Back to Journals » Journal of Inflammation Research » Volume 17
Immune Cell-Derived Exosomes in Inflammatory Disease and Inflammatory Tumor Microenvironment: A Review
Authors Zhang R, Li M, Li H, Ran X, Jin F, Tan Q, Chen Z
Received 5 July 2023
Accepted for publication 18 October 2023
Published 17 January 2024 Volume 2024:17 Pages 301—312
DOI https://doi.org/10.2147/JIR.S421649
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Runmin Zhang, Muzhe Li, Huiyun Li, Xun Ran, Fengtian Jin, Qingshan Tan, Zhiwei Chen
Department of Orthopaedics, The First Affiliated Hospital of University of South China, Hengyang, People’s Republic of China
Correspondence: Zhiwei Chen, Department of Orthopaedics, The First Affiliated Hospital of University of South China, 69| Chuanshan Road, Hengyang, Hunan, People’s Republic of China, Tel +86-13973409923, Email [email protected]
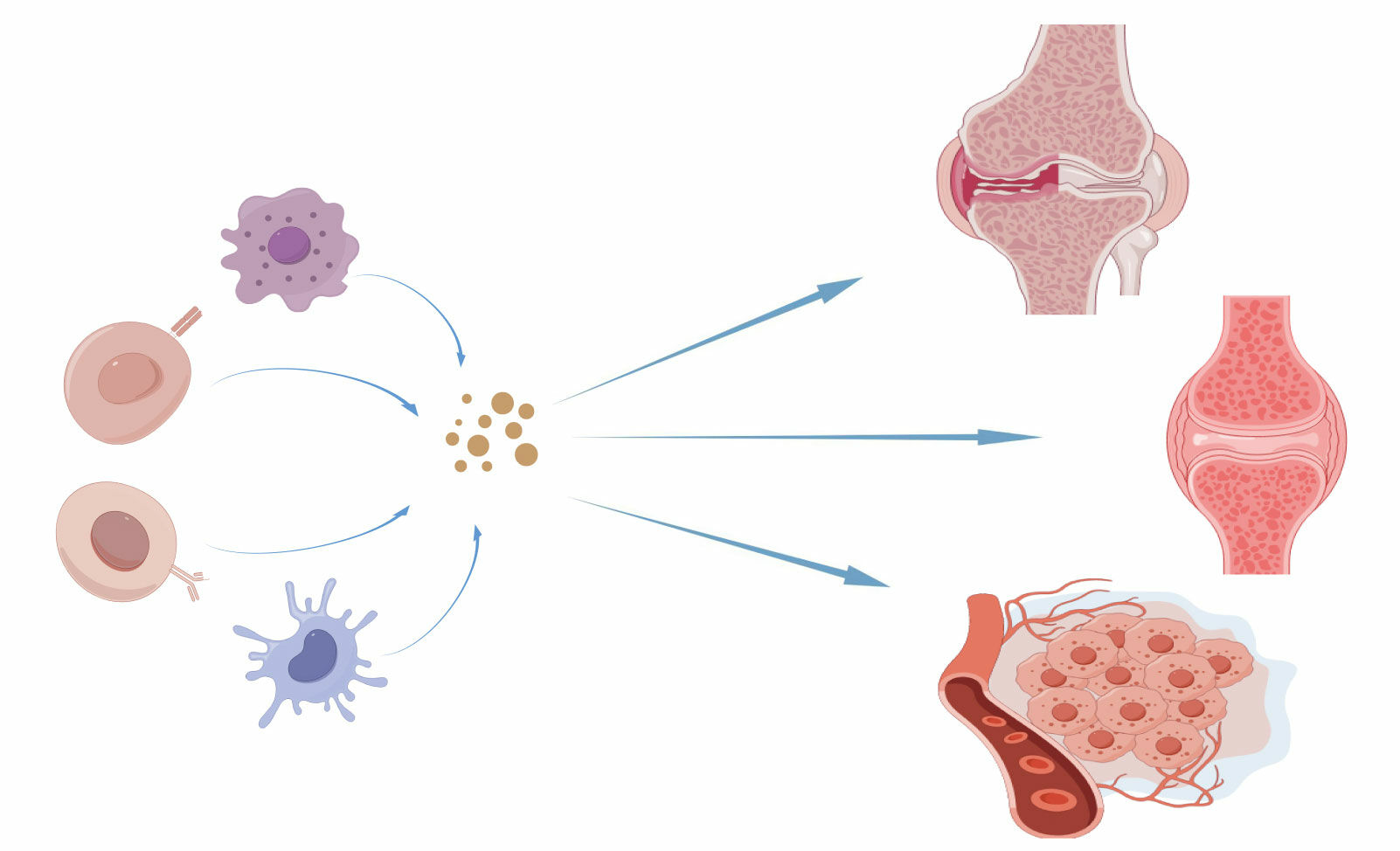
Abstract: Inflammation is a common feature of many inflammatory diseases and tumors, and plays a decisive role in their development. Exosomes are extracellular vesicles unleashed by assorted types of cells, and it is widely known that exosomes of different immune cell sources play different functions. Exosome production has recently been reported for immune cells comprising macrophages, T cells, B cells, and dendritic cells (DCs). Immune cell-derived exosomes are involved in a variety of inflammatory responses.Herein, we summarize and review the role of macrophages, T cells, B cells, and dendritic cells (DC) in inflammatory diseases, with a focus on the role of immune cell-derived exosomes in osteoarthritis, rheumatoid arthritis, and the inflammatory tumor microenvironment (TME).These findings are expected to be important for developing new treatments for inflammatory diseases and ameliorating tumor-related inflammation.
