Back to Journals » Journal of Inflammation Research » Volume 16
Immune-Related Genes in the Pathogenesis of Atherosclerosis: Based on Sex Differences
Authors Zhang P, Lin H, Guo Y, Peng F, Meng L
Received 29 July 2023
Accepted for publication 12 October 2023
Published 18 October 2023 Volume 2023:16 Pages 4713—4724
DOI https://doi.org/10.2147/JIR.S429247
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Peng Zhang,1,* Hui Lin,1,* Yan Guo,2 Fang Peng,1 Liping Meng1
1Department of Cardiology, Shaoxing People’s Hospital, Shaoxing, Zhejiang, 312000, People’s Republic of China; 2Department of Cardiology, Zhuji hospital of Traditional Chinese Medicine, Shaoxing, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Liping Meng; Fang Peng, Department of Cardiology, Shaoxing People’s Hospital, No. 568 Zhongxing North Road, Shaoxing, 312000, Zhejiang, People’s Republic of China, Email [email protected]; [email protected]
Purpose: Atherosclerosis is still a global public problem with increasing incidence rate and mortality. It has been found that gender factors play an important role in the progression of atherosclerosis. However, few people explore gender related atherosclerosis at the level of genes and immune cells. The purpose of this study was to determine genetic and immune cell differences between male and female samples.
Patients and Methods: This study aims to identify differential genes between male and female samples in the GSE43292 dataset. The focus will be on identifying immune-related genes (IRGs) among these differentially expressed genes. Subsequently, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis will be employed to explore the enrichment of IRGs in biological processes, molecular functions, cellular components, and pathways. Furthermore, a protein-protein interaction (PPI) network for the IRGs will be constructed using Cytoscape software. To estimate the degree of immune cell infiltration, single-sample gene set enrichment analysis (ssGSEA) will be conducted. Moreover, the identified IRGs will be validated using GSE28829 dataset. Finally, we validated in atherosclerotic mice.
Results: Seven IRGs (CCL13, IL1RN, FPR2, S100A8, CCL19, CXCL1, CXCL8) were identified as being overexpressed in male atherosclerosis. GO and KEGG analysis revealed that these IRGs are primarily enriched in inflammatory response pathways, cytokine signaling pathways, and cytokine- cytokine receptor interactions. Notably, when compared to females, there was a significant infiltration of immune cells in male specimens. Importantly, all seven IRGs demonstrated high diagnostic value in GSE28829 dataset. The use of animal samples supports our results.
Conclusion: This study demonstrates the effectiveness of seven IRGs and reveal sex differences in atherosclerosis. Notably, there is a significant presence of immune cells within the atherosclerotic plaque of men compared to women. These findings have potential implications for the development of personalized treatment approaches targeting gender-related atherosclerosis.
Keywords: atherosclerosis, immune related genes, sex related atherosclerosis, bioinformatics, immune infiltration
Introduction
Atherosclerosis is a cardiovascular disease caused by the accumulation of cells, cholesterol, and extracellular matrix, which thickens and hardens the arterial wall. An epidemiological survey1 showed that before the age of 75 years, the prevalence of atherosclerotic plaque accumulation in women is lower than that in men, and until the age of 75 years, the prevalence of atherosclerotic plaque in women is higher than that in women. Some studies2,3 have also shown that the total area of plaque accumulation is larger in men, and the incidence of ischemic events is higher as well. However, the reason for differences in atherosclerosis due to sex is still unknown. Some studies have explored sex differences in atherosclerosis to be associated with the levels of sex hormones,4 sex chromosomes5 and intestinal flora,6 and obtained preliminary results. However, no study has explored sex differences in atherosclerosis at the genetic level.
