilution, AB144P, Chemicon, USA) for cholinergic ACs, mouse anti-tyrosine hydroxylase (TH) monoclonal antibody (1:5,000 dilution, T1299, Sigma, USA) for dopaminergic ACs, and mouse anti-parvalbumin (PV) monoclonal antibody (1:1,000 dilution, 235, Swant, Switzerland) for glycinergic AII ACs. Goat anti-Brn3a polyclonal antibody (1:800 dilution, sc-31984, Santa Cruz Bio-technology, USA) was used for labeling GCs and mouse anti-melanopsin monoclonal antibody (1:200 dilution, sc-515838, Santa Cruz Bio-technology, USA) was used for labeling ipRGCs. Mouse anti-cellular retinaldehyde-binding protein (CRALBP) monoclonal antibody (1:1,000 dilution, ab15051, Abcam plc., UK) was used for labeling Müller cells.
Secondary antibodies used in this work were as follows: Alexa Fluor 488-linked donkey anti-rabbit IgG for labeling Mel1a receptor; Alexa Fluor 555-linked donkey anti-mouse IgG for Rho4D2, CB, TH, PV, melanopsin, and CRALBP; Alexa Fluor 555-linked donkey anti-goat IgG for S-opsin, L/M-opsin, ChAT, and Brn3a; Alexa Fluor 555-linked donkey anti-sheep IgG for ChX10 (all 1:200 dilution, Invitrogen, USA). Horseradish peroxidase-conjugated donkey anti-rabbit IgG (1:2,000, Santa Cruz Bio-technology, USA) was used for labeling Mel1a receptor in Western blot.
Immunohistochemistry on Retinal Slices
Experimental procedures were performed according to the method described previously8 with minor modifications. Briefly, tissues were pretreated in blocking solution for 2 hours to block non-specific bindings, and then treated with the Mel1a receptor antibody in an incubating medium at 4°C for 72 hours. Then the tissues were treated with donkey anti-rabbit IgG antibody for 2 hours. In control trials, the total operation was coincident except that the tissues were treated with a solution containing the primary antibody and the relevant antigen peptide. All fluorescent labeling trials were conducted on more than four pigeons.
Confocal Laser Scanning Microscopy and Data Analysis
A Fluoview FV1000 confocal laser scanning biological microscope (Olympus Corporation, Tokyo, Japan) was used to view the sections and acquire the images at 40× magnification. Images were resized and adjusted for global brightness and contrast using the software Photoshop CS3 (Adobe Systems, San Jose, CA, USA).
Western Blot
The concentration of protein from the retinas was determined using a BCA Protein Assay (Thermo Scientific). Extracted supernatants (20 μg/lane) were electrophoresed on 10% sodium dodecyl sulfate-polyacrylamide gel and electro-blotted onto nitrocellulose (NC) membranes. The membranes were then treated with block solution for 2 hours and dealt with the diluted Mel1a receptor antibody for 2 hours at room temperature and 12 hours at 4°C, and then followed by being incubated in HRP-conjugated secondary antibody (1:4,000 dilution, Millipore Corp., Billerica, MA, USA) for 2 hours. Specific binding was developed via Western blotting substrate and digital images were taken using ChemiDoc XRS System with Image Lab software (Bio-Rad, Hercules, CA, USA). In load control trials, the NC membranes were treated with the solution containing anti-Mel1a receptor antibody and the relevant immunizing peptide.
Results
Profile of Mel1a Receptor in Pigeon Retina
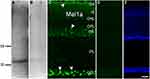
In the retinal supernatants from the birds, the Mel1a receptor antibody revealed a predominant band about 40 kDa (Figure 1A), which was consistent with the early report.13 No obvious band was detected if the antibody was pre-treated with the relevant immunizing peptide (Figure 1B). These results suggested the protein identified by our antibody might well be a Mel1a receptor.
In the retinal section (Figure 1C) Mel1a receptor immunoreactivity was detectable in the outer photoreceptor layer, corresponding to the outer segment (OS) of photoreceptors. Mel1a receptor-immunoreactivity clearly existed in many cells of the inner nuclear layer (INL) and they could be inhibitory neurons (such as HCs and ACs), excitability interneurons (such as BCs), output neurons (such as displaced GCs), and/or glia cells (such as Müller cells). Moreover, signals for the Mel1a receptor were strong in the ganglion cell layer (GCL), which might be displaced ACs (like cholinergic ACs) and/or GCs. In addition, very dispersive labeling for the Mel1a receptor was diffusely distributed throughout the full thickness of the IPL, while no clearly Mel1a receptor signal was visible in the outer plexiform layer (OPL). Note that the staining of the Mel1a receptor could be completely eliminated with the corresponding immunogen peptide (Figure 1D). Figure 1E describes the retinal layers definitely.
