Back to Journals » International Journal of General Medicine » Volume 16
Immune Checkpoint Inhibitor-Based Combination Therapy for Colorectal Cancer: An Overview
Received 12 February 2023
Accepted for publication 19 April 2023
Published 26 April 2023 Volume 2023:16 Pages 1527—1540
DOI https://doi.org/10.2147/IJGM.S408349
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Jingjing Li, Xuanfu Xu
Department of Gastroenterology, Shidong Hospital of Shanghai, Shanghai, People’s Republic of China
Correspondence: Xuanfu Xu, Department of Gastroenterology, Shidong Hospital of Shanghai, Shanghai, People’s Republic of China, Tel +86-021-25066666, Email [email protected]
Abstract: Colorectal cancer (CRC) is one of the most common diseases in the world. Tumor immunotherapy is an innovative cancer treatment that acts by activating the human body’s autoimmune system. Immune checkpoint block has been shown to be effective in DNA deficient mismatch repair/microsatellite instability-high CRC. However, the therapeutic effect for proficient mismatch repair/microsatellite stability patients still requires further study and optimization. At present, the main CRC strategy is to combine other therapeutic methods, such as chemotherapy, targeted therapy, and radiotherapy. Here, we review the current status and the latest progress of immune checkpoint inhibitors in the treatment of CRC. At the same time, we consider therapeutic opportunities for transforming cold to hot, as well as perspectives on possible future therapies, which may be in great demand for drug-resistant patients.
Keywords: immune checkpoint inhibitors, colorectal cancer, PD-1/PD-L1, combination therapy, DNA mismatch repair
Introduction
The incidence and mortality rates of colorectal cancer (CRC) are the third highest in the United States, representing a great threat to the health of the population.1 At present, CRC is treated with surgery, chemotherapy, radiotherapy, immunotherapy, and targeted therapy. However, because the initial symptoms are not obvious, patients often have poor prognosis in the late stages, so that the 5-year overall patient survival rate is less than 15%.2 Reducing the metastasis and recurrence in late-stage patients is of great significance. Immune checkpoints refer to a series of molecules that are expressed in the immune cells. They can regulate the degree of immune activation and play an important role in preventing the occurrence of autoimmune effects.3 The combination of programmed death receptor and its ligand results in T cell failure and inability to kill tumor cells. Moreover, the tumor cells can escape the immune surveillance of the host.4,5 Therefore, effectively blocking the activation of immune checkpoints can improve the aggressiveness of the host immune system to tumor cells.
Immune cells are common normal cell types that live in association with cancer cells. If there are more immune cells around the cancer, the tumor is categorized as hot. The opposite is true for cold tumors. Immunotherapy techniques for CRC have been developing rapidly. Clinical trials have included advanced, first-line, second-line, and various other types of adjuvant therapy.6,7 Continuous progress in precise tumor treatments, second-generation gene sequencing, and other technologies have obviated the original tumor division based on tumor location and organ, instead introducing the population screening method based on different new biomarkers. Recommendations for the treatment of the same cancer species of different molecular types are also different.8–10 The mismatch repair (MMR)/microsatellite instability (MSI) system is the most important indicator for CRC classification, which is used to formulate treatment strategies. MSI is a code-shifting mutation of microsatellites in tumor cells caused by the insertion or deletion of duplicate units.11 The DNA MMR system fights against these errors by identifying and repairing DNA damage and correcting base insertion, deletion, or mismatch caused by the wrong cycle in the DNA replication process. The defect of mismatch repair function (dMMR) is characterized by the lack of the MMR protein.12,13 When the MMR system is dysfunctional or mutated, these genetic errors will not be corrected, so that they will be permanently integrated into the tumor DNA, which is highly unstable (MSI-H). The MMR protein is normally expressed in mismatch repair proficient (pMMR), which is categorized by low instability (MSI-L) and stability (MSS).14,15 According to the above classification, CRC will enter a new era of differential treatment for the same disease.
Classic Immune Checkpoint Inhibitors (ICIs) for dMMR/MSI-H
T cells can fight against tumor immune responses by increasing cytotoxic responses after they receive an effective and lasting stimulus signal. This stimulus signal includes co-stimulatory signals to enhance immunity and co-inhibitory signals to suppress immunity, which are immune checkpoints.16 Tumors inhibit the immune system to promote tumor immune escape and tolerance through overexpression of immune checkpoints, thus promoting tumor cell growth.17,18 ICIs can effectively block the binding of inhibitory checkpoints and ligands, reactivate T cells, and monitor the invasion of immune tumor cells in the body.19 They include programmed cell death receptor 1 (PD-1), programmed cell death receptor ligand 1 (PD-L1), cytotoxic T lymphocyte associated antigen 4 (CTLA-4), and others.20,21 Studies have confirmed that ICIs have a significant effect in patients with dMMR/MSI-H who can benefit from them.
PD-1/PD-L1
PD-1, also known as CD279 (differentiation cluster 279), is an important immunosuppressive molecule. In 1992, Ishida et al first found and named PD-1 in gene screening involved in cell apoptosis.22 PD-1/PD-L1 can regulate the immune system and promote self-tolerance by downward regulating the immune system response to human cells, as well as by inhibiting the inflammatory activities of T cells.23 This can prevent autoimmune diseases. Tumor cells may induce tolerance and apoptosis of T cells through PD-1/PD-L1 and achieve immune escape by inhibiting the proliferation and activity of T cells and promoting epithelial mesenchymal transformation.24
In 2015, Le et al first found that dMMR/MSI-H patients benefit from ICIs, bringing them new hope.25 The representative ICIs drugs are pembrolizumab and nivolumab, which have been approved for the third-line or even second-line treatment of PD-L1-positive metastatic CRC (mCRC). The key Phase II study KEYNOTE-016 was the first to reveal the relationship between the MMR status and ICI efficacy. In this study, the objective response rate (ORR) in mCRC patients initially included in the dMMR and pMMR groups after receiving the pembrolizumab treatment was 40% and 0%, respectively. Later, the dMMR/MSI-H patients were further treated, resulting in an ORR of 52%. The 2-year progression-free survival (PFS) and overall survival (OS) rates were 59% and 72%, respectively.26 The KEYNOTE-028 study verified that among 20 different types of tumors, only the patients with MSI-H advanced CRC went into partial remission.27,28 The KEYNOTE-164 study further introduced PD-1 inhibitor into clinical application. Its research results suggested that the ORR of patients who had received lines 2 and 1 treatments before administering pembrolizumab were 32.8% and 34.9% respectively. Restarting the treatment after progress has been achieved, making some patients relieved or stable.29 Furthermore, the KEYNOTE-177 study compared the dual drug chemotherapy of pembrolizumab combined with vascular endothelial growth factor (VEGF) or epidermal growth factor receptor (EGFR) monoclonal antibody. The results showed that the median PFS of a single drug was 16.5 months and the ORR was 45.1%, while the values for the chemotherapy group were 8.2 months and 33.1%, respectively. The above experiments confirmed that pembrolizumab could be used as the first-line standard treatment for patients with dMMR/MSI-H. The National Comprehensive Cancer Network (NCCN) diagnosis and treatment guidelines have also been successfully rewritten based on this information.30,31 Another key phase II study CheckMate-142 single drug treatment cohort showed that the single drug treatment of nivolumab resulted in an ORR in the dMMR/MSI-H patients of 31.1%, with the median PFS of 14.3 months and the OS rate of 73% at 12 months.32 Current research studies have mainly focused on the combined application of PD-1 inhibitors with other immunosuppressants, which will significantly improve the therapeutic effect without increasing adverse reactions.
Atezolizumab is an important representative of PD-L1. The COMMIT trial is a Phase III open label in-progress study that compares the efficacy and safety of atezolizumab versus mFOLFOX6/bevacizumab, where PFS serves as the primary endpoint (NCT02997228). The latest research has demonstrated that many new PD-1 and PD-L1 inhibitors, such as doxalimab, cindilizumab, tirelizumab, and duvalizumab, showed effective anti-tumor activity in patients with advanced solid tumors.33–35
CTLA-4
CTLA-4, also known as CD152 (differentiation cluster 152), is a protein receptor that acts as an immune checkpoint that downregulates the immune response. Golstein et al identified CTLA-4 in 1987.36 In 1995, Tak Wah Mak and Arlene H. Sharpe independently published the discovery about the function of CTLA-4 as a negative regulator of T cell activation by knocking out mouse genes.37 CTLA4 is constitutively expressed in regulatory T cells, but is only up-regulated in conventional T cells after activation, which is particularly significant in cancer.38 Recombinant CTLA-4 Ig can effectively and specifically inhibit cellular and humoral immune responses in vivo and in vitro and has significant therapeutic effects on transplant rejection and various autoimmune diseases with very low toxicity and side effects.39,40 It is currently considered to be a promising new immunosuppressive drug, especially ipilimumab and tremelimumab.