In recent years, the rapid development of microarray and high-throughput technologies has become an effective tool for revealing disease pathogenesis. Using this method, researchers have identified several biomarkers related to cardiovascular diseases. CXCR2, CCN4, DLL1, PLXND2, APLN, NRP2, CCL2, and ANGPTL2 have helped clarify the pathogenesis and progression of diabetic cardiomyopathy.7 CD68, PAM, and GFBP6 can be used as diagnostic markers to identify unstable atherosclerotic plaques effectively.8 TP53, MAPK1, STAT3, HMOX1, and PTGS2 can be used as iron death-related atherosclerosis genes.9 In this study, we first identified the differentially expressed genes between men and women with advanced atherosclerosis, then calculated the infiltration of immune cells in the plaque using the ssGSEA algorithm, and finally intersected with immune-related genes to obtain immune-related hub genes, which were verified in atherosclerosis mouse models.
Materials and Methods
Data Collection
We collected the mRNA expression profiles of 32 advanced atherosclerotic plaques and 32 early atherosclerotic plaques from the GEO database (GSE43292). The expression profiles of 16 atherosclerotic plaques were verified using the GSE28829 dataset. Probes were converted into gene symbols according to the annotation files. If multiple probes corresponded to the same gene, the average value of these probes was taken as the gene expression level.
Differential Gene Analysis
In the GSE28829 dataset, we divided the samples into males and females according to “DEAD-box helicase 3 Y-linked (DDX3Y)” and other genes that only exist in the Y chromosome, and then in the samples of advanced atherosclerosis, we used the “limma” package to select the differential genes (P value>0.05 and |log2FoldChange| >0.75). Immune-related genes (IRGs) were obtained from an import database.
Construction of Protein-Protein Interaction (PPI) Network
To explore the interaction between proteins, we uploaded immune-related differential genes to the STRING (https://cn.string-db.org/) database, set the minimum interaction score to 0.4, and imported the results into the Cytoscape software using the mcode10 plug-in to identify the seven most relevant genes. The selected genes were displayed using the PPI network and subsequent analyses were conducted.
Functional Enrichment Analysis
The David database was used for enrichment analysis of The Gene Ontology (GO) and The Kyoto Encyclopedia of Genes and Genomes (KEGG) functions. GO can provide computable information about genes and their functional products. KEGG can be used to systematically study gene function. We used the Benjamin Hochberg test to correct the p-value of the multiple-hypothesis test. GO included cellular components (CC), molecular functions (MF), and biological processes (BP).
Immunocyte Infiltration Analysis
We used single-sample gene set enrichment analysis (ssGSEA) to identify the infiltration of different immune cells in the tissue cell expression profile.
Animal Model
According to the guidelines of the National Institutes of Health of the United States, the mice were raised in a suitable environment and the study protocol was approved by the Animal Research Ethics Review Committee of Shaoxing People’s Hospital (No.2021-016). All mice were raised under conditions of relative temperature (21–24°C) and relative humidity (50–60%), with a 12-hour light and dark cycle and free access to water and food. Ten male ApoE−/− mice and ten female ApoE−/− mice were purchased from the Nanjing Biomedical Research Institute of Nanjing University. The mice were randomly divided into four groups: the first group consisted of male mice fed a standard diet, the second group consisted of female mice fed a standard diet, the third group consisted of male mice fed a high-fat diet (20% fat, 20% sugar, and 1.25% cholesterol), and the fourth group comprised female mice fed a high-fat diet. All mice were raised for 16 weeks, and the serum, heart, and aorta were collected for follow-up operation.
Serum Biochemical Determination
Commercial kits were purchased from Nanjing Jiancheng Bioengineering Research Institute (Nanjing, China) to detect the levels of total cholesterol (TC), triglyceride (TG), and low-density lipoprotein cholesterol (LDL-C) in mouse serum.
RNA Extraction and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from plaque homogenates using a commercial kit (ES Science, China). A reverse transcription kit was used to convert all RNA into cDNA. RT-PCR was performed on a PCR instrument (Roche, Switzerland). To determine the expression of the corresponding genes, GAPDH was used as an internal parameter, and the expression of mRNA was calculated using the 2–ΔΔCt method. The primer sequences for each mRNA are listed in Table 1.
 |
Table 1 Primer Sequence |
Masson’s Staining
The roots of the mouse aorta were fixed with 4% paraformaldehyde for 12 h, embedded in paraffin, and cut into pieces. The aortic sections were stained with Masson’s trichrome to evaluate atherosclerotic lesions in the aorta. The ImageJ software was used to quantify the lesion area.
