Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Immune-Based Combination Therapies for Advanced Hepatocellular Carcinoma
Authors Carloni R, Sabbioni S , Rizzo A, Ricci AD, Palloni A, Petrarota C, Cusmai A, Tavolari S, Gadaleta-Caldarola G, Brandi G
Received 22 February 2023
Accepted for publication 29 August 2023
Published 6 September 2023 Volume 2023:10 Pages 1445—1463
DOI https://doi.org/10.2147/JHC.S390963
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ahmed Kaseb
Riccardo Carloni,1,2 Simone Sabbioni,1,2 Alessandro Rizzo,3 Angela Dalia Ricci,4 Andrea Palloni,1,2 Cataldo Petrarota,3 Antonio Cusmai,3 Simona Tavolari,1,2 Gennaro Gadaleta-Caldarola,5,* Giovanni Brandi1,2,*
1Department of Specialized, Experimental and Diagnostic Medicine, University of Bologna, Bologna, Italy; 2Division of Medical Oncology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy; 3Struttura Semplice Dipartimentale di Oncologia Medica per la Presa in Carico Globale del Paziente Oncologico “Don Tonino Bello”, I.R.C.C.S. Istituto Tumori “Giovanni Paolo II”, Bari, Italy; 4Medical Oncology Unit, National Institute of Gastroenterology, “Saverio de Bellis” Research Hospital, Bari, Italy; 5Unità Operativa Complessa di Oncologia Medica, Ospedale “Mons. A.R. Dimiccoli”, Barletta, Italy
*These authors contributed equally to this work
Correspondence: Alessandro Rizzo, Struttura Semplice Dipartimentale di Oncologia Medica per la Presa in Carico Globale del Paziente Oncologico “Don Tonino Bello”, I.R.C.C.S. Istituto Tumori “Giovanni Paolo II”, Viale Orazio Flacco 65, Bari, 70124, Italy, Tel +39-051-2144078, Fax +39-051-6364037, Email [email protected]
Abstract: Hepatocellular carcinoma (HCC) is the fourth most frequent cause of cancer-related death worldwide. HCC frequently presents as advanced disease at diagnosis, and disease relapse following radical surgery is frequent. In recent years, immune checkpoint inhibitors (ICIs) have revolutionized the treatment of advanced HCC, particularly with the introduction of atezolizumab/bevacizumab as the new standard of care for first-line treatment. Recently, dual immune checkpoint blockade with durvalumab plus tremelimumab has also emerged as an effective first-line treatment for advanced HCC and most of the research is currently focused on developing combination treatments based mainly on ICIs. In this review, we will discuss the rationale and ongoing clinical trials of immune-based combination therapies for the treatment of advanced HCC, also focusing on new immunotherapy strategies such as chimeric antigen receptor T cells (CAR-T) and anti-cancer vaccines.
Keywords: hepatocellular carcinoma, VEGF, PD-1, tislelizumab, atezolizumab, durvalumab
Introduction
Primary liver cancers represent the seventh most frequently occurring cancer in the world and the fourth most frequent cause of cancer-related death worldwide.1 Hepatocellular carcinoma (HCC) accounts for more than 75% of all primary liver cancers and its incidence is increasing both in the Americas and in most European countries.2 Hepatitis B virus (HBV) and hepatitis C virus (HCV) remain the most relevant risk factors worldwide, but their impact is decreasing due to HBV vaccination and effective antiviral treatments for HCV. By contrast, particularly in USA and Europe, non-alcoholic fatty liver disease (NAFLD) is becoming one of the most relevant risk factors, representing the most rapidly growing cause of HCC among patients listed for liver transplantation in USA.3
In most cases HCC is diagnosed at advanced stage and up to 70% of resected patients develop disease recurrence for which systemic treatment is required.4 For many years tyrosine kinase inhibitors represented the only systemic treatment available for advanced HCC. Improvement in terms of median overall survival (mOS) was modest with approximately 12 months with first-line sorafenib or lenvatinib, while cabozantinib, regorafenib and ramucirumab were used as second-line treatment with an improvement in terms of mOS of approximately two months compared to placebo.5–8 Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of several cancer types and their role has also been investigated in advanced HCC. Despite promising results of first Phase I/II trials with ICIs monotherapy, the subsequent large Phase III trials failed to demonstrate an advantage in terms of OS compared to