Ipilimumab is an important inhibitor acting on CTLA-4. After their combination, the interaction between CTLA-4 and its ligand CD80/CD86 is blocked and the activation and proliferation of T cells enhance the tumor immune response.41,42 However, the results of ipilimumab’s single use are not satisfactory. At present, it is believed that nivolumab plus ipilimumab has a very positive clinical effect in dMMR/MSI-H patients, such that 54.6% of them can objectively experience relief.32 Although the research on CTLA-4 has begun early on, there is a still a lack of drugs on the market, which indicates that the research progress is relatively slow. At present, many drugs are still in the exploration and adjustment Phases I and II and are generally used together with other PD-1 drugs to achieve better therapeutic effects. It is possible that other targets can replace CTLA-4 to develop more effective drugs.43–45
Other Immune Checkpoint Molecules
With the in-depth study of immune regulation and tumor microenvironment, more targets have been discovered and added to the existing clinical research investigations, such as T cell immune receptor with Ig and immunoreceptor tyrosine-based inhibitory motif domains (TIGIT), lymphocyte activation gene-3 (LAG-3), natural killer group 2 member A (NKG2A), and T cell immunoglobulin domain and mucin domain-3 (TIM-3). There are many current related clinical studies on this subject.23,46–48 A Phase I clinical trial on pembrolizumab combined with anti-LAG-3 antibody favezelimab in 89 previously treated MSS mCRC patients showed four patients with partial remission and one patient achieving complete remission. The median remission duration was 10.6 months and the toxicity was controllable, suggesting that combined immunotherapy may have survival benefits in patients with MSS type cancer.49
TIM-3 and TIGHT are also emerging immune checkpoint molecules, which might bring new hope to cancer patients who cannot benefit from PD-1 antibody use. Several drugs targeting TIM-3 and TIGHT, such as BMS-986258, relatimab, and tiragolumab, have entered clinical trials50–52 Recently, Tiganis et al suggested that there is a new immune checkpoint on the endoplasmic reticulum of tumor-infiltrating T cells known as protein tyrosine phosphatase 1b (PTP1B). The high expression level of PTP1B inhibits the proliferation, while its knockout promotes the activation of the STAT5 signaling pathway in T cells, thereby inhibiting tumor growth. It can also be combined with PD-1 inhibitor and chimeric antigen receptor T-cell immunotherapy (CAR-T) cells to achieve better anti-tumor effects.53 Recombinant tumor necrosis factor receptor superfamily member 9 agonist combined with PD-L1 can effectively activate and expand tumor-specific cytotoxic T cells and enhance tumor control and killing. Related drugs, such as RG-7827, urelumab, and utomilumab, are under development.54,55 These new targets are expected to greatly improve the progress of tumor immunotherapy. However, there has been no breakthrough in the effect of single-use drugs at the immune checkpoint. More and more studies have focused on the combination of immunotherapy schemes, and it has been confirmed that they do not cause a significant increase in adverse reactions.56,57
Combined Immunotherapy
In 2018, Overman et al reported the effect of dual immunotherapy of nivolumab plus ipilimumab in the second- and posterior-line treatments of dMMR/MSI-H mCRC in the CheckMate142 study.32 A total of 119 patients were included in the study, which had an ORR of 55%, and the OS rates after 9 and 12 months of 87% and 85%, respectively. The above results showed that nivolumab combined with ipilimumab had a higher ORR than nivolumab alone, suggesting that PD-1/PD-L1 inhibitors combined with other immune checkpoint blockers improve the treatment effect.32,58,59 Anti-CTLA4 therapy has resulted in an enhanced antigen-specific T cell-dependent immune response, while anti PD-1 seemed to reactivate the ability of CD8+T cells to cleave cancer cells. This experiment moves immunotherapy from the third-line selection to the first-line treatment. At present, pembrolizumab and nivolumab alone or in combination with CTLA-4 blockers (such as ipilimumab) have been approved by the US FDA for clinical use in the treatment of mCRC with dMMR/MSI-H. Additional experiments are still in progress. For example, CheckMate 8HW is exploring the efficacy of three regimens, including nivolumab alone, nivolumab combined with ipilimumab, and chemotherapy selected by the researchers (NCT04008030).60 The latest studies have found that blocking the expression of CD73 in immune circulation can improve the effect of combined immunity, and the molecular mechanism related to improving the effect of dual immunotherapy needs to be further explored.31
Adjuvant and Neoadjuvant Therapy
It is well known that MSI-H CRC has a poor postoperative chemotherapy response, and immunotherapy can be used to improve it. At present, there are three major clinical trials. The ATOMIC study (NCT02912559) has explored the effect of atezolizumab combined with single chemotherapy with DFS as the end point.61 The POLEM trial (NCT03827044) is a multicenter, three-stage, randomized clinical study on adjuvant treatment of dMMR colon cancer with avelumab and fluoropyrimidine.62 These studies are still in progress and their results are expected.
A new adjuvant therapy strategy is to generate a better immune response before tumor resection. NICHE is the first phase II clinical trial on early neoadjuvant therapy. The study patients underwent colon cancer surgery no later than 6 weeks after receiving the first immunotherapy treatment. The remission rate of dMMR colon cancer patients receiving neoadjuvant immunotherapy reached 100%, and only 10% of patients had related toxic reactions. However, the remission rate in pMMR was only 27%.63 The VOLTAGE study also showed that 60% of patients with MSI⁃H achieved pathological complete remission (pCR) after preoperative radiotherapy and chemotherapy combined with nivolumab, where only three patients experienced immune related adverse events (AEs) and all had recovered.64 The new adjuvant treatment monotherapy regimen of nivolumab plus ipilimumab or pembrolizumab has been added to the 2021 NCCN CRC guidelines.30 A small Phase 2 clinical trial has recently reported that 12 rectal cancer patients received dostarlimab treatment with a clinical complete remission rate of 100%. No disease recurrence was observed during the follow-up period, and none of the patients reported adverse reactions of grade 3 or above, which is a significant step in the fight against cancer.65 In addition, the combined new auxiliary scheme of star combination nivolumab and ipilimumab also produced a 100% response rate in patients with dMMR colon cancer.66
Although the dMMR/MSI-H population significantly benefited from the treatment with immunosuppressive agents at immune checkpoints, the total number of beneficiaries was about 5%. Treating patients with pMMR/MSS mCRC with ICIs alone is not ideal67 and relevant methods need to be further explored. On one hand, the dominant population has been screened based on the MMR classification. Moretto et al reported that homologous recombination defect tumors in CRC patients with pMMR/MSS were a significantly different subgroup with unique molecular and prognostic characteristics, which could be used to investigate the potential efficacy of drugs worth exploring.68 On the other hand, it is necessary to improve the ICI dosage selection and actively seek other drug combinations to improve the therapeutic effect.69,70
Combination Therapy for pMMR/MSS
The pMMR/MSS population has no obvious benefits due to its inherent immunosuppressive characteristics, low tumor lymphocyte infiltration level, and low tumor mutation burden (TMB). How to explore such populations, overcome their limitations, and transform cold tumors into hot is very important.71 The combination of ICIs and other treatment schemes for pMMR/MSS CRC patients may achieve a certain effect due to the change in tumor microenvironment, while the combination of ICIs and other treatment schemes for dMMR/MSI-H CRC patients may improve the effect of immunotherapy due to the improvement in TMB.72 From the aspects of tumorigenesis, angiogenesis, metastasis, tumor immunity, and other key carcinogenic pathways, using various joint means to regulate the immune microenvironment of such tumors so that drugs can have a synergistic anti-tumor effect with ICIs will eventually transform the inherently cold tumors into hot, which can be effectively recognized and targeted by the activated immune system.73,74
Combined Targeted Medicine
VEGF/VEGFR
Recognized basic research has confirmed that VEGF and VEGFR are important molecules that control tumor angiogenesis. Their inhibitors can reduce tumor angiogenesis, increase the degree of T cell response, and have a synergistic effect when combined with immunotherapy.75 Multi-target tyrosine kinase inhibitors (TKIs), which mainly inhibit VEGF or VEGFR, have become the most promising combination drugs.76
Regorafenib is a multi-kinase inhibitor that targets several receptor tyrosine kinases involved in angiogenesis and metastasis (VEGFR1, VEGFR2, VEGFR3, FGFR1, FGFR2, TIE2, and PDGFRs), tumorigenesis (KIT, RET, and RAF1), and tumor immunity (CSF1R). The Japanese REGONIVO study reported that the exploratory phase I b study using nivolumab combined with low-dose regorafenib for the treatment of refractory MSS type CRC and gastric cancer showed that the ORR in mCRC was as high as 33.3%, 1-year PFS rate was 41.8%, and 1-year OS rate was 68.0%.77 In the second phase of the French REGOMUNE and North American studies (NCT04126733), the results showed that PD-1 inhibitors combined with regorafenib did not achieve good results, with an ORR level of <10%.78–80 The REGNIVO5 and REGOTORI6 studies in North America have shown inconsistent results,81,82 largely due to the extensive study heterogeneity in terms of design, geographical region, patient characteristics, and sample size.83 Lenvatinib is also a TKI type, which can inhibit the kinase activity of VEGF receptor and pathological angiogenesis of other tumors. LEAP⁃005 study is a phase II multi cohort clinical trial on lenvatinib combined with pembrolizumab in previously treated patients with solid tumors. Its ORR and median OS were reported to be 22% and 10.6 months, respectively.84 At present, there are three phases in the LEAP-017 study (NCT04776148) and their results for using pembrolizumab in combination with lenvatinib in CRC patients are promising.