Results
Sex-Related Differentially Expressed Genes (DEGs) in Atherosclerosis
To explore the role of sex differences in atherosclerosis, we used the GSE43292 dataset in the GEO database to identify 141 differential genes by the “limma” method, including 45 upregulated genes and 96 downregulated genes (Figure 1A). A heat map is shown in Figure 1B. We identified 30 genes with the largest differences. The difference between male and female genes was evident.
 |
Figure 1 Sex-related differentially expressed genes (DEGs) in atherosclerosis. (A) DEGs between male and female samples. (B) Heatmap of Top 30 DEGs. |
Functional Enrichment Analyses of DEGs
We conducted GO and KEGG enrichment analyses on the DEGs, and the results are shown in Figure 2. Biological processes are mainly concentrated in cell adhesion, signal transduction, and negative regulation of smooth muscle cell proliferation. In terms of cellular components, it mainly focuses on the extracellular region, plasma membrane, and the extracellular space. Molecular functions mainly focus on signaling receptor activity, chemokine activity, and actin-binding. KEGG is mainly enriched in dilated cardiomyopathy, circadian entrainment, and amphetamine addiction.
 |
Figure 2 Functional enrichment analyses of DEGs. (A–C) GO enrichment analysis of DRGs. (D) KEGG enrichment analysis of DRGs. |
Enrichment and Analysis of IRGs and Construction of PPI Network
We found that DEGs were mainly enriched in the inflammatory response and neutrophil chemotaxis chemokine-mediated signaling pathways closely related to the immune response in Figure 2. Therefore, we aimed to explore the relationship between sex-related differential genes and immunity. We found 1793 immune-related genes in the Immport database (https://www.immport.org/home). After crossing these genes with the DEGs, we obtained 24 IRGs (Figure 3A). On this basis, we re-concentrated and analyzed IRGs (Figure 3B–E), which are mainly concentrated in inflammatory responses, extracellular spaces, chemokine activity, chemokine signaling pathways, and NF−kappa B signaling pathways. We then built a PPI network using the STRING database and Cytoscape and used the mcode plug-in to calculate the seven genes with the largest proportion of 24 IRGs (Figure 3F). The darker the color, the greater the proportion. These seven genes were C-C motif chemokine ligand 13 (CCL13), C-C motif chemokine ligand 19 (CCL19), C-X-C motif chemokine ligand 1 (CXCL1), C-X-C motif chemokine ligand 8 (CXCL8), formyl peptide receptor 2 (FPR2), S100 calcium-binding protein A8 (S100A8), and interleukin 1 receptor antagonist (IL1RN). In addition, we explored whether these gene differences also exist in normal samples. We analyzed the normal tissues and atherosclerotic plaque of individuals of different sexes (Figure 4). The expression of seven IRGs in normal tissues is not different, although male samples will show an upward trend. We used this to prove that these seven IRGs were formed in atherosclerosis, and that there was no difference before the lesions.
 |
Figure 4 Expression IRGs of each sample. *P<0.05. |
Immune Infiltration Analysis
We used the “ssGSEA” algorithm to calculate the infiltration of immune cells in normal and plaque samples. The heatmap results are shown in Figure 5A. The number of immune cells in the female samples was relatively low. Subsequently, we compared the immune cells in male and female samples, and the results are shown in Figure 5B. Most of the cells in the male plaque samples were higher than those in the female samples. Specifically, we found activated CD4 T cells, activated dendritic cells, CD56 bright natural killer cells, central memory CD4 T cells, central memory CD8 T cells, effector memory CD4 T cells, gamma delta T cells, immature dendritic cells, macrophages, myeloid-derived suppressor cells (MDSC), monocytes, natural killer T cells, neutrophils, T follicular helper cells, type 1 T helper cells, and type 17 T helper cells. Then, we carried out a correlation analysis between IRGs and immune cells, and the result was as expected, ie, there was a high correlation between IRGs and immune cells (Figure 5C). These results indicate that the inflammatory reaction of these immune cells may be related to atherosclerosis caused by sex differences.