sorafenib or lenvatinib. In the phase III randomized CheckMate-459 trial, nivolumab (an anti PD-1 antibody) was compared to sorafenib as first-line treatment in advanced HCC. Despite a clinically relevant improvement in mOS (16.4 vs 14.7 months) and in overall response rate (16% vs 7%), OS did not reach statistical significance.9 The results of the phase III RATIONALE-301 study have been recently presented; in this trial, 674 advanced HCC patients were randomized to receive tislelizumab (an anti-PD-1 antibody) or sorafenib as first-line treatment. The study, which was a non-inferiority trial, met its primary endpoints, with an mOS of 15.9 vs 14.1 months and a median progression free survival (mPFS) of 2.2 vs 3.6 months for tislelizumab and sorafenib respectively, while overall response rate (ORR) was markedly improved in tislelizumab arm (14.3% vs 5.4%) [ESMO Congress 2022, LBA36]. Additionally, durvalumab (an anti-PD-1 antibody) proved to be non-inferior compared to sorafenib in the phase III Himalaya trial which will be discussed later.10 Finally, pembrolizumab (an anti-PD-1 antibody) was tested as second-line treatment in advanced HCC patients whose disease progressed on sorafenib in the phase III KEYNOTE-240 trial. A total of 413 patients were randomized to receive pembrolizumab or placebo, and the experimental arm showed a clinically meaningful improvement in mOS (13.8 vs 10.6 months; HR 0.781; 95% CI, 0.611 to 0.998; p = 0.0238) and ORR (18.3% vs 4.4%; nominal one-sided p = 0.00007), but the study did not meet its primary endpoints as per specified criteria.11
Given the modest results shown by ICIs monotherapy, growing attention has been paid to immunotherapy-based combinations with the aim to increase ICIs efficacy. The phase III IMbrave150 trial demonstrated the superiority of atezolizumab (an anti-PD-L1 antibody) plus bevacizumab compared to sorafenib as first-line treatment, thus becoming the new standard of care, and other immunotherapy-based combinations are currently under investigation.12 In this review, we will discuss immunotherapy-based combinations under investigation for treatment of advanced HCC, focusing on the biological rationale and ongoing clinical trials.
Combination of ICIs Plus mTKI or Anti-VEGF Agents
The introduction of ICIs, such as anti-PD-1/PD-L1 and anti-CTL4, has revolutionized the therapeutic approach to solid tumors. Despite their increasingly widespread employment, the mechanisms which are responsible for the onset of primary and secondary resistance to ICIs have not been well identified and explored yet. Thus, it is also not surprising that the lack of specific and reliable predictors of tumor response to ICIs is still an unmet clinical need. In the attempt to unravel the determinants of tumor resistance, increasing interest has been directed toward tumor microenvironment (TME).13
Indeed, many studies suggest a thorough involvement of the TME - as well as paracrine and juxtacrine signaling - as pivotal player both in tumorigenesis and in the development of ICI resistance.14,15 It is enough to say that either hypoxic environment or the vascular endothelial growth factor (VEGF) secreted by tumor-associated cells induce PD-L1 upregulation, hence resulting in tumor immuno-resistance.16,17 Similarly, also the intrinsic mechanisms underpinning the tumor immune escape seem to be sustained by tyrosine kinase-dependent signaling pathways. In fact, several oncogenic pathways, such as PI3K/Akt-, MEK/ERK- and RAS-dependent signaling, are deemed to converge in PD-L1 upregulation.18 Nevertheless, TKIs should also have, per se, an anti-proliferative role, directly acting on HCC cells, as evidenced by in vitro assays.19 Altogether, these considerations represent a thorough biological rationale supporting the association of ICIs and TKIs as a valuable therapeutic strategy in HCC.
Over the years, several clinical trials have been designed, with some of these studies often showing contrasting results. In such a context, IMbrave-150 trial has revolutionized the therapeutic standard of care in advanced HCC, demonstrating that the combination of atezolizumab plus bevacizumab is the first-line treatment of choice. Conversely, in the same disease setting, other studies have failed to demonstrate an overall significant and general benefit of the association of ICI plus TKI over TKI alone.