Keywords: inflammatory diseases, immune cell-derived exosomes, osteoarthritis, rheumatoid arthritis, inflammatory tumor microenvironment
Graphical Abstract:
Introduction
The immune system plays a vital role in defending the body, but excessive immune responses can result in inflammatory diseases.1 Inflammation is a response to harmful stimuli, but prolonged and severe inflammation can lead to tissue damage and dysfunction.2 Exosomes are extracellular vesicles with diverse functions that were first identified in 1983.3,4 They have since become a promising therapeutic modality for inflammatory diseases. Immune-derived exosomes are a novel form of intercellular communication that are involved in various cellular processes, such as immune response, signal transduction, immune activation, antigen presentation, cell killing, and metabolism regulation.5 In this review, we summarize the roles of immune cell-derived exosomes, including those from macrophages, T cells, B cells, and dendritic cells, in inflammatory conditions such as osteoarthritis, rheumatoid arthritis, and tumors. We also discuss the potential applications of immune cell-derived exosomes in diagnosing and treating these diseases. This review provides new insights into the roles of immune cell-derived exosomes in inflammatory diseases and their potential therapeutic applications.
Exosome Composition and Formation Mechanism
Exosomes are small vesicles, typically measuring between 30–100 nm in length, that are secreted by cells and can be found in various biological fluids, including cell culture supernatants, serum, plasma, saliva, urine, and amniotic fluid.6,7 Exosomes have a phospholipid bilayer membrane that encapsulates DNA, RNA, proteins, lipids, and sugars.8 These vesicles contain a variety of specialized proteins, including tetrameric proteins such as CD9, CD63, CD81, and CD82, Alix, heat shock proteins (HSP), major histocompatibility complex (MHC-1/2), lipid rafts, and tumor susceptibility gene 101 (TSG101). Of these proteins, CD9 and CD63 are widely accepted as biomarkers and their expression levels have been shown to correlate with the prognosis of several tumors,9,10 Additionally, elevated CD63 levels have been found to be associated with the severity of organ-system dysfunction and mortality prediction in patients with severe sepsis.11 Alix is involved in exosome biogenesis and its expression has been shown to regulate the level of exosome-associated proteins in induced pluripotent stem cells (iPSC). Furthermore, the gradual decrease in Alix protein signaling during colorectal adenoma-carcinoma sequences promotes epithelial-stromal interactions that control tumor growth, metastatic invasion, and therapeutic response. HSP has both intracellular and extracellular functions in the immune system, including antigen presentation, expression of innate receptors, and promoting membrane deformation and exosome release by facilitating the fusion of multivesicular vesicle endosomes (MVEs) with the plasma membrane.12–14 MHC is an important molecule in the process of antigen recognition and plays a crucial role in antigen presentation and immune signaling. MHC class I molecules mainly interact with CD8+ cytotoxic T cells (Tc), while MHC class II molecules primarily interact with CD4+ helper T cells (Th), making them important biological markers for tumor immunotherapy.15
The process of exosome biogenesis is not yet fully understood, but it is believed to involve several steps (Figure 1). Initially, the plasma membrane invaginates to form a cup-shaped structure. Then, early sorted endosomes develop and mature into late sorted endosomes. During this process, the endosomal membrane buds into the cavity, forming intraluminal vesicles (ILVs) and transforming the endosomes into mature multivesicular endosomes (MVEs). MVEs can undergo two possible fates: fusion with lysosomes, leading to the degradation of the luminal vesicles, or transport to the plasma membrane, where they fuse and release their luminal vesicles outside the cell as exosomes.16,17
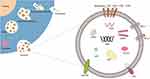 |
Figure 1 Secretion and structure of exosomes. |
Immune Cell-Derived Exosome Function
Macrophage-Derived Exosomes
Macrophages serve as both effectors and regulators in the immune response, contributing to innate immunity, as well as cellular and humoral immunity. Under the influence of different cytokines, macrophages can be classified as M1 or M2.18 M1-type macrophages are typically activated by Th1 cytokines (such as TNF-α and IFN-γ) or bacterial lipopolysaccharide (LPS), leading to the release of NO through inducible nitric oxide synthase, as well as high levels of pro-inflammatory cytokines (including IL-1α, IL-1β, IL-6, IL-12, IL-23, TNF-α, and cox-2). On the other hand, M2-type macrophages are polarized by Th2 cytokines (such as IL-4 and IL-13) and display heightened expression of scavenger receptors. These cells are capable of producing anti-inflammatory cytokines, such as IL-4, IL-13, IL-10, or transforming growth factor-β(TGF-β).19,20 Thus, macrophages have the potential to both promote and suppress inflammatory responses depending on the cytokine stimuli they receive.21
Recent studies have shown that macrophage-derived extracellular vesicles contribute to the activation of pro-inflammatory signaling pathways in endothelial cells, leading to increased levels of inflammation and ICAM expression.22 Additionally, exosomes derived from M2 macrophages can increase the percentage of M2 macrophages and decrease the percentage of M1 macrophages, whereas M1 macrophage-derived exosomes exhibit the opposite effect.23 Macrophage-derived exosomes have also been shown to suppress inflammation and accelerate diabetic wound healing by inhibiting the secretion of pro-inflammatory enzymes and cytokines in a diabetic rat model of skin defects.24 Conversely, macrophage-derived exosomes imbued with miR-155 have been found to promote the production of inflammatory cytokines, including IL-6, IL-23, and TNF-α, as well as increase the expression of CD40, CD63, CD81, and MCH-1 in H.pylori-infected macrophages, while downregulating inflammatory signaling pathway proteins such as MyD88 and NF-κB25 (Table 1). Overexpression of the trigger receptor-2 expressed on myeloid cells (TREM2) has been shown to inhibit corticosterone biosynthesis during the early stages of septic shock, potentially contributing to macrophage-derived exosomes.26 Mg2+ has also been found to induce macrophage autophagy and modulate M1/M2 polarization in macrophages, a process closely associated with macrophage-derived exosomes, particularly those containing miR-381.27 Inducing macrophage polarization from pro-inflammatory M1 to anti-inflammatory M2 via exosomes could be a promising target for treating inflammatory diseases.