Verification the IRGs of Atherosclerosis Caused by Sex Differences
First, we used the receiver operating characteristic (ROC) curve to verify the diagnostic value of IRGs in the GSE28829 dataset (Figure 6A and B). IRGs have a high diagnostic value in other atherosclerosis datasets, and the lowest area under the curve was 0.8.
 |
Figure 6 Receiver operating characteristic curve for seven IRGs. (A) ROC curve for four genes (CCL13, CCL19, CXCL1, CXCL8). (B) (A) ROC curve for three genes (FPR2, S100A8, IL1RN). |
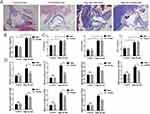
We then constructed atherosclerotic mouse models based on sex differences (Figure 7A and B). Masson staining showed that the atherosclerotic male and female mice had obvious plaque formation compared with the control group. We then tested their serum levels of TC, TG, and LDL-C (Figure 7C). Compared with the control group, the serum indices of atherosclerotic mice were significantly increased; however, there was no difference between male and female rats, which proves the success of the model. Based on this, we performed RT-PCR validation of the IRGs. Similar to our assumption, these IRGs rise in atherosclerosis, and the male sample shows a greater rise than the female sample, with a statistically significant difference (Figure 7D). In general, these results suggest that IRGs may be key genes for sex differences in atherosclerosis.
Discussion
Atherosclerosis is the primary cause of cardiovascular disease. However, few studies have explored the differences between men and women in the process of atherosclerosis at the genetic and immune cell level. In this study, based on the differential expression of the Y chromosome gene, we divided them into 27 male patients and five female patients. The differentially expressed genes found in 27 men with atherosclerosis and 5 women with atherosclerosis intersected with immune-related genes, and the seven IRGs were CCL13, CCL19, CXCL1, CXCL8, FPR2, S100A8, and IL1RN. We also found that some immune cells differed in sex-related atherosclerosis, and most of the immune cells in atherosclerotic plaques in men were higher than those in women.
CCL13 is a member of the CC chemokine family, also known as MCP4 (monocyte chemoattractant protein 4). It is upregulated in alopecia areata11 and in specific dermatitis.12 In addition, CC chemokine receptor 2 may interact with CCL13 and promote atherosclerosis as a link between platelets and monocytes.13 Some studies have also shown that CCL13 is a biological marker of obesity, which may aggravate subclinical atherosclerosis in obese patients by affecting the circulation level of major atherosclerosis markers.14 CCL19, similar to CCL13, is a member of the CC chemotaxis family. It is involved in many immune system diseases such as rheumatoid arthritis15 and Crohn’s disease.16 Some studies have reported that CCL19 increases atherosclerosis.17,18 CCL19 can accelerate the progression of atherosclerosis by promoting the formation of foam cells, activation of leukocytes and lipid uptake of macrophages.19 The CCL19 level at admission is also related to the development of heart failure.20 Although there are a large number of literatures proving the role of CCL19 in atherosclerosis, there are also literatures indicating that CCL19 cannot be used as a predictor of future adverse events in coronary heart disease population.21 Our research also shows that CCL19 is higher in male atherosclerosis patients than in women. CXCR1 is a member of the G-protein-coupled receptor family. It is the receptor of interleukin 8 (IL 8) and can bind to IL 8 with high affinity. The CXCR1-CXCR2 axis can change the course of the disease by affecting immune cells.22,23 In addition, some studies have reported sex-based differences in CXCR1. Male mice produce more CXCR1 in lung inflammation caused by nickel nanoparticles,24 but some studies also show that female patients produce more CXCR1 during antiretroviral treatment;25 however, no study has compared sex in patients with atherosclerosis. CXCL8 is a pro-inflammatory catalytic factor that is secreted by different cells, such as monocytes,26 macrophages,27 and fibroblasts.28 It is expressed after these cells have been stimulated by inflammation. CXCL8 plays an anti-inflammatory role, mainly through the CXCL8-CXCR1/2 axis. Some studies have shown that the expression of CXCL8 increases in atherosclerosis and that inhibition of CXCL8 can improve atherosclerosis.29 Based on this conclusion, we further confirmed that CXCL8 levels were higher in male patients than in female