The Phase III, global, open-label IMbrave-150 study enrolled up to 501 patients, who were randomized 2:1 to receive either atezolizumab plus bevacizumab or sorafenib treatment. Remarkably, both primary data and updated analysis have met the primary endpoints of the study, showing an improvement in mOS and mPFS - 19.1 months (HR 0.66) and 6.8 months, respectively, with atezolizumab plus bevacizumab superior over sorafenib (mOS: 13.4 months; mPFS: 4.3 months). The benefit duration, expressed as median duration of response, was longer in patients treated with combinatory therapy compared to sorafenib alone, settling at 18.1 months and 14.9 months, respectively. Moreover, atezolizumab plus bevacizumab treatment performed better also in terms of activity, showing 29.8% ORR and 7.7% CR, despite the unfavorable prognostic features of the study population (ie, macro-vascular or bile duct invasion). In addition, HCC patients treated with the co-administration of ICI and anti-VEGF experienced a better QoL, as witnessed by 11.2% of mTTD (median time to deterioration), compared to 3.6% with sorafenib.12,20 Similarly, camrelizumab/apatinib association (ie, an anti-PD-1 monoclonal antibody and a VEGFR-2 TKI) showed promising results, supporting its value as alternative first line treatment in advanced HCC. Based on the results of a successful Phase II pivotal study, the RESCUE trial, SHR-1210-III-310, a phase III trial, the results of which have been recently presented at ESMO 2022 Conference, evaluated the clinical benefit of camrelizumab plus apatinib over sorafenib. The study enrolled 543 patients who were randomized to receive either the combination treatment or TKI alone. The trial has shown the superiority of camrelizumab/apatinib in terms of mOS and mPFS, 22.1 months (HR 0.62) and 5.6 months, respectively. Nonetheless, the efficacy data are equally encouraging, exhibiting 25.4% of ORR21 [ESMO 2022, LBA35]. Unlike IMbrave-150 trial, characterized by more heterogeneous population, in SHR-1210-III-310 trial 83% of the study population is Asiatic and 75% patients have been affected by HBV-related HCC. Thus, further investigation is needed to better clarify the actual impact and the relevance of these results in a more assorted population, such as non-Asiatic people and non-viral HCC.
Unlike ground-breaking results evidenced by IMbrave-150 and SHR-1210-III-310 trials, LEAP-002 and COSMIC-312 trials did not meet their primary endpoints. LEAP-002 was aimed at assessing the effect of lenvatinib/pembrolizumab regimen in first-line treatment of advanced HCC patients. 743 eligible patients were enrolled and randomized 1:1 to receive either combo active treatment or lenvatinib/placebo. The coprimary endpoint of PFS and OS was not met because the pre-specified statistical significance was not reached. One of the most putative factors responsible for such an outcome might be found in using placebo in control arm; indeed, this choice could lower the drop-out rate and/or investigator-assessed clinical progression events [ESMO 2022, LBA34]. Nevertheless, the combination of lenvatinib/pembrolizumab achieved mOS and mPFS similar to that detected in control arm, 21.2 vs 19 months and 8.2 vs 8.1 months, respectively. As regards antitumor activity, the doublet lenvatinib/pembrolizumab displayed an advantage in ORR compared to lenvatinib alone - 26.1% vs 17.5%, respectively. Although the study was negative, the mOS of 19.0 months with lenvatinib monotherapy supports its role as a valuable standard of care in first-line advanced HCC.