 |
Table 1 The Role of Immune Cell-Derived Exosomes in the Inflammatory Response |
T Cell-Derived Exosomes
T cells are a crucial component of the immune system and play an essential role in transmitting immunological information and directly killing infected cells. There are three primary types of T cells: CD8+ killer T cells, CD4+ helper T cells, and regulatory T cells. Exosomes derived from T cells can transfer inhibitory or promotive factors to other cells and have emerged as novel regulators of intercellular signal transduction in inflammatory responses, autoimmune, and infectious diseases. Researchers have identified specific proteins in the RAS/MAPK signaling pathway in exosomes produced by activated T cells, which can serve as biomarkers for inflammation by inducing ERK phosphorylation in recipient immune cells.28 Additionally, exosomes produced by bovine mammary epithelial T cells may stimulate macrophage polarization to M1 during inflammatory responses.29
CD8+ T cells play a vital role in defending against infection by killing infected cells through recognition of microbial peptides presented by MHC class I molecules on the surface of target cells. Granzyme and perforin, found in exosomes derived from CD8+ T cells, can assist in killing target cells.36 CD4+ T cell-derived exosomes interact with NK cells, macrophages, CD8+ T cells, and other cells, and MHCII-like peptides can stimulate the TCR of CD4+ T cells and enable their functions. Regulatory T cells (Tregs) are responsible for controlling autoimmune responses. A low number or hypofunction of Tregs can lead to an overreaction of the immune system and the development of autoimmune diseases, while a high number or abnormal activation can suppress the immune response, which is associated with the development of numerous tumors. Exosomes derived from Tregs have been shown to have immunosuppressive capacity, and exosomes from Tregs can suppress acute rejection and inhibit T-cell proliferation.30,37 Treg cells secrete more exosomes containing membrane-bound molecules, including CD25, CTLA4, and CD73, compared to other T cell subtypes. Exosomes derived from Tregs mainly suppress inflammation by releasing adenosine and CD73.31,38 Also, it was demonstrated that MiR-150-5p and MiR-142-3p from exosomes derived from tregs were transferred to DCs, where they were expressed more strongly.Moreover, miRNAs, such as MiR-150-5p and MiR-142-3p, transferred to DCs via exosomes derived from Tregs may regulate DC function38 (Table 1).
B Cell-Derived Exosomes
B cells are essential effector cells in humoral immunity and are a major source of extracellular vesicles. During their differentiation into effector cells, B cells undergo a wide range of physiological changes, with exosome production being a key feature. The activation of B cells by CD40 and IL-4 signaling is known to facilitate the production of exosomes.32 The exosomes formed by B cells contain immunoglobulins, which are transported via the surface B cell receptor (BCR) to the endosomal/exosomal pathway and then into the extracellular space.40 Using B-cell-derived exosomes, exogenic miRNA-155 mimics or inhibitors were found to be delivered into hepatocytes or macrophages, respectively, and stimulation of B cells resulted in an effective immune response.41
B-cell activation leads to the release of large amounts of MHC II-positive exosomes, which play a crucial role in antigen presentation.42 B-cell-derived exosomes have been found to enhance metabolic activity, leading to the suppression of cytotoxic T cells.43 They also express functional integrins that mediate the anchoring of ECM and cell surface adhesion molecules, representing a novel mode of conveying adhesion signals during inflammation beyond the distance of direct cell-cell contact33 (Table 1). In the tumor microenvironment, B-cell-derived exosomes inhibit CD8+ T-cell responses and interfere with the antitumor effects of chemotherapeutic agents. The levels of CD19+ B-cell-derived exosomes in the peripheral blood of tumor patients have been shown to correlate with worse tumor prognosis, and their levels may serve as a predictor of chemotherapeutic drug efficacy. Therefore, controlling the secretion of B-cell-derived exosomes could be a promising strategy for tumor treatment.
DC Cell-Derived Exosomes
As well as macrophages, dendritic cells (DCs) are powerful antigen-presenting cells, and the role of the exosomes should not be overlooked. Mature dendritic cell-derived exosomes highly express HLA-II-like molecules and CD40, CD80 and CD86 co-stimulatory molecules on their surface. In peripheral lymph nodes, m Dex interacts with DCs and T cells to promote the activation of initial T cells into effector T cells and initiate specific immune responses.In contrast, immature dendritic cell-derived exosomes (im Dex) with low surface expression of HLA-II class antigen-presenting molecules and CD40, CD80 and CD86 costimulatory molecules are not activated to induce immune tolerance and are not only associated with t cells,44,45 but DC-derived exosomes are also capable of mediating T and B cell activation and inducing antitumor immunity through complement activation and antigen shuttling.46,47 DCs can also receive exosomes from B cells and regulate T cell polarization, while T cells can use exosomes carrying genomic or mitochondrial DNA to induce antiviral responses in DCs through the cGAS/STING cytoplasmic DNA sensing pathway with IRF3-dependent interferon regulation of gene expression.48 In addition DC-derived exosomes mainly activate Natural killer cells (NK) and promote the proliferation of NK cells. dC cells are directly involved in IL-15R and NKG2D-dependent Nk cell proliferation and activation by secreting exosomes expressing interleukin-15Ra and NKG2D ligands,34 and it has also been studied that Dc cell-derived exosomes are also able to contribute to the proliferation and secretion of IFNc by NK cells and inhibit tumor metastasis.49 It has been reported that inflammatory factors can affect DCs-derived exosome activity, and if the inflammatory environment contains high amounts of pro-inflammatory factors such as IL-6 and TNF-α, the precursors of DCs differentiate into mature DCs, and their exosomes exert pro-inflammatory effects; on the contrary, if the microenvironment contains factors such as IL-10, the precursors of DCs differentiate into immature DCs, and their exosomes exert immunosuppressive effects.50 During parasitic infection, antigen-stimulated DCs are able to resist the inflammatory response by means of exosomes35 (Table 1).Therefore, DC-derived exosomes play an important role in antigen presentation, regulation of immune response outcome, antitumor, and play different functions influenced by local inflammatory microenvironment inflammatory factors, activating T cells, B cells, NK cells and interacting with each other. The diversity of DC-derived exosome functions also The functional diversity of DC-derived exosomes also provides researchers with new ideas for targeted drug delivery in inflammatory diseases.