Bevacizumab is a monoclonal antibody that can inhibit VEGF production. The BACCI study in 2019 has reported that the combination of atezolizumab with bevacizumab and other chemotherapy drugs effectively improved ORR, but the median PFS and OS were not significantly improved.85 The AtezoTRIBE study at the ESMO meeting in 2021 showed that significant differences between groups were generated after the treatment with atezolizumab plus bevacizumab combined with FOLFOXIRI or fluorouracil. However, clinician approval still needs further discussion.86 At present, some RCT studies are still exploring whether the combination of standard first-line chemotherapy and bevacizumab-based immunotherapy is effective.87–89
Fruquintinib is a new, oral, highly selective targeting drug for VEGFR. It can inhibit the formation of tumor neovascularization by inhibiting VEGFR phosphorylation and downstream signals on the surface of vascular endothelial cells and play a role in inhibiting tumor growth and metastasis. The results of a phase I b study suggested that the ORR in patients with mCRC who failed to receive second-line treatment with fruquintinib and cindilimab was 22.7%, while the median PFS was 5.6 months.90 Another study confirmed that the ORR and median PFS after the mCRC treatment with fruquintinib combined with a new PD-1 inhibitor geptanolimab reached 26.7% and 7.33, respectively, and 25% and 5.45 months, respectively, in MSS patients.91 Although its current sample size is small, this study has great potential.
The specific mechanism for the difference in efficacy between anti-angiogenic targeted drugs with different mechanisms and immunotherapy is unknown. It is speculated that the reason may be that TKI drugs are multi-target. In addition to inhibiting the VEGF/VEGFR pathway, TKI drugs may also inhibit targets related to immune regulation, such as platelet-derived growth factor receptor (PDGFR), angiopoietin-2 receptor (TIE2), and colony-stimulating factor 1 receptor, and have stronger synergy with immunotherapy.92–94
EGFR
EGFR is a member of the epidermal growth factor receptor (HER) family, which includes HER1 (erbB1, EGFR), HER2 (erbB2, NEU), HER3 (erbB3), and HER4 (erbB4).95,96 Studies have shown that EGFR is overexpressed or abnormally expressed in many solid tumors.97,98 It is also related to the inhibition of tumor cell proliferation, angiogenesis, tumor invasion, metastasis, and apoptosis.99
Panitumumab is a monoclonal antibody targeting EGFR. The LCCC1632 study (NCT03442569) evaluated the efficacy and safety of panitumumab and nivolumab plus ipilimumab in the treatment of patients with KRAS, NRAS, BRAF wild-type, and MSS metastatic CRC. The ORR was 35%, and the median PFS was 5.7 months.100 The AVETUX (NCT03174405) study explored the effects of cetuximab, avelumab, and conventional chemotherapy. The median PFS was 11.1 months, but the rate of adverse reactions was high, mainly including dryness, acne-like dermatitis, rash, hypomagnesemia, and vomiting.101,102 Therefore, more clinical trials will be needed to optimize the dosage and joint use plan and determine the minimum toxicity plan, which will bring health benefits to patients and not damage their quality of life.
MAPK/MEK
Mitogen-activated protein kinase (MAPK) pathway has three levels of signal transmission: MAPK, MAPK kinase, and kinase of MAPK kinase. These three kinases can be activated in turn and jointly regulate many important physiological/pathological effects, such as cell growth, differentiation, stress, and inflammatory reaction. MEK is a key factor in this pathway, and its abnormal function leads to serious tumor diseases.103 Many studies have confirmed that MEK inhibitors can enhance tumor immunity, while helper T cells can maximize the synergistic effect on immunotherapy. However, further exploration of the CRC treatment is needed.104,105 The IMblaze370 test showed that the combination of atezolizumab and cobimetinib had an effective rate of 8%. Compared to the single-drug group of atezolizumab and regorafenib, the OS for the combination of atezolizumab and cobimetinib was not significantly different, and the incidence of grade 3–4 AEs increased significantly to 64%.106
BRAF/RAS
Kirsten rat sarcoma viral oncogene (KRAS) is the most common mutation member of the RAS family and the most common oncogene driver in human cancer. About 30–40% of patients with CRC have a KRAS mutation.107 Previous CheckMate 142 and KEYNOTE-164 studies have both investigated the KRAS/RAS mutation. CheckMate 142 results suggested that the ORR in mutant patients using nivolumab was lower than that in wild-type patients, while KEYNOTE-164 results showed that the ORR in different patients using pembrolizumab was similar.29,32,59 Therefore, it is of great significance to further determine the joint role of KRAS and PD-1.
In 2018, a Phase 1b/2 study evaluated the safety and effect of binimetinib combined with nivolumab or nivolumab plus ipilimumab. The results showed that although the adverse reaction rate for KRAS inhibitor combined with a single drug was 44.44%, while that of two combined drugs was 40.75% with a median ORR of 7.4 months, the treatment showed preliminary efficacy, although its safety needs to be further optimized and improved.108 A trial report on BRAF V600E mutation mCRC patients in 2020 has shown that the ORR for PD-1 monoclonal antibody combined with BRAF inhibitor (dabrafenib)+MEK inhibitor (trametinib) was 35%, while the ORR for MSS patients who had not received targeted therapy before was 45%. In 2022, a new KRAS inhibitor adagrasib (MRTX849) showed that MRTX849 alone achieved an ORR of 87%, while cetuximab combined with targeted drug achieved a stronger anti-cancer effect. Furthermore, 100% of patients were in stable condition and their tumors shrank to varying degrees.109 The pMMR patients with BRAF/RAS mutations can significantly benefit from combined immune targeted therapy and even use double target therapy to improve the effect.110
Other Targets
Based on statistical analysis, human epidermal growth factor receptor-2 (HER2) amplification or overexpression is found in 3–5% of patients with advanced or metastatic CRC.111 In 2022, the FDA granted priority approval to the new combination therapy of tucatinib and trastuzumab. In the treatment of adult patients with HER2-positive CRC, the confirmed ORR was 38.1% in 84 patients receiving tucatinib and trastuzumab, although other new drugs were also under study.112 In addition, about 1–5% of colon cancer patients have neurotrophin receptor kinase fusion, especially with MSI-H. The results of the first targeted drug larotrectinib, which is not limited to cancer species, showed that the ORR in CRC patients was as high as 47%, which means that nearly half of all CRC patients’ lesions were significantly reduced or even disappeared. In addition, 42% of patients were in stable condition, and the disease control rate reached 89%.113 There will be more effective and targeted drugs with the improvement of gene detection technology in the future, which will be used in clinical standardization and help tumor diagnosis and treatment.
Combined Conventional Chemotherapy
Theoretically, there is a synergistic effect between chemotherapy and immunotherapy. Tumor cells are exposed to antigens after being attacked by chemotherapy drugs to induce an immune response, which can also reduce the inhibition of tumor cells in immunity.68,101 Therefore, whether immunotherapy plays an effective role in pMMR patients with the assistance of chemotherapy drugs has piqued scientists’ interest, but the results have been disappointing.
In the KEYNOTE-651 study (NCT03374254), pembrolizumab combined with mFOLFOX7 (cohort B) or FOLFIRI (cohort D) has been used as the first- or second-line treatment for advanced CRC, respectively. The results showed that one case in cohort B progressed, the PFS in cohort D was 17.4 months, and the ORR was 16%.114 The BACCI study (NCT02873195) included patients with standard treatment failure, and capecitabine plus bevacizumab and atezolizumab were used as the posterior-line treatment. The OS and PFS in the two groups were not significantly improved, but the ORR increased from 4.35% to 8.54%.85 The METIMMOX study adopted the FLOX protocol plus nivolumab group to compare to the FLOX protocol alone. The results showed that the median PFS in the combined group was 6.6 months and 5.6 months in the single FLOX group. These results were not encouraging.115 The AtezoTRIBE and MODUL studies were conducted to investigate the first-line treatment for unresectable advanced CRC. Atezolizumab plus bevacizumab combined with FOLFOXIRI or fluorouracil was used for treatment, indicating that its combination with FOLFOXIRI prolonged the PFS of patients with metastatic CRC. However, PFS and OS were not significantly improved by fluorouracil use.86,116
Combined Radiotherapy
Radiotherapy induces immunogenic cell death using radiation to enable T cell response with anti-tumor activity. This method can promote the release of tumor-related antigens. Many antigen-presenting cells enhance the presentation of tumor antigens, recruit more immune cells, change the tumor microenvironment, and improve the anti-tumor effect.117,118
A previous phase II trial suggested that the distant tumors in MSS mCRC patients treated with a combination of ipilimumab and nivolumab and radiotherapy were reduced in size, while an ORR of 12.5% was observed in patients. This study showed its potential feasibility.119 The VOLTAGE study showed that 30% of MSS patients achieved pCR after preoperative radiotherapy and chemotherapy followed by five cycles of nivolumab and surgical resection. Moreover, 60% of patients with MSI-H reached pCR.64 A multicenter phase II study AVANA (NCT03854799) found that 23% of patients reached pCR with preoperative radiotherapy and chemotherapy combined with avelumab, and 61.5% of them had major pathological reactions.120 The PANDORA study recruited 60 patients who had a clinical response percentage of 81.8%, while 23 patients (41.8%) experienced AEs related to the durvalumab treatment. This study showed a promising activity of neoadjuvant chemo-radiotherapy plus durvalumab in terms of pCR rate and safe toxicity profile.121 Other trials included AveRec (NCT03299660), INNATE (NCT04130854), and TARZAN (NCT04017455).122 This shows that immune combined chemotherapy can have a certain effect. The choice of radiotherapy mode (dose, segmentation mode, and treatment sequence) remains under investigation and more research is expected.