Expression of Mel1a Receptor in Outer Photoreceptors
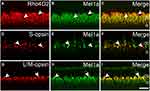
To study the expression of melatonin receptor Mel1a in the outer photoreceptors, we performed double labeling studies. Figure 2A–C shows localization of the Mel1a receptor in rods. Double immunolabeling revealed Rho4D2-labeled OS of the rod observed in seven vertical sections were strongly labeled by the Mel1a receptor antibody with a 100% positive rate (356 out of 356 cells). Figure 2D–F shows localization of the Mel1a receptor in blue cones. Double immunolabeling revealed that the Mel1a receptor immunoreactivity was undetectable on the S-opsin-labeled OS of the blue cone (133 out of 133 cells from six sections, 0% positive rate). Figure 2G–I shows localization of the Mel1a receptor in red/green cones. Double immunolabeling shows the Mel1a receptor signal was strong on the OS of L/M-opsin-labeled red/green cone (284 out of 286 cells from six sections, 99% positive rate), but not on the inner segment (IS).
Expression of Mel1a Receptor in the HCs and BCs
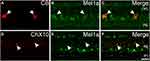
We made more investigations to explore the localization of Mel1a receptor in HCs and BCs. Figure 3A–C shows the localization of the Mel1a receptor in HCs. CB-immunolabeled HCs characterized by large somata were situated in the distal edge of the INL. Double immunolabeling indicated that the CB-labeled HCs were clearly Mel1a receptor positive. A total of 25 CB-labeled neurons in 15 retinal slices were identified in the outer INL, and they were co-labeled by Mel1a receptor (25 out of 25 cells, 100% positive rate). It was noteworthy that the processes of these cells were negative for Mel1a receptor. Figure 3D–F shows the localization of the Mel1a receptor in BCs. Double immunolabeling displayed that the somata of the BCs were not labeled by Mel1a receptor (Figure 3F). Our results demonstrated 33 ChX10-positive neurons in four retinal slices appeared to be Mel1a receptor negative (0 out of 33 cells, 0% positive rate).
Expression of Mel1a Receptor in the ACs
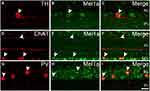
To explore the expression of the Mel1a receptor in ACs, we performed fluorescent labeling studies using the Mel1a receptor antibody together with specific and reliable markers for different types of ACs. Dopaminergic neurons solely existed in the innermost layer of the INL, while their dendrites were mostly located in the outermost layer of the IPL. Fluorescent immunolabeling suggested that TH-labeled dopaminergic neurons were found to be Mel1a receptor immunoreactivity (Figure 4A–C). Thirty-two cells with obvious TH immunostaining were observed in 25 retinal slices, and they were mostly Mel1a receptor-positive (31 out of 32 cells, 97% positive rate). Simultaneously, the processes of the TH-positive cells were not clearly labeled by Mel1a receptor. The cholinergic neurons were located in both the GCL and INL and mirror-symmetrically arranged, while their dendrites constituted two obvious braids in the IPL. Double labeling in Figure 4D–F showed ChAT-immunoreactive cholinergic ACs were also Mel1a receptor immunoreactive, while no clearly Mel1a receptor immunoreactivity was found in the two braids. In a quantitative analysis involving 119 ChAT-positive neurons collected from 14 retinal slices, none of these ChAT-positive ACs were not Mel1a immunoreactive. PV-positive AII neuron, characterized by these processes ended at distinct layers of the IPL was the mainly well-known subpopulation of glycinergic neuron. Double-labeling studies unambiguously indicated that Mel1a receptor obviously existed in AII ACs, while their dendrites were not marked by Mel1a receptor prominently (Figure 4G–I). Our results demonstrated that all the PV-immunolabeled AII neurons observed in this study were co-labeled by Mel1a receptor (38 out of 38 cells from four retinal slices with the positive rate being 100%).