Immune Cell-Derived Exosomes in Inflammatory Diseases
Role of Immune Cell-Derived Exosomes in Osteoarthritis
Osteoarthritis (OA) is a degenerative disease that affects the joints, causing pain, mobility problems, disability and deformity, particularly in older adults. Although several treatment methods exist for osteoarthritis (OA), including nonsteroidal anti-inflammatory drugs, corticosteroid injections, and stem cell therapy, recently patient education (PE), exercise therapy, weight management, and manual therapy (MT) have been recommended as first-line interventions for OA.51–53 Joint replacement is commonly used to treat advanced-stage osteoarthritis.The knee and hip joints, being the most commonly affected joints in osteoarthritis, undergo significant impact. A recent study examining preoperative and postoperative blood samples revealed that both total hip and knee replacement surgeries exert similar effects on early postoperative inflammatory markers in patients.54 Inflammation remains a consistent presence throughout the entirety of the treatment process, emphasizing the importance of effectively controlling and eliminating inflammation for the successful interventions of osteoarthritis.However, the underlying mechanisms of this disease are still unclear, and currently, there is still no effective treatment method available.Recent studies have shown that exosomes from mesenchymal stem cells (MSCs) can reduce inflammation and protect cartilage in OA. For instance, exosomes from adipose-derived MSCs can down-regulate inflammatory factors and reduce the production of prostaglandin E2 (PGE2), which plays a chondroprotective role.55 Bone marrow-derived MSCs (BM-MSCs) secrete exosomal microRNA-9-5p that inhibits inflammation and reduces oxidative stress injury associated with OA. Moreover, exosomes from human umbilical cord MSCs (hUCMSCs) can polarize macrophages and release anti-inflammatory cytokines.56 Inhibition of NF-B signaling by exosomes from human bone MSCs (hBMSCs) reduces chondrocyte damage and matrix metalloproteinase (MMP) levels.57 While MSC-derived exosomes have been widely studied in OA, immune cell-derived exosomes remain understudied. M2 macrophage-derived exosomes (M2-exo) have been shown to reduce pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β and exert anti-inflammatory effects via the PI3K/AKT/mTOR signaling pathway.58 M2-exo also regulate the production of pro-inflammatory factors and facilitate bone differentiation while inhibiting MMP13 levels.59,60 These findings suggest that macrophage exosomes may become a new therapeutic direction for OA treatment, particularly for synovial inflammation.
The Role of Immune Cell-Derived Exosomes in Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a chronic inflammatory disorder characterized by synovitis, destruction of articular cartilage and bones. The pathogenesis and progression of RA are believed to involve immune cells, autoantigens, and inflammatory mediators. In recent years, the role of immune cell-derived exosomes in RA has attracted increasing attentionStudies have shown that exosomes from RA patients contain higher levels of miR-17 compared to healthy exosomes. MiR-17 has been found to inhibit Treg induction by suppressing the expression of transforming growth factor β receptor II (TGFBR II) in T cells.61 Additionally, TNF-α-stimulated exosomes expressing upregulated miRNAs, including miR-155-5p, miR-146a-5p, miR-323a-5p, and miR-1307-3p, have been identified in RA patients. Among these miRNAs, miR-146a was expressed on CD4+ T cells from RA patients and overexpression of miR-146a suppressed Jurkat T cells by affecting FAS-related factor 1 (FAF1) apoptosis.62
RA has also been associated with changes in NK cells and CD38-expressing T cells. As CD38+NK cell ratios increase in RA, they inhibit Treg cell differentiation by stimulating mTOR signaling in CD4+ T cells.63 In addition, downregulation of miR-204-5p in exosomes has been observed in RA patients, and reduced exosomal miR-204-5p abundance has been observed in mice with collagen-induced arthritis (CIA), whereas human T lymphocytes release exosomes containing high amounts of miR-204-5p that can be transferred to synovial fibroblasts and inhibit FLS proliferation. Overexpression of miR-204-5p in synovial fibroblasts inhibits synovial fibroblast activation by targeting genes associated with FLS proliferation and invasion, making it a potential biomarker for the diagnosis and treatment of RA.In a mouse model of RA, plasmids encoding the anti-inflammatory cytokines interleukin 10 and betamethasone sodium phosphate (BSP) encapsulated into the bionic vector M2 exosome (M2 Exo), derived from M2 macrophages, have been shown to reduce the secretion of the pro-inflammatory cytokines IL-1β and TNF-α, increase the expression of IL-10 cytokines, promote M1 to M2 macrophage polarization, and attenuate RA.64 It has also been studied that over-activated B cells in autoimmune diseases can be involved in immune damage at inflammatory sites by releasing exosomes. Moreover, it has been suggested that blocking the release of B-cell-derived and dendritic cell-derived exosomes may suppress the overactive immune response of the body, offering a new avenue for the treatment of autoimmune diseases.