Combined Oncolytic Virus
Oncolytic viruses are natural or recombinant viruses that can selectively infect and kill cancer cells, but do not harm normal cells. Their principle is to selectively infect tumor cells via genetic modification of viruses with weak pathogenicity using the inactivation or defect of tumor suppressor gene in target cells to replicate in large numbers and destroy tumor cells.123 At the same time, they can also stimulate the immune response and attract more immune cells to continue to kill residual cancer cells.124,125 The combination of T-VEC and pembrolizumab, an oncolytic virus drug, in the treatment of melanoma showed an ORR of up to 62%, of which 33% of patients were in complete remission.126 A phase I/II study on the treatment of MSS mCRC with Pexa Vec combined with durvalumab, a tumor lytic virus drug, preliminarily demonstrated that it is well tolerated in the treatment of pMMR patients and showed a possible activity.127 In addition, a phase I b trial on the oncolytic virus drug talimogene laherparepvec combined with atezolizumab in the treatment of CRC patients with liver metastasis is presenting being investigated.
Combined Tumor Vaccine
Tumor vaccine development has become a research focus in recent years. Its principle is to introduce tumor antigens into patients in various forms, overcome the immunosuppressive state caused by tumors, enhance immunogenicity, activate patients’ own immune system, and induce cellular and humoral immune responses to control or eliminate tumors. In 2010, the FDA approved the first tumor vaccine sipuleucel-T for the treatment of prostate cancer.128 OncoVAX is under development in the field of CRC. A phase III randomized clinical study included 254 patients with phase II and III colon cancer. It demonstrated that the recurrence risk in the vaccine group patients was lower than that in the surgery group patients. Further research confirmed that OncoVAX had no significant effect on patients with stage III colon cancer. It can prolong the recurrence-free interval (RFI) in patients with stage II colon cancer, reduce the total recurrence risk, significantly prolong the recurrence-free survival period in the vaccine group patients, reduce the risk of recurrence or death, and improve the overall OS.129 A subsequent phase III clinical study on colon cancer found that the OncoVAX RFI was significantly delayed, and the 5-year OS and relapse-free survival rates were improved.130 PolyPEPI1018 is a ready-made polypeptide vaccine that contains 12 immunogenic epitopes derived from seven kinds of cancer-testis antigen (CTA) frequently expressed in CRC patients. The OBERTO study has evaluated the safety and effectiveness of PolyPEPI1018 vaccine in maintenance treatment. The results showed that all patients had no vaccine-related serious adverse reactions, and some patients experienced sustained clinical benefits.131 Further experiments confirmed that PolyPEPI1018 can effectively restore the immune response to CTA. The use of PolyPEPI1018 vaccine and maintenance therapy is safe and has demonstrated the early clinical activity of MSS mCRC tumors.132 Based on the above evidence, the application value of tumor vaccine in MSS type mCRC patients is worth studying.
Combined CAR-T Cell Therapy
CAR-T treatment involves collection and isolation of T cells from the patient’s blood, followed by gene modification to enhance their targeting and killing ability against cancer cells. After many T cells are cultured and expanded in vitro, they are imported into the patient’s body where they continue to reproduce, finally identifying cancer cells in vivo and destroying them.133–135
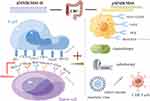
Carcinoembryonic antigen (CEA) is an important tumor marker in CRC. It is highly expressed in CRC cells and is a potential target for CRC treatment.136,137 In a phase I clinical trial on CEA, three mCRC patients received the CAR-T treatment, and their serum CEA levels decreased significantly. One patient had lung metastasis and liver metastasis, and all patients had severe colitis.138 Safety and effectiveness tests were also conducted in another experiment, where no obvious treatment-related toxic reactions were found.139 Three CUAD-101 dose levels were evaluated in the AlloSHRINK I study. The results showed that nine cases obtained a median PFS of 3.9 months. The treatment was well tolerated, and no treatment-related AEs above grade 3 occurred.140 Another study included 21 mCRC patients, where the ORR for dose level 1 (1x106 cells/kg) was 15.4% and 50% for dose level 2 (2x106 cells/kg). The most common AEs were cytokine release syndrome and diarrhea.141 The Immunological checkpoints and combination therapies are shown in Figure 1.
Future Perspectives
There is growing evidence that CRC is associated with altered gut microbiota. Specifically, the microbiota shapes the host’s immune system by modulating local and systemic immune responses, influencing the efficacy of cancer treatment. While many of the studies are still in their early stages, the results presented are strongly correlated. Therefore, in the future, scientists should combine various disciplines, such as microbiology and molecular biology, to establish a reasonable ecological model and promote the development of precision treatment to benefit patients.
More and more basic experiments and clinical studies suggest that immunosuppression has potential in the treatment of CRC.142 At present, the MMR or MSI status is still considered to be the most important distinguishing indicator. Further exploration of immune markers is necessary for more targeted clinical application of drugs. In addition, immune drug resistance and immune escape are the main obstacles to achieving effective immunotherapy. Even though there have been many related studies addressing this issue, it is difficult to control all aspects of the immunosuppression process due to its dynamic continuity. Future basic research studies with a strong foundation are needed to develop more accurate strategies.143 From traditional radiotherapy and chemotherapy to targeted therapy and immunotherapy, basic research studies have resulted in continuous progress in the area of clinical tumor treatment. More targets have been identified and more drugs have been explored.144,145 At the same time, combination therapy has also become more prominent. Strategies for adjusting the order and dosage of ICIs and combination drugs in various ways, achieving the best outcomes, and reducing the side effects while maintaining a safety profile still require a lot of research. It is expected that more optimized and accurate treatment techniques will be of benefit to CRC patients.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi:10.3322/caac.21708
2. Hoxha M, Zappacosta B. A review on the role of fatty acids in colorectal cancer progression. Front Pharmacol. 2022;13:1032806. doi:10.3389/fphar.2022.1032806
3. Peng X, Zhao G, Lin J, Li C. Interaction of mannose binding lectin and other pattern recognition receptors in human corneal epithelial cells during Aspergillus fumigatus infection. Int Immunopharmacol. 2018;63:161–169. doi:10.1016/j.intimp.2018.08.003
4. Zhang Q, Tang L, Zhou Y, He W, Li W. Immune checkpoint inhibitor-associated pneumonitis in non-small cell lung cancer: current understanding in characteristics, diagnosis, and management. Front Immunol. 2021;12:663986. doi:10.3389/fimmu.2021.663986
5. Lentz RW, Colton MD, Mitra SS, Messersmith WA. Innate immune checkpoint inhibitors: the next breakthrough in medical oncology? Mol Cancer Ther. 2021;20(6):961–974. doi:10.1158/1535-7163.MCT-21-0041
6. Dai W, Xu L, Yu X, et al. OGDHL silencing promotes hepatocellular carcinoma by reprogramming glutamine metabolism. J Hepatol. 2020;72(5):909–923. doi:10.1016/j.jhep.2019.12.015
7. Ghidini M, Fusco N, Salati M, et al. The emergence of immune-checkpoint inhibitors in colorectal cancer therapy. Curr Drug Targets. 2021;22(9):1021–1033. doi:10.2174/1389450122666210204204415