patients. FPR2 is a neutrophil chemokine. Previous studies have found that FPR2 signal transduction can increase the recruitment of monocytes and neutrophils in early atherosclerosis.30 However, another study revealed that FPR2 has dual effects on atherosclerosis. It not only contributes to the progression of atherosclerosis itself but also plays a role in enhancing plaque stability by affecting smooth muscle cells. This suggests that FPR2 may have complex and multifaceted involvement in the development and maintenance of atherosclerotic plaques.31 A recent study also reported that the expression of FPR2 is different in hepatocytes.32 However, they found that the expression of FPR2 in female hepatocytes was higher than that in male hepatocytes, which may be due to different animal models and differences in protein expression in organs. The role of FPR2 in gender-related atherosclerosis warrants further discussion and investigation. IL1RN is a leukocyte receptor antagonist. Many biochemical studies have confirmed that it plays an important role in atherosclerosis33,34 and is expressed in the endothelial cells of atherosclerosis. However, some studies have shown that there is a sex difference in the expression of IL1RN,35 with IL1RN * 1 being more common in men. Nevertheless, we also compared the expression of IL1RN in the normal tissues of patients and found no difference. Therefore, we need to further explore the role of these genes in sex.
Later, we carried out immune infiltration analysis, and found that the number of immune cells, including macrophages, monocytes, and many different types of T cells, increased in male patients. The infiltration of immune cells in male plaques was far greater than that in female plaques, which was consistent with previous results, Some studies have found that in the mouse model of atherosclerosis, male mice show more T cells,36 and macrophages,37 and more intense plaque inflammation than female mice. In addition, some studies have shown that male mice with atherosclerosis have more CD22 staining on behalf of B cells in the aortic root than female mice.38 We attempted to analyze the reasons for this difference. First, estrogen secreted by women may slow atherosclerosis. Some studies have shown that the degree of pathological changes in female monkeys after oophorectomy increases 2–10 times, and estrogen replacement treatment can alleviate this situation. Second, smoking, diabetes, hyperlipidemia, and other factors likely to promote atherosclerosis are not the same in men and women. Generally, the risk factor characteristics of men are worse than those of women, and this difference may promote gene expression and immune cell infiltration. Finally, physical differences between men and women will also affect the occurrence of atherosclerosis, and the size of male and female blood vessels will also affect the progression of atherosclerosis. Understanding sex-related atherosclerosis is critical for future precision medical strategies to provide personalized treatment for men and women.
Gender-based disease research has gained significant attention in recent years.39,40 In this study, seven IRGs associated with gender were identified. In future research, it would be beneficial to construct a hybrid polygenic risk score based on these genes to assess the progression of atherosclerosis in different sexes. Additionally, by obtaining specific drugs or adeno-associated viruses, we can inhibit gene expression in atherosclerotic mice, thereby exploring the specific mechanisms underlying gender-related atherosclerosis.
Our study had some advantages. First, in the past, the analysis of biographical information was based on disease and non-disease, and few people have analyzed the impact of sex on disease. Second, we found that some genes are specifically increased in male patients with atherosclerosis, but these genes have not been reported to differ according to sex; therefore, we can carry out personalized treatment according to sex based on this idea.
Our study had some limitations. First, we performed secondary mining based on the datasets. Although the analytical methods and ideas are innovative, we did not verify these genes in human samples. Second, although our results proved that there is no sex difference in the selected genes in normal samples, many genes have an upward trend in men, and the role of these genes should be further explored. Third, we cannot determine whether these gene changes are the outcome or the process, which needs to be further proven by experiments.