Role of Immune Cell-Derived Exosomes in Tumor Inflammatory Environment
Normal cells typically exist in a homeostatic internal environment, while tumour cells require a specific external microenvironment conducive to their growth and proliferation. This includes inducing tissue hypoxia and acidosis, producing large amounts of growth factors and protein hydrolases, and triggering immune inflammatory responses, among other factors. Cancer cells interact with their surrounding stromal and inflammatory cells to create an inflammatory tumour microenvironment (TME). The inflammatory microenvironment affects all stages of tumour development, and several important cytokines and chemokines such as IL-1β, IL-6, CSF-1, and TNF-α are involved in this process. Furthermore, the tumour microenvironment perpetuates an inflammatory response, enhancing the invasive and metastatic abilities of the tumour cells. Numerous studies have indicated that exosomes participate in tumour-associated inflammation and regulate inflammation by facilitating intercellular communication.
Tumor-associated macrophages (TAMs) are the most abundant immune cells that infiltrate tumor tissue and have been reported to play an important role in the development of osteosarcoma. Studies have shown that TAM-derived exosomal lncRNA LIFR-AS1 promotes osteosarcoma cell proliferation, invasion, and inhibits apoptosis through the miR-29a/NFIA axis.65 Tumor-associated macrophages (TAMs) usually have two opposing phenotypes: antitumor M1 and primary M2. Molecular features of TAM-EV are associated with a more favorable patient prognosis with enhanced inflammatory and immune responses compared to source TAM with a Th1 / M1 polarized molecular profile.Enriched TAM-EV preparations promote proliferation and activation of isolated T cells, and TAM-EV also contains bioactive lipids and biosynthetic enzymes that may alter pro-inflammatory signaling in cancer cells.TAM is largely immunosuppressive, but its exosomes largely stimulate the immune response.66 Exosomes derived from M1 macrophages can regulate the progression of hepatocellular carcinoma (HCC) through microRNAs (miRs).For example, miR-326, an M1 macrophage-derived exosome, inhibits HCC proliferation, migration, and invasion by downregulating NF-κB expression and promoting apoptosis.67 In contrast, exosomal miR-487a derived from M2 macrophages was found to promote proliferation and tumorigenesis in gastric cancer,68 Additionally, exosomes derived from M1 macrophages (M1NVs) can convert M2 TAMs into M1 macrophages, release pro-inflammatory cytokines, and induce anti-tumor immune responses.69
Tumor-derived exosomes (TDEs) have been reported to alter differentiation, maturation, and function of dendritic cells (DCs). TDEs contain a variety of biomolecules, including COX-2 (cyclooxygenase-2), PGE2 (prostaglandin E2), TGF-β (transforming growth factor-β), IL-6, HSP70, HSP72, HLA-G, and glycolytic enzymes, thereby affecting bone marrow progenitor cells and inhibiting the differentiation of DCs and monocytes while promoting the polarization of myeloid-derived suppressor cells (MDSCs). TDEs can also induce DCs to express TGF-β, which further increases TGF-β expression in an autocrine loop, and robustly inhibits anti-tumor immunity. Therefore, DCs are closely related to the tumor inflammatory environment, and exosomes from DCs may play a role in the tumor inflammatory environment. A pilot study showed that in hepatocellular carcinoma (HCC) mice treated with exosomes of DCs expressing alpha-fetoprotein (AFP) (DEXAFP), the tumor microenvironment was improved with a significant increase in CD8+ T lymphocytes expressing gamma-interferon (IFN-γ), increased levels of IFN-γ and interleukin-2, decreased CD25+Foxp3+ regulatory T (Treg) cells, and reduced levels of interleukin 10 and transforming growth factor. While other immune cell sources in the tumor inflammatory environment have been less studied, regulation of the tumor inflammatory microenvironment by immune-derived exosomes may become a direction of tumor therapy.
Clinical Implications
Inflammation is associated with the pathogenesis of many diseases, and both acute and chronic immune responses can lead to functional organ dysfunction. Inflammation and autoimmune diseases share common mechanisms, as both occur due to the inability to control the host’s immune response. Therefore, the treatment of these conditions involves targeting immune regulation.
Anti-inflammatory agents that can modulate inflammatory signaling pathways are commonly used to treat inflammation. For example, nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used for inflammatory conditions such as osteoarthritis and rheumatoid arthritis. They exert their effects by inhibiting prostaglandin synthesis and release in the inflammatory response, thereby alleviating inflammation-related pain and discomfort. Additionally, research is underway on anti-inflammatory small molecules, microRNAs (miRNAs), and other approaches.Therapeutic strategies that disrupt autoreactive responses are being pursued in autoimmune diseases. Monoclonal antibodies targeting B cells are being used to treat autoimmune diseases. In addition to these methods, signal pathway inhibitors, stem cell transplantation, tumor necrosis factor (TNF) biologics (such as infliximab and adalimumab), interleukin-1 receptor antagonists (such as anakinra), and other approaches are extensively studied. The use of these drugs is limited due to their toxic reactions and immunosuppressive side effects. The use of mesenchymal stem cells for the treatment of inflammation and autoimmune diseases faces challenges such as low cell survival rates, impaired paracrine effects, and limited homing ability. Extracellular vesicles secreted by immune cells, which possess potent immunomodulatory activity, have emerged as an alternative to cell-based therapies. Compared to cell-based therapies, extracellular vesicles are cell-free and therefore have lower toxicity and fewer immune reactions. Moreover, extracellular vesicles can protect immunomodulatory biomolecules from degradation, making them suitable as drug delivery systems. Thus, immune cell-derived extracellular vesicles hold potential clinical applications in the treatment of inflammation-related diseases.