8. Ding S, Han L. Newborn screening for genetic disorders: current status and prospects for the future. Pediatr Investig. 2022;6(4):291–298. doi:10.1002/ped4.12343
9. Zheng Y, Li Y, Feng J, et al. Cellular based immunotherapy for primary liver cancer. J Exp Clin Cancer Res. 2021;40(1):250. doi:10.1186/s13046-021-02030-5
10. Xu YP, Zhou YQ, Zhao YJ, et al. High level of CD73 predicts poor prognosis of intrahepatic cholangiocarcinoma. J Cancer. 2021;12(15):4655–4660. doi:10.7150/jca.51038
11. Amodio V, Lamba S, Chila R, et al. Genetic and pharmacological modulation of DNA mismatch repair heterogeneous tumors promotes immune surveillance. Cancer Cell. 2022;41:196–209.e5. doi:10.1016/j.ccell.2022.12.003
12. Caglayan M, Wilson SH. Pol mu dGTP mismatch insertion opposite T coupled with ligation reveals promutagenic DNA repair intermediate. Nat Commun. 2018;9(1):4213. doi:10.1038/s41467-018-06700-5
13. Baretti M, Le DT. DNA mismatch repair in cancer. Pharmacol Ther. 2018;189:45–62. doi:10.1016/j.pharmthera.2018.04.004
14. Jiricny J. The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol. 2006;7(5):335–346. doi:10.1038/nrm1907
15. Olave MC, Graham RP. Mismatch repair deficiency: the what, how and why it is important. Genes Chromosomes Cancer. 2022;61(6):314–321. doi:10.1002/gcc.23015
16. Gemelli M, Cortinovis DL, Baggi A, et al. Immune checkpoint inhibitors in malignant pleural mesothelioma: a systematic review and meta-analysis. Cancers. 2022;14(24):6063. doi:10.3390/cancers14246063
17. Ren B, Shen J, Qian Y, Zhou T. Sarcopenia as a determinant of the efficacy of immune checkpoint inhibitors in non-small cell lung cancer: a meta-analysis. Nutr Cancer. 2022;1–11. doi:10.1080/01635581.2022.2153879
18. Jacoberger-Foissac C, Allard B, Allard D, Stagg J. Assessing the efficacy of immune checkpoint inhibitors in preclinical tumor models. Methods Mol Biol. 2023;2614:151–169. doi:10.1007/978-1-0716-2914-7_11
19. Waissengrin B, Leshem Y, Taya M, et al. The use of medical cannabis concomitantly with immune checkpoint inhibitors in non-small cell lung cancer: a sigh of relief? Eur J Cancer. 2022;180:52–61. doi:10.1016/j.ejca.2022.11.022
20. Roccuzzo G, Giordano S, Fava P, et al. Immune check point inhibitors in primary cutaneous T-cell lymphomas: biologic rationale, clinical results and future perspectives. Front Oncol. 2021;11:733770. doi:10.3389/fonc.2021.733770
21. Hernaez R, Avila MA. Immunogenomic classification of hepatocellular carcinoma patients for immune check-point inhibitors therapy: cui bono? Gut. 2022. doi:10.1136/gutjnl-2022-327132
22. Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–3895. doi:10.1002/j.1460-2075.1992.tb05481.x
23. Makaremi S, Asadzadeh Z, Hemmat N, et al. Immune checkpoint inhibitors in colorectal cancer: challenges and future prospects. Biomedicines. 2021;9(9):1075. doi:10.3390/biomedicines9091075
24. Gessani S, Belardelli F. Immune dysfunctions and immunotherapy in colorectal cancer: the role of dendritic cells. Cancers. 2019;11(10):1491. doi:10.3390/cancers11101491
25. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. doi:10.1126/science.aan6733
26. Diaz LA
27. Ott PA, Piha-Paul SA, Munster P, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with recurrent carcinoma of the anal canal. Ann Oncol. 2017;28(5):1036–1041. doi:10.1093/annonc/mdx029
28. O’Neil BH, Wallmark JM, Lorente D, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS One. 2017;12(12):e0189848. doi:10.1371/journal.pone.0189848
29. Le DT, Kim TW, Van Cutsem E, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol. 2020;38(1):11–19. doi:10.1200/JCO.19.02107
30. Benson AB, Venook AP, Al-Hawary MM, et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(3):329–359. doi:10.6004/jnccn.2021.0012
31. Andre T, Shiu KK, Kim TW, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383(23):2207–2218. doi:10.1056/NEJMoa2017699
32. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182–1191. doi:10.1016/S1470-2045(17)30422-9
33. Oaknin A, Tinker AV, Gilbert L, et al. Clinical activity and safety of the anti-programmed death 1 monoclonal antibody dostarlimab for patients with recurrent or advanced mismatch repair-deficient endometrial cancer: a nonrandomized phase 1 clinical trial. JAMA Oncol. 2020;6(11):1766–1772. doi:10.1001/jamaoncol.2020.4515
34. Andre TBD, Curigliano G, Curigliano G. Safety and efficacy of anti–PD-1 antibody dostarlimab in patients (pts) with mismatch repair-deficient (dMMR) solid cancers: results from GARNET study. J Clin Oncol. 2021;39(suppl3):9. doi:10.1200/JCO.2021.39.3_suppl.9
35. Bhamidipati D, Raghav KPS, Morris VK, et al. Prognostic role of systemic inflammatory markers in patients with metastatic MSI-h/dMMR colorectal cancer receiving immunotherapy. J Clin Oncol. 2022;40(16_suppl):3524. doi:10.1200/JCO.2022.40.16_suppl.3524
36. Golstein P. Cytolytic T-cell melodrama. Nature. 1987;327(6117):12. doi:10.1038/327012a0
37. Mak TW. Gaining insights into the ontogeny and activation of T cells through the use of gene-targeted mutant mice. J Inflamm. 1995;45(2):79–84.
38. Sobhani N, Tardiel-Cyril DR, Davtyan A, Generali D, Roudi R, Li Y. CTLA-4 in regulatory T cells for cancer immunotherapy. Cancers. 2021;13(6):1440. doi:10.3390/cancers13061440
39. Michelson DA, Benoist C, Mathis D. CTLA-4 on thymic epithelial cells complements Aire for T cell central tolerance. Proc Natl Acad Sci U S A. 2022;119(48):e2215474119. doi:10.1073/pnas.2215474119
40. Janman D, Hinze C, Kennedy A, et al. Regulation of CTLA-4 recycling by LRBA and Rab11. Immunology. 2021;164(1):106–119. doi:10.1111/imm.13343
41. Horzum U, Yanik H, Taskiran EZ, Esendagli G. Effector Th1 cells under PD-1 and CTLA-4 checkpoint blockade abrogate the upregulation of multiple inhibitory receptors and by-pass exhaustion. Immunology. 2022;167(4):640–650. doi:10.1111/imm.13560
42. Fox TA, Houghton BC, Petersone L, et al. Therapeutic gene editing of T cells to correct CTLA-4 insufficiency. Sci Transl Med. 2022;14(668):eabn5811. doi:10.1126/scitranslmed.abn5811
43. Berezhnoy A, Sumrow BJ, Stahl K, et al. Development and preliminary clinical activity of PD-1-guided CTLA-4 blocking bispecific DART molecule. Cell Rep Med. 2020;1(9):100163. doi:10.1016/j.xcrm.2020.100163
44. Andrews LP, Yano H, Vignali DAA. Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: breakthroughs or backups. Nat Immunol. 2019;20(11):1425–1434. doi:10.1038/s41590-019-0512-0
45. Abunasser AAA, Xue J, Balawi EJA, Zhu Y. Combination of the EP and anti-PD-1 pathway or anti-CTLA-4 for the phase III trial of small-cell lung cancer: a meta-analysis. J Oncol. 2021;2021:6662344. doi:10.1155/2021/6662344
46. Bredin P, Naidoo J. The gut microbiome, immune check point inhibition and immune-related adverse events in non-small cell lung cancer. Cancer Metastasis Rev. 2022;41(2):347–366. doi:10.1007/s10555-022-10039-1
47. Zhang R, Peng X, Lin J, et al. The role of SREC-I in innate immunity to aspergillus fumigatus keratitis. Invest Ophthalmol Vis Sci. 2021;62(9):12. doi:10.1167/iovs.62.9.12
48. Peng XD, Zhao GQ, Lin J, et al. Fungus induces the release of IL-8 in human corneal epithelial cells, via Dectin-1-mediated protein kinase C pathways. Int J Ophthalmol. 2015;8(3):441–447. doi:10.3980/j.issn.2222-3959.2015.03.02
49. Garralda E, Sukari A, Lakhani NJ, et al. A phase 1 first-in-human study of the anti-LAG-3 antibody MK4280 (favezelimab) plus pembrolizumab in previously treated, advanced microsatellite stable colorectal cancer. J Clin Oncol. 2021;39(15_suppl):3584. doi:10.1200/JCO.2021.39.15_suppl.3584
50. Huang S, Zhao Y, Liao P, et al. Different expression patterns of Vista concurrent with PD-1, Tim-3, and TIGIT on T cell subsets in peripheral blood and bone marrow from patients with multiple myeloma. Front Oncol. 2022;12:1014904. doi:10.3389/fonc.2022.1014904