Conclusion
Based on our current study, we identified seven sex-related atherosclerosis genes. These genes were CCL13, CCL19, CXCL1, CXCL8, FPR2, S100A8, and IL1RN. Our in vivo experiments also showed that there are differences in these sex-related atherosclerosis genes.
Abbreviations
DDX3Y, DEAD-box helicase 3 Y-linked; IRGs, Immune-related genes; GO, The Gene Ontology; KEGG, The Kyoto Encyclopedia of Genes and Genomes; CC, cellular components; MF, molecular functions; BP, biological processes; ssGSEA, single-sample gene set enrichment analysis; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; RT-PCR, Reverse Transcription-Polymerase Chain Reaction; DEGs, differentially expressed genes; ROC, the receiver operating characteristic curve.
Ethics
The study was approved by the Animal Research Ethics Review Committee of Shaoxing People’s Hospital (No.2021-016).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study is supported by the National Natural Science Foundation of China (No. 82000252, 82200390); Zhejiang Province Medical and Health Science and Technology Program (2021RC032); Medical and Health Science and Technology Plan Project of Shaoxing City (2020A13018, 2022KY104); Postdoctoral program of Shaoxing People’s Hospital(2021BSQDJ01); The 551 talent project of Zhejiang Province.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Joakimsen O, Bonaa KH, Stensland-Bugge E, Jacobsen BK. Age and sex differences in the distribution and ultrasound morphology of carotid atherosclerosis: the Tromsø Study. Arterioscler Thromb Vasc Biol. 1999;19(12):3007–3013. doi:10.1161/01.atv.19.12.3007
2. Ota H, Reeves MJ, Zhu DC, et al. Sex differences of high-risk carotid atherosclerotic plaque with less than 50% stenosis in asymptomatic patients: an in vivo 3T MRI study. AJNR Am J Neuroradiol. 2013;34(5):1049–55, s1. doi:10.3174/ajnr.A3399
3. Wendorff C, Wendorff H, Pelisek J, et al. Carotid plaque morphology is significantly associated with sex, age, and history of neurological symptoms. Stroke. 2015;46(11):3213–3219. doi:10.1161/strokeaha.115.010558
4. Hodis HN, Mack WJ, Lobo RA, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135(11):939–953. doi:10.7326/0003-4819-135-11-200112040-00005
5. Link JC, Chen X, Prien C, et al. Increased high-density lipoprotein cholesterol levels in mice with XX versus XY sex chromosomes. Arterioscler Thromb Vasc Biol. 2015;35(8):1778–1786. doi:10.1161/atvbaha.115.305460
6. Ahmed S, Spence JD. Sex differences in the intestinal microbiome: interactions with risk factors for atherosclerosis and cardiovascular disease. Biol Sex Differ. 2021;12(1):35. doi:10.1186/s13293-021-00378-z
7. Zhong Z, Zhang H, Xu T, et al. Identification and verification of immune-related biomarkers and immune infiltration in diabetic heart failure. Front Cardiovasc Med. 2022;9:931066. doi:10.3389/fcvm.2022.931066
8. Wang J, Kang Z, Liu Y, Li Z, Liu Y, Liu J. Identification of immune cell infiltration and diagnostic biomarkers in unstable atherosclerotic plaques by integrated bioinformatics analysis and machine learning. Front Immunol. 2022;13:956078. doi:10.3389/fimmu.2022.956078
9. Huang T, Wang K, Li Y, et al. Construction of a novel ferroptosis-related gene signature of atherosclerosis. Front Cell Dev Biol. 2021;9:800833. doi:10.3389/fcell.2021.800833
10. Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi:10.1101/gr.1239303