Conclusions and Future Directions
In order for the immune system to maintain cellular homeostasis and provide host defense, exosomes play an integral role in the establishment of communication between immune cells and the maintenance of a relatively stable immune environment. Exosomes play an important role in many physiological and pathological processes by being released into the extracellular environment and delivering their cargo molecules to target cells. Exosomes of immune origin have become key players in shaping the immune response, such as immune activation, immune suppression, and maintaining the body’s immune homeostasis. Inflammation is one of the pathogenesis of many diseases, and the relative stability of immunity is closely related to inflammatory diseases. With the discovery of exosomes and the advancement of research, exosomes have become a new therapeutic modality for inflammatory diseases Different cell-derived exosomes play different roles, while we focus on the mutual regulation of immune cell-derived exosomes and their application in diseases, including macrophage-derived exosomes, B-cell-derived exosomes, T cell-derived exosomes, dc cell-derived exosomes, and in common inflammatory diseases. In conclusion, immune cell-derived exosomes have great potential for the treatment of a variety of inflammatory diseases. Understanding the function of immune-derived exosomes and using the immune characteristics of exosomes to modulate immune responses may have applications in the specific pathogenesis of inflammatory diseases. However, further research is needed to fully understand the mechanisms underlying exosome-mediated immune regulation and to develop safe and effective exosome-based therapies for inflammatory diseases.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The work was supported by the Health Commission of Hunan Province (NO: 20200476, 20201907, 202102080160).
Disclosure
The authors have no relevant financial or non-financial interests to disclose for this work.
References
1. Iain BM, Ellen MG. Immune-mediated inflammatory disease therapeutics: past, present and future. Nat Rev Immunol. 2021. doi:10.1038/s41577-021-00603-1
2. Tian Y, Cheng C, Wei Y, Yang F, Li G. The role of exosomes in inflammatory diseases and tumor-related inflammation. Cells. 2022;11(6):1005. doi:10.3390/cells11061005
3. Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–978. doi:10.1016/0092-8674(83)90040-5
4. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412–9420. doi:10.1016/S0021-9258(18)48095-7
5. Veerman RE, Gucluler Akpinar G, Eldh M, Gabrielsson S. Immune cell-derived extracellular vesicles - functions and therapeutic applications. Trends Mol Med. 2019;25(5):382–394. doi:10.1016/j.molmed.2019.02.003
6. Anguluri NVLK, Sundarrajan S, Seeram R. Therapeutic applications of exosomes in various diseases: a review. Mat Sci Engin. 2021. doi:10.1016/j.msec.2021.112579
7. Sangiliyandi G, Min-Hee K, Jin-Hoi K. A comprehensive review on factors influences biogenesis, functions, therapeutic and clinical implications of exosomes. Int J Nanomedicine. 2021. doi:10.2147/ijn.s291956
8. Weiping Z, Zhengbo W, Honglin C, Yuyou D. Exosomes as carriers for drug delivery in cancer therapy. Pharm Res. 2022. doi:10.1007/s11095-022-03224-y
9. Ben M, Pranitha P, Wadad M, et al. Exosomal markers (CD63 and CD9) expression and their prognostic significance using immunohistochemistry in right-sided and left-sided colon cancer. J Clin Oncol. 2020. doi:10.1200/jco.2020.38.4_suppl.182
10. Moh’d MK, Arun B, Girijesh KP, et al. The prognostic significance of exosomal markers (CD63 and CD9) expression using immunohistochemistry in patients with pancreatic ductal adenocarcinoma. J Clin Oncol. 2018. doi:10.1200/jco.2018.36.4_suppl.342
11. Yunjoo I, Hongseok Y, Ryoung-Eun K, Jin Young L, Junseon P, Kyeongman J. Exosomal CD63 in critically ill patients with sepsis. Sci Rep. 2021. doi:10.1038/s41598-021-99777-w
12. Azam B, Elnaz A. Heat shock proteins in infection. Clin Chimica Acta. 2019. doi:10.1016/j.cca.2019.08.015
13. Stuart KC, Jianlin G, Ayesha M. Extracellular HSPs: the complicated roles of extracellular HSPs in immunity. Front Immunol. 2016. doi:10.3389/fimmu.2016.00159
14. Elsa L, Yu-Chun W, Rodrigo G, et al. Hsp90 mediates membrane deformation and exosome release. Mol Cell. 2018. doi:10.1016/j.molcel.2018.07.016
15. Francesco S, Luigi L, Giovanna P, et al. Role of human leukocyte antigen system as a predictive biomarker for checkpoint-based immunotherapy in cancer patients. Int J Mol Sci. 2020. doi:10.3390/ijms21197295
16. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367. doi:10.1126/science.aau6977
17. Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. doi:10.1038/s41556-018-0250-9
18. Shapouri‐Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–6440. doi:10.1002/jcp.26429
19. Murray PJ. Macrophage Polarization. Annu Rev Physiol. 2016. doi:10.1146/annurev-physiol-022516-034339
20. Kim H, Wang SY, Kwak G, Yang Y, Kwon IC, Kim SH. Exosome‐guided phenotypic switch of M1 To M2 macrophages for cutaneous wound healing. Advan Sci. 2019;6. doi:10.1002/advs.201900513
21. Mosser DM, Hamidzadeh K, Goncalves R. Macrophages and the maintenance of homeostasis. Cell Mol Immunol. 2021;18(3):579–587. doi:10.1038/s41423-020-00541-3
22. Osada-Oka M, Shiota M, Izumi Y, et al. Macrophage-derived exosomes induce inflammatory factors in endothelial cells under hypertensive conditions. Hypert Res. 2016. doi:10.1038/hr.2016.163
23. Peng P, Hao Y, Cong X, et al. Exosomes-mediated phenotypic switch of macrophages in the immune microenvironment after spinal cord injury. Bio Pharmacoth. 2021;144:112311. doi:10.1016/j.biopha.2021.112311
24. Mengdie L, Tao W, He T, Guohua W, Liang Z, Yijie S. Macrophage-derived exosomes accelerate wound healing through their anti-inflammation effects in a diabetic rat model. Artif Cells, Nanomed Biotechnol. 2019. doi:10.1080/21691401.2019.1669617
25. Jianjun W, Zhiyong D, Zeyou W, et al. MicroRNA-155 in exosomes secreted from helicobacter pylori infection macrophages immunomodulates inflammatory response. Am J Transl Res. 2016:2016:1.