51. Chen Y, Zhang Y, Wang B, et al. Blood clot scaffold loaded with liposome vaccine and siRNAs targeting PD-L1 and TIM-3 for effective DC activation and cancer immunotherapy. ACS Nano. 2022. doi:10.1021/acsnano.2c10797
52. Brauneck F, Fischer B, Witt M, et al. TIGIT blockade repolarizes AML-associated TIGIT(+) M2 macrophages to an M1 phenotype and increases CD47-mediated phagocytosis. J Immunother Cancer. 2022;10(12). doi:10.1136/jitc-2022-004794
53. Wiede F, Lu KH, Du X, et al. PTP1B is an intracellular checkpoint that limits T-cell and CAR T-cell antitumor immunity. Cancer Discov. 2022;12(3):752–773. doi:10.1158/2159-8290.CD-21-0694
54. Geuijen C, Tacken P, Wang LC, et al. A human CD137xPD-L1 bispecific antibody promotes anti-tumor immunity via context-dependent T cell costimulation and checkpoint blockade. Nat Commun. 2021;12(1):4445. doi:10.1038/s41467-021-24767-5
55. Keck JG, He W, Buetow BS, et al. Validation of a clinically relevant humanized mouse model for the safety assessment of 4-1BB agonists utomilumab and urelumab. J Clin Oncol. 2022;40(16_suppl):e14602–e14602. doi:10.1200/JCO.2022.40.16_suppl.e14602
56. Crupi MJF, Taha Z, Janssen TJA, et al. Oncolytic virus driven T-cell-based combination immunotherapy platform for colorectal cancer. Front Immunol. 2022;13:1029269. doi:10.3389/fimmu.2022.1029269
57. Abedi Kiasari B, Abbasi A, Ghasemi Darestani N, et al. Combination therapy with nivolumab (anti-PD-1 monoclonal antibody): a new era in tumor immunotherapy. Int Immunopharmacol. 2022;113(PtA):109365. doi:10.1016/j.intimp.2022.109365
58. Antonia S, Goldberg SB, Balmanoukian A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17(3):299–308. doi:10.1016/S1470-2045(15)00544-6
59. Lenz HJ, Van Cutsem E, Luisa Limon M, et al. First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the phase II checkmate 142 study. J Clin Oncol. 2022;40(2):161–170. doi:10.1200/JCO.21.01015
60. Abdullaev S, André T, Lei M, et al. A phase III study of nivolumab (NIVO), NIVO + ipilimumab (IPI), or chemotherapy (CT) for microsatellite instability-high (MSI-H)/mismatch repair-deficient (dMMR) metastatic colorectal cancer (mCRC): checkmate 8HW. J Clin Oncol. 2020;38(4_suppl):TPS266–TPS266. doi:10.1200/JCO.2020.38.4_suppl.TPS266
61. Sinicrope FA, Ou F-S, Zemla T, et al. Randomized trial of standard chemotherapy alone or combined with atezolizumab as adjuvant therapy for patients with stage III colon cancer and deficient mismatch repair (ATOMIC, Alliance A021502). J Clin Oncol. 2019;37(15_suppl):e15169–e15169. doi:10.1200/JCO.2019.37.15_suppl.e15169
62. Lau D, Cunningham D, Gillbanks A, et al. POLEM: avelumab plus fluoropyrimidine-based chemotherapy as adjuvant treatment for stage III dMMR or POLE exonuclease domain mutant colon cancer—A phase III randomized study. J Clin Oncol. 2019;37(15_suppl):TPS3615–TPS3615. doi:10.1200/JCO.2019.37.15_suppl.TPS3615
63. Chalabi M, Fanchi LF, Dijkstra KK, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 2020;26(4):566–576. doi:10.1038/s41591-020-0805-8
64. Yuki S, Bando H, Tsukada Y, et al. Short-term results of VOLTAGE-A: nivolumab monotherapy and subsequent radical surgery following preoperative chemoradiotherapy in patients with microsatellite stable and microsatellite instability-high locally advanced rectal cancer. J Clin Oncol. 2020;38(15_suppl):4100. doi:10.1200/JCO.2020.38.15_suppl.4100
65. Cercek A, Lumish M, Sinopoli J, et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med. 2022;386(25):2363–2376. doi:10.1056/NEJMoa2201445
66. Verschoor YL, Berg JVD, Beets G, et al. Neoadjuvant nivolumab, ipilimumab, and celecoxib in MMR-proficient and MMR-deficient colon cancers: final clinical analysis of the NICHE study. J Clin Oncol. 2022;40(16_suppl):3511. doi:10.1200/JCO.2022.40.16_suppl.3511
67. Chen L, Jiang X, Li Y, et al. How to overcome tumor resistance to anti-PD-1/PD-L1 therapy by immunotherapy modifying the tumor microenvironment in MSS CRC. Clin Immunol. 2022;237:108962. doi:10.1016/j.clim.2022.108962
68. Moretto R, Elliott A, Zhang J, et al. Homologous recombination deficiency alterations in colorectal cancer: clinical, molecular, and prognostic implications. J Natl Cancer Inst. 2022;114(2):271–279. doi:10.1093/jnci/djab169
69. Wang D, Zhang H, Xiang T, Wang G. Clinical application of adaptive immune therapy in MSS colorectal cancer patients. Front Immunol. 2021;12:762341. doi:10.3389/fimmu.2021.762341
70. Baraibar I, Mirallas O, Saoudi N, et al. Combined treatment with immunotherapy-based strategies for MSS Metastatic Colorectal Cancer. Cancers. 2021;13(24):6311. doi:10.3390/cancers13246311
71. Ganesh K, Stadler ZK, Cercek A, et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16(6):361–375. doi:10.1038/s41575-019-0126-x
72. Borelli B, Antoniotti C, Carullo M, Germani MM, Conca V, Masi G. Immune-Checkpoint Inhibitors (ICIs) in Metastatic Colorectal Cancer (mCRC) patients beyond microsatellite instability. Cancers. 2022;14(20):4974. doi:10.3390/cancers14204974
73. Li Y, Ma Y, Wu Z, et al. Tumor mutational burden predicting the efficacy of immune checkpoint inhibitors in colorectal cancer: a systematic review and meta-analysis. Front Immunol. 2021;12:751407. doi:10.3389/fimmu.2021.751407
74. Huang K, Lin B, Liu J, et al. Predicting colorectal cancer tumor mutational burden from histopatholog ical images and clinical information using multi-modal deep learning. Bioinformatics. 2022;38(22):5108–5115. doi:10.1093/bioinformatics/btac641
75. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15(5):325–340. doi:10.1038/nrclinonc.2018.29
76. Kim CW, Chon HJ, Kim C. Combination immunotherapies to overcome intrinsic resistance to checkpoint blockade in microsatellite stable colorectal cancer. Cancers. 2021;13(19):4906. doi:10.3390/cancers13194906
77. Fukuoka S, Hara H, Takahashi N, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38(18):2053–2061. doi:10.1200/JCO.19.03296
78. Li J, Cong L, Liu J, et al. The efficacy and safety of regorafenib in combination with anti-PD-1 antibody in refractory microsatellite stable metastatic colorectal cancer: a retrospective study. Front Oncol. 2020;10:594125. doi:10.3389/fonc.2020.594125
79. Cousin S, Cantarel C, Guegan JP, et al. Regorafenib-avelumab combination in patients with biliary tract cancer (REGOMUNE): a single-arm, open-label, phase II trial. Eur J Cancer. 2022;162:161–169. doi:10.1016/j.ejca.2021.11.012
80. Cousin S, Cantarel C, Guegan JP, et al. Regorafenib-avelumab combination in patients with microsatellite stable colorectal cancer (REGOMUNE): a single-arm, open-label, phase II trial. Clin Cancer Res. 2021;27(8):2139–2147. doi:10.1158/1078-0432.CCR-20-3416
81. Wang F, He MM, Yao YC, et al. Regorafenib plus toripalimab in patients with metastatic colorectal cancer: a phase Ib/II clinical trial and gut microbiome analysis. Cell Rep Med. 2021;2(9):100383. doi:10.1016/j.xcrm.2021.100383
82. Cousin S, Bellera CA, Guégan JP, et al. REGOMUNE: a phase II study of regorafenib plus avelumab in solid tumors—Results of the non-MSI-H metastatic colorectal cancer (mCRC) cohort. J Clin Oncol. 2020;38(15_suppl):4019. doi:10.1200/JCO.2020.38.15_suppl.4019
83. Akin Telli T, Bregni G, Vanhooren M, Saude Conde R, Hendlisz A, Sclafani F. Regorafenib in combination with immune checkpoint inhibitors for mismatch repair proficient (pMMR)/microsatellite stable (MSS) colorectal cancer. Cancer Treat Rev. 2022;110:102460. doi:10.1016/j.ctrv.2022.102460
84. Gomez⁃Roca C, Yanez Ruiz E, Im S. LEAP-005: aphase 2 multicohort study of lenvatinib plus pembrolizumab in patients with previously treated selected solid tumors—results from the colorectal cancer cohort. J Clin Oncol. 2021;39(Suppl15):abstr3564. doi:10.1200/JCO.2021.39.15_suppl.3564
85. Mettu NB, Twohy E, Ou FS, et al. BACCI: a phase II randomized, double-blind, multicenter, placebo-controlled study of capecitabine (C) bevacizumab (B) plus atezolizumab (A) or placebo (P) in refractory metastatic colorectal cancer (mCRC): an ACCRU network study - ScienceDirect. Annals Oncol. 2019;30:v203.