Expression of Mel1a Receptor in the GCs
The Mel1a receptor-immunoreactive neurons situated in the innermost cell layer might be ACs and/or GCs. More experiments were conducted to investigate whether the Mel1a receptor existed in GCs (Figure 5). Our results demonstrated that 432 GCs with strong immunofluorescence signal for Brn3a were observed in 16 retinal slices, and all of them were Mel1a-positive (100% positive rate). The ipRGC was a kind of Brn3a-negative and melanopsin-positive GC. Immunofluorescence labeling experiment in 14 retinal slices indicated the Mel1a receptor was situated in melanopsin-containing ipRGC (13 out of 13 cells with a 100% positive rate, Figure 5D–F).
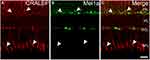
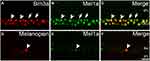 |
Figure 5 Mel1a receptor immunoreactivity in retinal GCs. (A–C) Representative data from retinal slice double-labeled by Brn3a and Mel1a receptor. Clear Mel1a receptor immunostaining is observed in all Brn3a-positive GCs. Some Mel1a receptor-positive cells (locations marked by the arrows) in the GCL are not immunoreactive for Brn3a, and they may be displaced ACs (cholinergic neurons in Figure 4D were typical examples) or Brn3a-negative GCs (such as inner photoreceptor ipRGC). (D–F) Representative data of a retinal slice double-labeled by melanopsin and Mel1a receptor. Note that Mel1a receptor immunostaining is observed in soma of melanopsin-containing photoreceptor. Representative neurons are marked by white arrowheads and arrows. |
Expression of Mel1a Receptor in the Müller Cells
The retinal glia Müller cells, the main gliocyte, can be labeled by CRALBP. Double-immunolabeling experiment (Figure 6A–C) was performed for assessing the expression of Mel1a receptor in CRALBP-labeled Müller cell. Intense staining for the Mel1a receptor was detected in the somata of Müller cells (100 out of 101 cells from 15 retinal slices, with a 99% positive rate), while no obvious Mel1a receptor-immunoreactivity was visible in the inner and outer stem processes.
Discussion
Melatonin, eliciting its biological functions with Mel1a and Mel1b receptors (corresponding to mammalian MT1 and MT2 receptors, respectively), is a significant neuromodulator in circadian rhythm systems.1,14,15 Numerous research studies have mainly stayed at the retina of rat, mouse, and others, which belong to nocturnal animals. In the adult rat retina, the melatonin receptor MT1 immunocytochemically locates in the IPL, OPL, and ipRGCs,1,6,7 while MT1 receptor immunoreactivity is found in many mouse retinal neurons, including rod and cone photoreceptors and ipRGCs.9,16 Fujieda et al’s17 studies indicate the MT1 receptor is detectable in the GCs, ACs, IPL, and OPL in guinea pig retina. However, we’d better investigate the melatonin system both in the nocturnal animals and diurnal animals. In the human retina, the MT1 receptor transcript is immunoreactive in the INL, GCL, and in the IS of outer photoreceptors.11,18,19 Natesan and Cassone’s12 work shows us the Mel1a receptor mRNA is primarily located in the IS of photoreceptors, inner part of the INL, and GCL in the chick retina. However, the pinpoint cellular localization of the Mel1a receptor at the protein level in the retina of diurnal animals remains unclear.