26. Hui Y, Qian Z, Ping F, et al. Triggering receptor expressed on myeloid cells-2 (TREM2) inhibits steroidogenesis in adrenocortical cell by macrophage-derived exosomes in lipopolysaccharide-induced septic shock. Mol Cell Endocrinol. 2021. doi:10.1016/j.mce.2021.111178
27. Yong Z, Shushan Z, Liang C, et al. Mg2+ -mediated autophagy-dependent polarization of macrophages mediates the osteogenesis of bone marrow stromal stem cells by interfering with macrophage-derived exosomes containing miR-381. J Orthop Res. 2021. doi:10.1002/jor.25189
28. Inbar AA, Adam M. Proteomic analysis of human T cell‐derived exosomes reveals differential RAS/MAPK signaling. Eur J Immunol. 2018. doi:10.1002/eji.201847655
29. Mingcheng C, Wenqiao F, Xiaoying L, et al. The regulation of S. aureus induced inflammatory responses in bovine mammary epithelial cells. Front Vet Sci. 2021. doi:10.3389/fvets.2021.683886
30. Xuesong Y, Chibing H, Bo S, et al. CD4+CD25+ regulatory T cells-derived exosomes prolonged kidney allograft survival in a rat model. Cell Immunol. 2013. doi:10.1016/j.cellimm.2013.06.010
31. Smyth LA, Ratnasothy K, Tsang JY, Boardman D, Warley A, Lechler R, Lombardi G. CD73 expression on extracellular vesicles derived from CD4+ CD25+ Foxp3+ T cells contributes to their regulatory function. Eur J Immunol. 2013;43(9):2430–40. doi:10.1002/eji.201242909
32. !!! INVALID CITATION !!! [33].
33. Saunderson SC, Schuberth PC, Dunn AC, et al. Induction of exosome release in primary B cells stimulated via CD40 and the IL-4 receptor. J Immunol. 2008;180:8146–8152. doi:10.4049/jimmunol.180.12.8146
34. Aled C, Atilla T, Sharon D, Robert S, Malcolm DM, Maurice BH. Adhesion and signaling by B cell-derived exosomes: the role of integrins. FASEB J. 2004. doi:10.1096/fj.03-1094fje
35. Sophie V, Magali T, Caroline F, et al. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha. PLoS One. 2009. doi:10.1371/journal.pone.0004942
36. Lifu W, Zilong Y, Shuo W, et al. Exosomes derived from dendritic cells treated with schistosoma japonicum soluble egg antigen attenuate DSS-induced colitis. Front Pharmacol. 2017. doi:10.3389/fphar.2017.00651
37. Peters PJ, Geuze HJ, Van der Donk HA, et al. Molecules relevant for T cell-target cell interaction are present in cytolytic granules of human T lymphocytes. Eur J Immunol. 1989;19(8):1469–1475. doi:10.1002/eji.1830190819
38. Akansha A, Giorgia F, Marilena L, et al. Regulatory T cell-derived exosomes: possible therapeutic and diagnostic tools in transplantation. Front Immunol. 2014. doi:10.3389/fimmu.2014.00555
39. Sim LT, Dominic AB, Monica S, et al. Regulatory T cell-derived extracellular vesicles modify dendritic cell function. Sci Rep. 2018. doi:10.1038/s41598-018-24531-8
40. McLellan AD. Exosome release by primary B cells. Crit Rev Immunol. 2009;29(3):203–217. doi:10.1615/critrevimmunol.v29.i3.20
41. Momen-Heravi F, Bala S, Bukong T, Szabo G. Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages. Nanomedicine. 2014;10(7):1517–1527. doi:10.1016/j.nano.2014.03.014
42. Liu W, Lu J, Zhou H, et al. B细胞来源外泌体的免疫调控作用研究进展 [Role of B cell-derived exosomes in immunoregulation: review]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2021;37(2):174–177. Chinese.