86. Cremolini C, Rossini D, Antoniotti C. FOLFOXIRIplus bevacizumab(Bev) plus atezolizumab(Atezo) versus FOLFOXIRI plus bev as first-line treatment of unresectable metastatic colorectal cancer(mCRC) patients: results of the phase II randomized AtezoTRIBE study by GONO. Ann Oncol. 2021;32(Suppl 5):S1283–S1346. doi:10.1016/j.annonc.2021.08.2094
87. Zhou LN, Feng CX, Zhang Y, et al. The bevacizumab plus oxaliplatin-based chemotherapy regimen is more suitable for metastatic colorectal cancer patients with a history of schistosomiasis: a clinical retrospective analysis. J Gastrointest Oncol. 2022;13(3):1086–1096. doi:10.21037/jgo-22-207
88. Wang F, Dai G, Deng Y, et al. Efficacy and safety of chemotherapy combined with bevacizumab in Chinese patients with metastatic colorectal cancer: a prospective, multicenter, observational, non-interventional Phase IV trial. Chin J Cancer Res. 2021;33(4):490–499. doi:10.21147/j.issn.1000-9604.2021.04.06
89. Dell’Aquila E, Rossini D, Fulgenzi CAM, et al. Bone metastases are associated with worse prognosis in patients affected by metastatic colorectal cancer treated with doublet or triplet chemotherapy plus bevacizumab: a subanalysis of the TRIBE and TRIBE2 trials. ESMO Open. 2022;7(6):100606. doi:10.1016/j.esmoop.2022.100606
90. Guo Y, Zhang W, Ying J, Zhang Y, Li J. Preliminary results of a phase 1b study of fruquintinib plus sintilimab in advanced colorectal cancer. J Clin Oncol. 2021;39(15_suppl):2514. doi:10.1200/JCO.2021.39.15_suppl.2514
91. Bai Y, Xu N, An S, Chen W, Gao C, Zhang D. A phase ib trial of assessing the safety and preliminary efficacy of a combination therapy of geptanolimab (GB 226) plus fruquintinib in patients with metastatic colorectal cancer (mCRC). J Clin Oncol. 2021;39(15_suppl):e15551–e15551. doi:10.1200/JCO.2021.39.15_suppl.e15551
92. Sturrock M, Miller IS, Kang G, et al. Anti-angiogenic drug scheduling optimisation with application to colorectal cancer. Sci Rep. 2018;8(1):11182. doi:10.1038/s41598-018-29318-5
93. Lai E, Cascinu S, Scartozzi M. Are all anti-angiogenic drugs the same in the treatment of second-line metastatic colorectal cancer? Expert opinion on clinical practice. Front Oncol. 2021;11:637823. doi:10.3389/fonc.2021.637823
94. Cao M, Wang Y, Lu G, et al. Classical angiogenic signaling pathways and novel anti-angiogenic strategies for colorectal cancer. Curr Issues Mol Biol. 2022;44(10):4447–4471. doi:10.3390/cimb44100305
95. Doleschal B, Petzer A, Rumpold H. Current concepts of anti-EGFR targeting in metastatic colorectal cancer. Front Oncol. 2022;12:1048166. doi:10.3389/fonc.2022.1048166
96. Chu J, Fang X, Sun Z, et al. Non-coding RNAs regulate the resistance to anti-EGFR therapy in colorectal cancer. Front Oncol. 2021;11:801319. doi:10.3389/fonc.2021.801319
97. Park YL, Kim HP, Ock CY, et al. EMT-mediated regulation of CXCL1/5 for resistance to anti-EGFR therapy in colorectal cancer. Oncogene. 2022;41(14):2026–2038. doi:10.1038/s41388-021-01920-4
98. Janani B, Vijayakumar M, Priya K, et al. EGFR-based targeted therapy for colorectal cancer-promises and challenges. Vaccines. 2022;10(4). doi:10.3390/vaccines10040499
99. Zhang W, Han X, Yang L, et al. Safety, pharmacokinetics and efficacy of SCT200, an anti-EGFR monoclonal antibody in patients with wild-type KRAS/NRAS/BRAF metastatic colorectal cancer: a phase I dose-escalation and dose-expansion study. BMC Cancer. 2022;22(1):1104. doi:10.1186/s12885-022-10147-9
100. Lee MS, Loehrer PJ, Imanirad I, et al. Phase II study of ipilimumab, nivolumab, and panitumumab in patients with KRAS/NRAS/BRAF wild-type (WT) microsatellite stable (MSS) metastatic colorectal cancer (mCRC). J Clin Oncol. 2021;39(3_suppl):7. doi:10.1200/JCO.2021.39.3_suppl.7
101. Stein A, Binder M, Goekkurt E, et al. Avelumab and cetuximab in combination with FOLFOX in patients with previously untreated metastatic colorectal cancer (MCRC): final results of the phase II AVETUX trial (AIO-KRK-0216). J Clin Oncol. 2020;38(4_suppl):96. doi:10.1200/JCO.2020.38.4_suppl.96
102. Boland PM, Hutson A, Maguire O, Minderman H, Fountzilas C, Iyer RV. A phase Ib/II study of cetuximab and pembrolizumab in RAS-wt mCRC. J Clin Oncol. 2018;36(4_suppl):834. doi:10.1200/JCO.2018.36.4_suppl.834
103. Cooper ZA, Reuben A, Austin-Breneman J, Wargo JA. Does it MEK a difference? Understanding immune effects of targeted therapy. Clin Cancer Res. 2015;21(14):3102–3104. doi:10.1158/1078-0432.CCR-15-0363
104. Goldman C, Tchack J, Robinson EM, et al. Outcomes in melanoma patients treated with BRAF/MEK-directed therapy or immune checkpoint inhibition stratified by clinical trial versus standard of care. Oncology. 2017;93(3):164–176. doi:10.1159/000475715
105. Buhle A, Johnson N, Grider D, Phillips M. Early onset drug-induced hypersensitivity syndrome with lymphopenia, hepatitis, and normal eosinophils induced by BRAF/MEK inhibitor after immune checkpoint inhibitor therapy. Dermatol Online J. 2022;28(1). doi:10.5070/D328157062
106. Schröder C, Lawrance M, Li C, et al. Building external control arms from patient-level electronic health record data to replicate the randomized IMblaze370 control arm in metastatic colorectal cancer. JCO Clin Cancer Infor. 2021;(5):450–458. doi:10.1200/cci.20.00149
107. Schuch G, Kobold S, Bokemeyer C. Evolving role of cetuximab in the treatment of colorectal cancer. Cancer Manag Res. 2009;1:79–88. doi:10.2147/CMAR.S4750
108. Bendell JC, Kopetz S, Middleton MR, et al. Phase 1b/2 study of binimetinib (BINI) in combination with nivolumab (NIVO) or NIVO plus ipilimumab (IPI) in patients (pts) with previously treated microsatellite-stable (MSS) metastatic colorectal cancer (mCRC) with RAS mutation. J Clin Oncol. 2018;36(4_suppl):TPS870–TPS870. doi:10.1200/JCO.2018.36.4_suppl.TPS870
109. Ou SI, Janne PA, Leal TA, et al. First-in-human phase I/IB dose-finding study of adagrasib (MRTX849) in patients with advanced KRAS(G12C) solid tumors (KRYSTAL-1). J Clin Oncol. 2022;40(23):2530–2538. doi:10.1200/JCO.21.02752
110. Corcoran R, Giannakis M, Allen J. SO-26 Clinical efficacy of combined BRAF, MEK, and PD −1 inhibition in BRAFV600E colorectal cancer patients. Ann Oncol. 2020;31:S226–S227. doi:10.1016/j.annonc.2020.04.041
111. Xu T, Wang X, Xin Y, et al. Trastuzumab combined with irinotecan in patients with HER2-positive metastatic colorectal cancer: a phase II single-arm study and exploratory biomarker analysis. Cancer Res Treat. 2022;55:626–635. doi:10.4143/crt.2022.1058
112. Strickler JH, Ng K, Cercek A, et al. MOUNTAINEER: open-label, phase II study of tucatinib combined with trastuzumab for HER2-positive metastatic colorectal cancer (SGNTUC-017, trial in progress). J Clin Oncol. 2021;39(3_suppl):TPS153–TPS153. doi:10.1200/JCO.2021.39.3_suppl.TPS153
113. Suh K, Carlson JJ, Xia F, Williamson TE, Sullivan SD. The potential long-term comparative effectiveness of larotrectinib versus entrectinib for treatment of metastatic TRK fusion colorectal cancer. J Clin Oncol. 2022;40(4_suppl):40. doi:10.1200/JCO.2022.40.4_suppl.040
114. Yamada N, Karasawa T, Wakiya T, et al. Iron overload as a risk factor for hepatic ischemia-reperfusion injury in liver transplantation: potential role of ferroptosis. Am J Transplant. 2020;20(6):1606–1618. doi:10.1111/ajt.15773