High-resolution structures of two subtypes of melatonin receptor are demonstrated recently using XFEL analysis.20 The research on circadian rhythm-related melatonin receptors will be beneficial to the study on pathogenesis of rhythm-related discomfort or diseases and staying natural rhythms. So the studies on cellular localization of melatonin receptors should be placed on the agenda urgently. In the present work, we demonstrated the cellular localization of Mel1a receptor in pigeon retina by double immunofluorescence labeling. Mel1a receptor-positive signals were found in photoreceptors (OS of rods and red/green cones, somata of ipRGC), and somata of HCs, three subtypes of ACs (dopaminergic neurons, cholinergic neurons, glycinergic AII neurons) and GCs, while faint signals were observed in the IPL and no clearly Mel1a receptor signal was detected in the OS of blue cones, somata of BCs and OPL. At present, no research demonstrated retinal glia cells expressing melatonin receptor definitely, and our results have proved melatonin receptor Mel1a might be positive in Müller cells, especially in the somata. Regarding the issue of cytoplasmic staining of Mel1a receptor, we reported similar cases before,7,8 and the staining pattern appearing to localize to the cytoplasm, rather than to the membrane, is similar to those previous studies.21,22
Interestingly, the Mel1a receptor is expressed in red/green cone and rod photoreceptors, but not in blue cones, which suggests melatonin may prefer to modulate the red/green cone pathway and rod pathway via activating the Mel1a receptor. More, the expression of melatonin receptor in inhibitory neurons HCs and ACs, but not BCs, suggests a role of melatonin in the inhibitory neuromodulation pathway rather than in the excitatory neuromodulation pathway. According to our studies, the Mel1a receptor and Mel1b receptor are co-expressed in rods, red/green cones, dopaminergic neurons, cholinergic neurons, glycinergic AII neurons, conventional ganglion cells, and intrinsically photosensitive retinal ganglion cells, while the two receptors seem to be negative in bipolar cells in pigeon retina. Localizational heterogeneity of the Mel1a receptor and Mel1b receptor is mainly behaved in blue cones, horizontal cells, and Müller cells. Specifically, the Mel1a receptor prefers to be expressed in horizontal cells and Müller cells, when the Mel1b receptor favors being localized in blue cones.
Corresponding to other diurnal animals, such as mammalian human and monkey, the MT1 receptor is expressed in photoreceptors, HCs, ACs, and GCs. That indicates no matter in mammalian or non-mammalian animals MT1 or Mel1a receptor owns same or similar cellular localization in diurnal animals.11,18,19,23 However, it is noteworthy that MT1 or Mel1a receptors may be predominantly expressed in rods, cones, ACs, and GCs in the diurnal animals,11,12,18,19,23,24 while this receptor frequently appears in the IPL, OPL, and GCs in the nocturnal animals.6,7,11–13,17–19 The different characters on localization of MT1 or Mel1a receptor may indeed contribute to the divergent circadian rhythms. How melatonin modulates these pathways differentially and how such modulation is integrated to modify retinal rhythmicity, or even circadian rhythm in the central nervous system will be an issue of great interest.
Acknowledgments
We gratefully acknowledge financial support from the Youth Fund of Shandong Academy of Sciences (No. 2019QN001, 2019QN005), Natural Science Foundation of Shandong Province (No. ZR2018PC013), International Science and Technology Cooperation Program of Shandong Academy of Sciences (No. 2019GHZD10), and National Key R&D Program of China (No. 2018YFC1707300).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Huang H, Wang Z, Weng SJ, Sun XH, Yang XL. Neuromodulatory role of melatonin in retinal information processing. Prog Retin Eye Res. 2013;32:64–87. doi:10.1016/j.preteyeres.2012.07.003
2. Cosci B, Longoni B, Marchiafava PL. Melatonin induces membrane conductance changes in isolated retinal rod receptor cells. Life Sci. 1997;60:1885–1889. doi:10.1016/S0024-3205(97)00150-1
3. Fischer TW, Zmijewski MA, Wortsman J, Slominski A. Melatonin maintains mitochondrial membrane potential and attenuates activation of initiator (casp-9) and effector caspases (casp-3/casp-7) and PARP in UVR-exposed HaCaT keratinocytes. J Pineal Res. 2008;44:397–407. doi:10.1111/j.1600-079X.2007.00542.x