11. Glickman JW, Dubin C, Renert-Yuval Y, et al. Cross-sectional study of blood biomarkers of patients with moderate to severe alopecia areata reveals systemic immune and cardiovascular biomarker dysregulation. J Am Acad Dermatol. 2021;84(2):370–380. doi:10.1016/j.jaad.2020.04.138
12. Brunner PM, Suárez-Fariñas M, He H, et al. The atopic dermatitis blood signature is characterized by increases in inflammatory and cardiovascular risk proteins. Sci Rep. 2017;7(1):8707. doi:10.1038/s41598-017-09207-z
13. Breland UM, Michelsen AE, Skjelland M, et al. Raised MCP-4 levels in symptomatic carotid atherosclerosis: an inflammatory link between platelet and monocyte activation. Cardiovasc Res. 2010;86(2):265–273. doi:10.1093/cvr/cvq044
14. Gentili A, Zaibi MS, Alomar SY, et al. Circulating levels of the adipokines monocyte chemotactic protein-4 (MCP-4), macrophage inflammatory protein-1β (MIP-1β), and eotaxin-3 in severe obesity and following bariatric surgery. Horm Metab Res. 2016;48(12):847–853. doi:10.1055/s-0042-108731
15. Burman A, Haworth O, Hardie DL, et al. A chemokine-dependent stromal induction mechanism for aberrant lymphocyte accumulation and compromised lymphatic return in rheumatoid arthritis. J Immunol. 2005;174(3):1693–1700. doi:10.4049/jimmunol.174.3.1693
16. Middel P, Raddatz D, Gunawan B, Haller F, Radzun HJ. Increased number of mature dendritic cells in Crohn’s disease: evidence for a chemokine mediated retention mechanism. Gut. 2006;55(2):220–227. doi:10.1136/gut.2004.063008
17. Halvorsen B, Dahl TB, Smedbakken LM, et al. Increased levels of CCR7 ligands in carotid atherosclerosis: different effects in macrophages and smooth muscle cells. Cardiovasc Res. 2014;102(1):148–156. doi:10.1093/cvr/cvu036
18. Salem MK, Butt HZ, Choke E, et al. Gene and protein expression of chemokine (C-C-Motif) Ligand 19 is upregulated in unstable carotid atherosclerotic plaques. Eur J Vasc Endovasc Surg. 2016;52(4):427–436. doi:10.1016/j.ejvs.2016.05.018
19. Akhavanpoor M, Gleissner CA, Gorbatsch S, et al. CCL19 and CCL21 modulate the inflammatory milieu in atherosclerotic lesions. Drug Des Devel Ther. 2014;8:2359–2371. doi:10.2147/dddt.S72394
20. Caidahl K, Hartford M, Ravn-Fischer A, et al. Homeostatic chemokines and prognosis in patients with acute coronary syndromes. J Am Coll Cardiol. 2019;74(6):774–782. doi:10.1016/j.jacc.2019.06.030
21. Katra P, Hennings V, Nilsson J, et al. Plasma levels of CCL21, but not CCL19, independently predict future coronary events in a prospective population-based cohort. Atherosclerosis. 2023;366:1–7. doi:10.1016/j.atherosclerosis.2023.01.004
22. Wang L, Zhang YL, Lin QY, et al. CXCL1-CXCR2 axis mediates angiotensin II-induced cardiac hypertrophy and remodelling through regulation of monocyte infiltration. Eur Heart J. 2018;39(20):1818–1831. doi:10.1093/eurheartj/ehy085
23. Wang S, Bai J, Zhang YL, et al. CXCL1-CXCR2 signalling mediates hypertensive retinopathy by inducing macrophage infiltration. Redox Biol. 2022;56:102438. doi:10.1016/j.redox.2022.102438
24. You DJ, Lee HY, Taylor-Just AJ, Linder KE, Bonner JC. Sex differences in the acute and subchronic lung inflammatory responses of mice to nickel nanoparticles. Nanotoxicology. 2020;14(8):1058–1081. doi:10.1080/17435390.2020.1808105