115. Anne H, Harme H, Kersten C. Repeat sequential oxaliplatin⁃based chemotherapy (FLOX) and nivolumab versus FLOX alone as first⁃line treatment of microsatellite⁃stable (MSS) metastatic colorectal cancer (mCRC): initial results from the randomized METIMMOX study. J Clin Oncol. 2021;39(Suppl15):abstr3556. doi:10.1200/JCO.2021.39.15_suppl.3556
116. Tabernero J, Grothey A, Arnold D. Exploratory biomarker findings from cohort 2 of MODUL: An adaptable, phase 2, signal⁃seeking trial of fluoropyrimidine + bevacizumab ± atezolizumab maintenance therapy for BRAFwt metastatic colorectal cancer. J Clin Oncol. 2021;39(Suppl 15):S3570. doi:10.1200/JCO.2021.39.15_suppl.3570
117. Xue M, Tian Y, Sui Y, et al. Protective effect of fucoidan against iron overload and ferroptosis-induced liver injury in rats exposed to alcohol. Bio Pharmaco. 2022;153:113402. doi:10.1016/j.biopha.2022.113402
118. Voronova V, Vislobokova A, Mutig K, et al. Combination of immune checkpoint inhibitors with radiation therapy in cancer: a hammer breaking the wall of resistance. Front Oncol. 2022;12:1035884. doi:10.3389/fonc.2022.1035884
119. Parikh AR, Clark JW, Wo JY-L, et al. A phase II study of ipilimumab and nivolumab with radiation in microsatellite stable (MSS) metastatic colorectal adenocarcinoma (mCRC). J Clin Oncol. 2019;37(15_suppl):3514. doi:10.1200/JCO.2019.37.15_suppl.3514
120. Salvatore L, Bensi M, Corallo S, et al. Phase II study of preoperative (PREOP) chemoradiotherapy (CTRT) plus avelumab (AVE) in patients (PTS) with locally advanced rectal cancer (LARC): the AVANA study. J Clin Oncol. 2021;39(15_suppl):3511. doi:10.1200/JCO.2021.39.15_suppl.3511
121. Tamberi S, Grassi E, Zingaretti C, et al. A phase II study of capecitabine plus concomitant radiation therapy followed by durvalumab (MEDI4736) as preoperative treatment in rectal cancer: PANDORA study final results. J Clin Oncol. 2022;40(17_suppl):LBA3513–LBA3513. doi:10.1200/JCO.2022.40.17_suppl.LBA3513
122. Michael M, Wong R, Gill SS, et al. Phase II trial PD-L1/PD-1 blockade avelumab with chemoradiotherapy for locally advanced resectable T3B-4/N1-2 rectal cancer: the Ave-Rec trial. J Clin Oncol. 2019;37(15_suppl):TPS3622–TPS3622. doi:10.1200/JCO.2019.37.15_suppl.TPS3622
123. Wu Q, Hu X, Zhang X, et al. Single-cell transcriptomics of peripheral blood reveals anti-tumor systemic immunity induced by oncolytic virotherapy. Theranostics. 2022;12(17):7371–7389. doi:10.7150/thno.74075
124. Nelson A, Gebremeskel S, Lichty BD, Johnston B. Natural killer T cell immunotherapy combined with IL-15-expressing oncolytic virotherapy and PD-1 blockade mediates pancreatic tumor regression. J Immunother Cancer. 2022;10(3). doi:10.1136/jitc-2021-003923
125. Svensson-Arvelund J, Cuadrado-Castano S, Pantsulaia G, et al. Expanding cross-presenting dendritic cells enhances oncolytic virotherapy and is critical for long-term anti-tumor immunity. Nat Commun. 2022;13(1):7149. doi:10.1038/s41467-022-34791-8
126. Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2018;174(4):1031–1032. doi:10.1016/j.cell.2018.07.035
127. Monge B MC, Xie C, Steinberg SM, et al. A phase I/II study of Pexa-Vec oncolytic virus in combination with immune checkpoint inhibition in refractory colorectal cancer. J Clin Oncol. 2020;38(4_suppl):117. doi:10.1200/JCO.2020.38.4_suppl.117
128. Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17(11):3520–3526. doi:10.1158/1078-0432.CCR-10-3126
129. de Weger VA, Turksma AW, Voorham QJ, et al. Clinical effects of adjuvant active specific immunotherapy differ between patients with microsatellite-stable and microsatellite-instable colon cancer. Clin Cancer Res. 2012;18(3):882–889. doi:10.1158/1078-0432.CCR-11-1716
130. Uyl-de Groot CA, Vermorken JB, Hanna MG Jr, et al. Immunotherapy with autologous tumor cell-BCG vaccine in patients with colon cancer: a prospective study of medical and economic benefits. Vaccine. 2005;23(17–18):2379–2387. doi:10.1016/j.vaccine.2005.01.015
131. Hubbard JM, Cremolini C, Graham RP, et al. A phase I study of PolyPEPI1018 vaccine plus maintenance therapy in patients with metastatic colorectal cancer with a predictive biomarker (OBERTO). J Clin Oncol. 2019;37(15_suppl):3557. doi:10.1200/JCO.2019.37.15_suppl.3557
132. Hubbard JM, Cremolini C, Graham RP, et al. Evaluation of safety, immunogenicity, and preliminary efficacy of PolyPEPI1018 off-the-shelf vaccine with fluoropyrimidine/bevacizumab maintenance therapy in metastatic colorectal cancer (mCRC) patients. J Clin Oncol. 2020;38(15_suppl):4048. doi:10.1200/JCO.2020.38.15_suppl.4048
133. O’Leary K. CAR T cells beyond cancer. Nat Med. 2022;28(12):2450. doi:10.1038/s41591-022-02150-1
134. Bailey SR, Berger TR, Graham C, Larson RC, Maus MV. Four challenges to CAR T cells breaking the glass ceiling. Eur J Immunol. 2022;e2250039. doi:10.1002/eji.202250039
135. Ghazi B, El Ghanmi A, Kandoussi S, Ghouzlani A, Badou A. CAR T-cells for colorectal cancer immunotherapy: ready to go? Front Immunol. 2022;13:978195. doi:10.3389/fimmu.2022.978195
136. Wang L, Zhang G, Shen J, Shen Y, Cai G, Liu Y. Elevated CEA and CA 19-9 levels within the normal ranges increase the likelihood of CRC recurrence in the Chinese han population. Appl Bionics Biomech. 2022;2022:8666724. doi:10.1155/2022/8666724
137. Koyel B, Priyabrata D, Rittwika B, et al. Deterministic role of CEA and MSI status in predicting outcome of CRC patients: a perspective study amongst hospital attending Eastern Indian Populations. Indian J Surg Oncol. 2017;8(4):462–468. doi:10.1007/s13193-017-0651-4
138. Parkhurst MR, Yang JC, Langan RC, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19(3):620–626. doi:10.1038/mt.2010.272
139. Katz SC, Burga RA, McCormack E, et al. Phase I hepatic immunotherapy for metastases study of intra-arterial chimeric antigen receptor-modified T-cell therapy for CEA+ liver metastases. Clin Cancer Res. 2015;21(14):3149–3159. doi:10.1158/1078-0432.CCR-14-1421
140. Prenen H, Dekervel J, Hendlisz A, et al. Updated data from alloSHRINK phase I first-in-human study evaluating CYAD-101, an innovative non-gene edited allogeneic CAR-T in mCRC. J Clin Oncol. 2021;39(3_suppl):74. doi:10.1200/JCO.2021.39.3_suppl.74
141. Cui J, Chen N, Pu C, et al. A phase 1 dose-escalation study of GCC19 CART a novel coupled CAR therapy for subjects with metastatic colorectal cancer. J Clin Oncol. 2022;40(16_suppl):3582. doi:10.1200/JCO.2022.40.16_suppl.3582
142. Morse MA, Hochster H, Benson A. Perspectives on treatment of metastatic colorectal cancer with immune checkpoint inhibitor therapy. Oncologist. 2020;25(1):33–45. doi:10.1634/theoncologist.2019-0176
143. Peng X, Zhao G, Lin J, Qu J, Zhang Y, Li C. Phospholipase Cgamma2 is critical for Ca(2+) flux and cytokine production in anti-fungal innate immunity of human corneal epithelial cells. BMC Ophthalmol. 2018;18(1):170. doi:10.1186/s12886-018-0847-6
144. Dai W, Li Y, Sun W, et al. Silencing of OGDHL promotes liver cancer metastasis by enhancing hypoxia inducible factor 1 alpha protein stability. Cancer Sci. 2022. doi:10.1111/cas.15540
145. Yorita N, Yuge R, Takigawa H, et al. Stromal reaction inhibitor and immune-checkpoint inhibitor combination therapy attenuates excluded-type colorectal cancer in a mouse model. Cancer Lett. 2021;498:111–120. doi:10.1016/j.canlet.2020.10.041
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.