COSMIC-312, a phase III randomized trial, explored the efficacy and safety of cabozantinib/atezolizumab over sorafenib with regard to mOS and mPFS as primary end-points. The ad-interim analysis showed no benefit of the combo therapy over sorafenib in terms of mOS. Conversely, mPFS at final analysis was significantly improved by the co-administration of cabozantinib/atezolizumab (6.8 vs 4.2 months), and a specific sub-set of patients (ie, Asiatic and HBV-affected population) benefited from the drug association compared to control arm also in terms of mOS. Of note, ICI/TKI co-administration offers higher ORR (11% vs 4%), comparable CR and longer duration of response (10.6 vs 8.8 months).22
Finally, a special mention should be reserved for a population-specific recent clinical trial. Indeed, the phase II/II ORIENT-32 study aimed to assess sintilimab plus IBI305, an anti-PD-1 and bevacizumab biosimilar, respectively, versus sorafenib as a first-line treatment for advanced HBV-associated HCC. The study randomized 595 Chinese HCC patients to receive either combination treatment or sorafenib alone, in a ratio 2:1. The co-primary endpoints were OS and mPFS; the trial was positive, demonstrating a superiority of combinatorial treatment in both endpoints. In particular, sintilimab/IBI305 showed longer mPFS, (4.6 months vs 2.8 months) and mOS, at least at the first ad interim analysis (median not reached vs 10.4 months).23 By comparing ORIENT-32 study and the other two positive phase III trials, IMbrave-150 and SHR-1210-III-310, it emerged that mPFS and mOS of control arm (ie, sorafenib treated patients) in Chinese study were shorter. This phenomenon might be attributable to the different population enrolled in the trials. In fact, in IMbrave-150, SHR-1210-III-310, and ORIENT-32 the ratio of Asians vs non-Asians (including Japanese people) progressively increases from 40% to around 83% and 100%, respectively. Moreover, the subset of HBV-positive patients, which significantly varied among the studies, ranging from 49% in IMbrave-150 to 94% in ORIENT-32, could have impact on both mPFS and mOS. Therefore, the major caveat of this study is the homogeneity of the enrolled population. Further investigation is needed to clarify the actual role of sintilimab/IBI305 co-administration in a wider and more heterogeneous population, with careful attention to non-viral HCC and Caucasian ethnicity.
Besides the previously mentioned studies, several clinical trials are currently ongoing. Table 1 displays a list of most recent studies and their details.
 |
Table 1 Clinical Trials Investigating the Association of Immune Checkpoint Inhibitors with Multikinase Inhibitors or Anti-VEGFR Agents |
Dual Immune Checkpoint Blockade
It has been demonstrated that agents targeting PD-1/PD-L1 and antibodies against CTLA-4, even if they present some points of convergence in their respective downstream pathways, lead to distinct patterns of immune activation in vivo.24 Given this, in recent years the association of two ICIs has received growing attention, with several clinical trials investigating this therapeutic approach and it has been demonstrated to be an effective strategy in the treatment of several malignancies such as melanoma, renal cell carcinoma and non-small cell lung cancer.25–27 The open-label phase I/II CheckMate 040 study was one of the first studies to investigate this therapeutic strategy in HCC. In the fourth cohort of this trial, 148 advanced HCC patients, previously treated with sorafenib, were randomized to receive nivolumab (an anti-PD-1 antibody) plus ipilimumab (an anti-CTLA-4 antibody) at three different dosages, and obtaining an ORR of 32% with an mOS and a 3-year OS of 22.2 months and 42% respectively (arm A: nivolumab 1 mg/kg + ipilimumab 3 mg/kg every 3 weeks, 4 doses, followed by nivolumab 240 mg every 2 weeks).28,29 Interestingly, an increased dosage of ipilimumab seems to be associated with an improved outcome, even if the study was not powered enough to detect differences between arms.30 Safety profile was acceptable with 53% of patients in arm A who reported a grade 3–4 adverse event (AE).28 The association of nivolumab plus ipilimumab is currently under investigation in the phase III CheckMate 9DW trial as first-line treatment for advanced HCC compared to monotherapy with sorafenib or lenvatinib.31