43. LeBleu VS, Kalluri R. Exosomes exercise inhibition of anti-tumor immunity during chemotherapy. Immunity. 2019;50(3):547–549. doi:10.1016/j.immuni.2019.02.019
44. Tkach M, Kowal J, Zucchetti AE, et al. Qualitative differences in T-cell activation by dendritic cell-derived extracellular vesicle subtypes. EMBO J. 2017;36(20):3012–3028. doi:10.15252/embj.201696003
45. Peche H, Renaudin K, Beriou G, Merieau E, Amigorena S, Cuturi MC. Induction of tolerance by exosomes and short-term immunosuppression in a fully MHC-mismatched rat cardiac allograft model. Am J Transplant. 2006;6(7):1541–1550. doi:10.1111/j.1600-6143.2006.01344.x
46. Tanja IN, Ulf G, Khaleda RQ, Mikael CIK, Susanne G. Dendritic cell-derived exosomes need to activate both T and B cells to induce antitumor immunity. J Immunol. 2013. doi:10.4049/jimmunol.1203082
47. Chao W, Zhengyuan L, Zhongli Z, et al. Allogeneic dendritic cells induce potent antitumor immunity by activating KLRG1+CD8 T cells. Sci Rep. 2019. doi:10.1038/s41598-019-52151-3
48. Daniel T, Francesc B, Carolina V-B, et al. Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nat Commun. 2018. doi:10.1038/s41467-018-05077-9
49. Jonathan MP, Mélinda C, Sophie V, et al. Dendritic cell-derived exosomes as immunotherapies in the fight against cancer. J Immunol. 2014. doi:10.4049/jimmunol.1400703
50. Robbins PD, Dorronsoro A, Booker CN. Regulation of chronic inflammatory and immune processes by extracellular vesicles. J Clin Invest. 2016;126(4):1173–1180. doi:10.1172/JCI81131
51. Sinatti P, Sánchez Romero EA, Martínez-Pozas O, Villafañe JH. Effects of patient education on pain and function and its impact on conservative treatment in elderly patients with pain related to hip and knee osteoarthritis: a systematic review. Int J Environ Res Public Health. 2022;19:6194. doi:10.3390/ijerph19106194
52. Sánchez-Romero EA, González-Zamorano Y, Arribas-Romano A, et al. Efficacy of manual therapy on facilitatory nociception and endogenous pain modulation in older adults with knee osteoarthritis: a case series. Appl Sci. 2021. doi:10.3390/app11041895
53. Sánchez Romero EA, Fernández-Carnero J, Calvo-Lobo C, Ochoa Sáez V, Burgos Caballero V, Pecos-Martín D. Is a combination of exercise and dry needling effective for knee OA? Pain Medicine. 2020. doi:10.1093/pm/pnz036
54. Sánchez-Romero EA, Battaglino A, Campanella W, Turroni S, Bishop MD, Villafañe JH. Impact on blood tests of lower limb joint replacement for the treatment of osteoarthritis: hip and knee. Top Geriatr Rehabil. 2021;37(4):227–229. doi:10.1097/TGR.0000000000000337
55. Tofiño-Vian M, Guillén MI, Pérez Del Caz MD, Silvestre A, Alcaraz MJ. Microvesicles from human adipose tissue-derived mesenchymal stem cells as a new protective strategy in osteoarthritic chondrocytes. Cellular Physiol Biochem. 2018;47:11–25. doi:10.1159/000489739
56. Li K, Yan G, Huang H, et al. Anti-inflammatory and immunomodulatory effects of the extracellular vesicles derived from human umbilical cord mesenchymal stem cells on osteoarthritis via M2 macrophages. J Nanobiotechnology. 2022. doi:10.1186/s12951-021-01236-1
57. Yunxia T, Jing Z, Zhen W, et al. Human bone mesenchymal stem cells-derived exosomal miRNA-361-5p alleviates osteoarthritis by downregulating DDX20 and inactivating the NF-κB signaling pathway. Bioorg Chem. 2021. doi:10.1016/j.bioorg.2021.104978
58. Zha Xi D-W, Ma J, Liu C-Z, et al. Exosomes derived from M2 macrophages exert a therapeutic effect via inhibition of the PI3K/AKT/mTOR pathway in rats with knee osteoarthritic. Biomed Res Int. 2021. doi:10.1155/2021/7218067
59. Ziyi L, Yafei W, Shilun L, Yukun L. Exosomes derived from M2 macrophages facilitate osteogenesis and reduce adipogenesis of BMSCs. Front Endocrino. 2021. doi:10.3389/fendo.2021.680328
60. Kun L, Xin L, Zhao-Yong L, et al. Macrophage-derived exosomes promote bone mesenchymal stem cells towards osteoblastic fate through microRNA-21a-5p. Front Bioeng Biotechnol. 2022. doi:10.3389/fbioe.2021.801432
61. Liping W, Chunyan W, Xuqiang J, Jing Y. Circulating Exosomal miR-17 inhibits the induction of regulatory T cells via suppressing TGFBR II expression in rheumatoid arthritis. Cell Physiol Biochem. 2018. doi:10.1159/000494793
62. Yosuke T, Wataru A, Atsushi S, Seiji S, Mitsuyoshi U. Small RNAs detected in exosomes derived from the MH7A synovial fibroblast cell line with TNF-α stimulation. PLoS One. 2018. doi:10.1371/journal.pone.0201851
63. Wang H, Fang K, Yan W, Chang X. T-cell immune imbalance in rheumatoid arthritis is associated with alterations in NK cells and NK-Like T cells expressing CD38. J Innate Immun. 2021. doi:10.1159/000516642
64. Hui L, Yue F, Xiu Z, et al. M2-type exosomes nanoparticles for rheumatoid arthritis therapy via macrophage re-polarization. J Contro Rel. 2021. doi:10.1016/j.jconrel.2021.11.019
65. Hongliang Z, Yiyang Y, Jun W, et al. Macrophages-derived exosomal lncRNA LIFR-AS1 promotes osteosarcoma cell progression via miR-29a/NFIA axis. Cancer Cell Int. 2021. doi:10.1186/s12935-021-01893-0
66. Chiara C, Tim B, Florent D, et al. Molecular profiling and functional analysis of macrophage-derived tumor extracellular vesicles. Cell Rep. 2019. doi:10.1016/j.celrep.2019.05.008
67. Zhen-zi B, Hong-yan L, Cheng-hua L, Chuan-lun S, Xiao-nan Z. M1 macrophage-derived exosomal MicroRNA-326 suppresses hepatocellular carcinoma cell progression via mediating NF-κB signaling pathway. Nanoscale Res Lett. 2020. doi:10.1186/s11671-020-03432-8
68. Yusheng W, Kun S, Ninggang Z, Jian Z, Bangwei C. Tumor-associated macrophage-derived exosomes promote the progression of gastric cancer by regulating the P38MAPK signaling pathway and the immune checkpoint PD-L1. Cancer Biother Radiopharm. 2021. doi:10.1089/cbr.2021.0218
69. Yeon Woong C, Mikyung K, Han Young K, et al. M1 macrophage-derived nanovesicles potentiate the anticancer efficacy of immune checkpoint inhibitors. ACS Nano. 2018. doi:10.1021/acsnano.8b02446
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