4. Yang XF, Miao Y, Ping Y, Wu HJ, Yang XL, Wang Z. Melatonin inhibits tetraethylammonium-sensitive potassium channels of rod ON type bipolar cells via MT2 receptors in rat retina. Neuroscience. 2011;173:19–29. doi:10.1016/j.neuroscience.2010.11.028
5. Tosini G, Baba K, Hwang CK, Iuvone PM. Melatonin: an underappreciated player in retinal physiology and pathophysiology. Exp Eye Res. 2012;103:82–89. doi:10.1016/j.exer.2012.08.009
6. Pack W, Hill DD, Wong KY. Melatonin modulates M4-type ganglion-cell photoreceptors. Neuroscience. 2015;303:178–188. doi:10.1016/j.neuroscience.2015.06.046
7. Sheng WL, Chen WY, Yang XL, Zhong YM, Weng SJ. Co-expression of two subtypes of melatonin receptor on rat M1-type intrinsically photosensitive retinal ganglion cells. PLoS One. 2015;10:e0117967. doi:10.1371/journal.pone.0117967
8. Sheng W, Jin M, Pan G, et al. Cellular localization of melatonin receptor Mel1b in pigeon retina. Neuropeptides. 2019;78:101974. doi:10.1016/j.npep.2019.101974
9. Sengupta A, Baba K, Mazzoni F, et al. Localization of melatonin receptor 1 in mouse retina and its role in the circadian regulation of the electroretinogram and dopamine levels. PLoS One. 2011;6:e24483. doi:10.1371/journal.pone.0024483
10. Ikegami T, Motohashi E, Doi H, Hattori A, Ando H. Synchronized diurnal and circadian expressions of four subtypes of melatonin receptor genes in the diencephalon of a puffer fish with lunar-related spawning cycles. Neurosci Lett. 2009;462:58–63. doi:10.1016/j.neulet.2009.06.076
11. Scher J, Wankiewicz E, Brown GM, Fujieda H. AII amacrine cells express the MT1 melatonin receptor in human and macaque retina. Exp Eye Res. 2003;77:375–382. doi:10.1016/S0014-4835(03)00123-4
12. Natesan AK, Cassone VM. Melatonin receptor mRNA localization and rhythmicity in the retina of the domestic chick, Gallus domesticus. Vis Neurosci. 2002;19:265–274. doi:10.1017/S0952523802192042
13. Fujieda H, Hamadanizadeh SA, Wankiewicz E, Pang SF, Brown GM. Expression of mt1 melatonin receptor in rat retina: evidence for multiple cell targets for melatonin. Neuroscience. 1999;93:793–799. doi:10.1016/S0306-4522(99)00111-6
14. Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27:101–110. doi:10.1385/ENDO:27:2:101
15. Pandi-Perumal SR, Trakht I, Srinivasan V, et al. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurobiol. 2008;85:335–353. doi:10.1016/j.pneurobio.2008.04.001
16. Baba K, Pozdeyev N, Mazzoni F, et al. Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor. Proc Natl Acad Sci U S A. 2009;106:15043–15048. doi:10.1073/pnas.0904400106
17. Fujieda H, Scher J, Hamadanizadeh SA, Wankiewicz E, Pang SF, Brown GM. Dopaminergic and GABAergic amacrine cells are direct targets of melatonin: immunocytochemical study of mt1 melatonin receptor in guinea pig retina. Vis Neurosci. 2000;17:63–70. doi:10.1017/S0952523800171068
18. Savaskan E, Wirz-Justice A, Olivieri G, et al. Distribution of melatonin MT1 receptor immunoreactivity in human retina. J Histochem Cytochem. 2002;50:519–526. doi:10.1177/002215540205000408
19. Scher J, Wankiewicz E, Brown GM, Fujieda H. MT(1) melatonin receptor in the human retina: expression and localization. Invest Ophthalmol Vis Sci. 2002;43:889–897.
20. Stauch B, Johansson LC, McCorvy JD, et al. Structural basis of ligand recognition at the human MT1 melatonin receptor. Nature. 2019;569:284–288. doi:10.1038/s41586-019-1141-3
21. Wu YH, Ursinus J, Zhou JN, et al. Alterations of melatonin receptors MT1 and MT2 in the hypothalamic suprachiasmatic nucleus during depression. J Affect Disord. 2013;148:357–367. doi:10.1016/j.jad.2012.12.025
22. Hunt AE, Al-Ghoul WM, Gillette MU, Dubocovich ML. Activation of MT(2) melatonin receptors in rat suprachiasmatic nucleus phase advances the circadian clock. Am J Physiol Cell Physiol. 2001;280:C110–C118. doi:10.1152/ajpcell.2001.280.1.C110
23. Meyer P, Pache M, Loeffler KU, et al. Melatonin MT-1-receptor immunoreactivity in the human eye. Br J Ophthalmol. 2002;86:1053–1057. doi:10.1136/bjo.86.9.1053
24. Wu YH, Zhou JN, Balesar R, et al. Distribution of MT1 melatonin receptor immunoreactivity in the human hypothalamus and pituitary gland: colocalization of MT1 with vasopressin, oxytocin, and corticotropin-releasing hormone. J Comp Neurol. 2006;499:897–910. doi:10.1002/cne.21152
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.