25. Vanpouille C, Wells A, Wilkin T, et al. Sex differences in cytokine profiles during suppressive antiretroviral therapy. Aids. 2022;36(9):1215–1222. doi:10.1097/qad.0000000000003265
26. Peveri P, Walz A, Dewald B, Baggiolini M. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med. 1988;167(5):1547–1559. doi:10.1084/jem.167.5.1547
27. Strieter RM, Kunkel SL, Showell HJ, Marks RM. Monokine-induced gene expression of a human endothelial cell-derived neutrophil chemotactic factor. Biochem Biophys Res Commun. 1988;156(3):1340–1345. doi:10.1016/s0006-291x(88)80779-4
28. Zhai J, Shen J, Xie G, et al. Cancer-associated fibroblasts-derived IL-8 mediates resistance to cisplatin in human gastric cancer. Cancer Lett. 2019;454:37–43. doi:10.1016/j.canlet.2019.04.002
29. Hedayati-Moghadam M, Hosseinian S, Paseban M, et al. The role of chemokines in cardiovascular diseases and the therapeutic effect of curcumin on CXCL8 and CCL2 as pathological chemokines in atherosclerosis. Adv Exp Med Biol. 2021;1328:155–170. doi:10.1007/978-3-030-73234-9_11
30. Butcher MJ, Galkina EV. wRAPping up early monocyte and neutrophil recruitment in atherogenesis via Annexin A1/FPR2 signaling. Circ Res. 2015;116(5):774–777. doi:10.1161/circresaha.115.305920
31. Petri MH, Laguna-Fernández A, Gonzalez-Diez M, Paulsson-Berne G, Hansson GK, Bäck M. The role of the FPR2/ALX receptor in atherosclerosis development and plaque stability. Cardiovasc Res. 2015;105(1):65–74. doi:10.1093/cvr/cvu224
32. Lee C, Kim J, Han J, et al. Formyl peptide receptor 2 determines sex-specific differences in the progression of nonalcoholic fatty liver disease and steatohepatitis. Nat Commun. 2022;13(1):578. doi:10.1038/s41467-022-28138-6
33. Su W, Zhao Y, Wei Y, Zhang X, Ji J, Yang S. Exploring the pathogenesis of psoriasis complicated with atherosclerosis via microarray data analysis. Front Immunol. 2021;12:667690. doi:10.3389/fimmu.2021.667690
34. Wang Y, Su W, Li Y, et al. Analyzing the pathogenesis of systemic lupus erythematosus complicated by atherosclerosis using transcriptome data. Front Immunol. 2022;13:935545. doi:10.3389/fimmu.2022.935545
35. Bessler H, Osovsky M, Beilin B, Alcalay Y, Sirota L. The existence of gender difference in IL-1Ra gene polymorphism. J Interferon Cytokine Res. 2007;27(11):931–935. doi:10.1089/jir.2007.0029
36. Moss ME, Lu Q, Iyer SL, et al. Endothelial mineralocorticoid receptors contribute to vascular inflammation in atherosclerosis in a sex-specific manner. Arterioscler Thromb Vasc Biol. 2019;39(8):1588–1601. doi:10.1161/atvbaha.119.312954
37. Wang Y, Lu H, Huang Z, et al. Apolipoprotein E-knockout mice on high-fat diet show autoimmune injury on kidney and aorta. Biochem Biophys Res Commun. 2014;450(1):788–793. doi:10.1016/j.bbrc.2014.06.060
38. Hernández-Vargas P, Ortiz-Muñoz G, López-Franco O, et al. Fcgamma receptor deficiency confers protection against atherosclerosis in apolipoprotein E knockout mice. Circ Res. 2006;99(11):1188–1196. doi:10.1161/01.RES.0000250556.07796.6c
39. Shankhwar V, Urvec J, Steuber B, et al. Association of gender with cardiovascular and autonomic responses to central hypovolemia. Front Cardiovasc Med. 2023;10:1211774. doi:10.3389/fcvm.2023.1211774
40. Wenger NK. Special issue on atherosclerotic cardiovascular disease: sex and gender differences. Atherosclerosis. 2023;117271. doi:10.1016/j.atherosclerosis.2023.117271
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.