One of the most relevant novelties in the field of dual immune checkpoint blockade for the treatment of advanced HCC is represented by the recently published results of the phase III Himalaya trial. A total of 1171, previously untreated, advanced HCC patients were randomized to receive the so-called STRIDE regimen (one dose of tremelimumab 300 mg plus durvalumab 1500 mg, followed by durvalumab 1500 mg every 4 weeks), durvalumab 1500 mg every 4 weeks or sorafenib until disease progression. STRIDE regimen significantly improved mOS compared to sorafenib (HR 0.78; 96% CI 0.65–0.93; p=0.0035) with an mOS of 16.4 months, 16.6 months and 13.8 months for STRIDE regimen, durvalumab, and sorafenib respectively. ORR and 3-year OS were 20.1% and 30.7% for STRIDE regimen, 17.0% and 24.7% for durvalumab, while sorafenib-treated patients had 5.1% and 20.2%.10 Grade 3–4 AEs were 25.8%, 12.9% and 36.9% for STRIDE regimen, durvalumab and sorafenib respectively.10 Even if it is not possible to compare different studies, STRIDE regimen seems to be associated with a reduced risk of grade 3–4 AEs compared to nivolumab plus ipilimumab, where hepatitis was the second most frequent immune-mediated adverse event requiring immune-modulating medication.28 This difference may be explained by the reduced dose of anti-CTLA-4 administered in the STRIDE regimen where there was only a single priming dose of tremelimumab.32 Currently, a phase III study (NCT05557838) is investigating the efficacy of durvalumab plus tremelimumab also in Child Pugh B patients, who were excluded from Himalaya trial.33 Interestingly, dual immune checkpoint blockade seems to exert comparable efficacy also in nonviral-related HCC. In the phase I/II study 22 the association of durvalumab plus tremelimumab showed comparable mOS between HBV-related HCC and nonviral HCC (14.4 and 13.8 months, respectively), while in HCV-related HCC mOS was further increased reaching 22.3 months.34 This trend seems to be confirmed also in the subsequent Himalaya trial where the advantage of durvalumab plus tremelimumab over sorafenib was maintained also in nonviral HCC subgroup (HR: 0.74; 95% CI: 0.57–0.95).10 Similar results were obtained in second-line setting in the CheckMate 040 study with an mOS of 14.7, 15.2 and 21.9 months in the nonviral, HBV and HCV subgroups respectively.28 Even if the previously mentioned studies were not designed to detect differences in terms of efficacy based on HCC etiology, this observation could be quite relevant especially if we consider that nonviral-related HCC seems to derive less benefit from atezolizumab/bevacizumab or monotherapy with ICIs. In the IMbrave 150 trial atezolizumab plus bevacizumab showed no benefit over sorafenib in the nonviral HCC subgroup (mOS 17.0 vs 18.1; HR 1.05; 95% CI 0.68–1.63), while also single-agent nivolumab and pembrolizumab, in their respective phase III trials demonstrated a reduced OS benefit in nonviral HCC compared to sorafenib (nivolumab HR: 0.91; 95% CI: 0.72–1.16; pembrolizumab HR: 0.88; 95% CI: 0.64–1.20).9,11 These results are supported by two recently published meta-analyses which showed that therapy with anti PD-1/PD-L1 alone, or in combination with bevacizumab, resulted in lower OS in nonviral HCC compared to that in viral HCC.35,36 In addition, a recently published large retrospective study suggests that lenvatinib is associated with a survival benefit compared to atezolizumab/bevacizumab treatment in patients with NAFLD-related HCC.37 These clinical data are supported by preclinical evidence demonstrating the presence of a subgroup of exhausted, unconventionally activated CD8+PD-1+ T cells in NASH-mice models. These cells had tissue-damaging functions and are increased by treatment with anti-PD-1 agents without leading to tumor regression. Interestingly, a similar subgroup of cells has been found also in human NASH-affected livers, thus hypothesizing a possible contribution to the unfavorable effects of anti-PD-1 treatment.36 Stratification of HCC patients based on etiology is warranted in future clinical trials in order to better understand the real advantage of dual immune checkpoint blockade over anti-PD-1/PD-L1 monotherapy and anti-PD-1/PD-L1 plus anti-VEGF agents in nonviral HCC.
IBI310, an anti-CTLA-4 monoclonal antibody, in association with sintilimab (an anti-PD- antibody) has demonstrated promising antitumor activity and a manageable safety profile in a Phase I study enrolling advanced HCC patients.38 Currently sintilimab plus IBI310 is under evaluation as first-line treatment in a randomized phase III trial compared to sorafenib.39
Dual immune checkpoint blockade has demonstrated promising antitumor activity after progression to prior ICI monotherapy in melanoma, non-small-cell lung cancer and renal cell carcinoma.40–42 Nivolumab or pembrolizumab plus ipilimumab has been tested as second line therapy, after progression to monotherapy with ICIs, also in HCC patients in a small retrospective study. Twenty-five patients were included and an ORR of 16% with an mOS of 10.9 months were reported. ORR did not differ between primary resistance group and acquired resistance group while mOS was 4.4 and 11.4 months respectively; all responders were Child Pugh A HCC patients.43 If this suggestion is confirmed in larger prospective studies, dual immune checkpoint blockade could represent a promising candidate for second-line treatment after atezolizumab plus bevacizumab in advanced HCC patients. Currently, a phase II trial is evaluating the association of nivolumab plus ipilimumab in advanced HCC patients after progression to atezolizumab plus bevacizumab.44
In recent years, other new inhibitory immune checkpoint molecules turned out to play an important role in immune tolerance in HCC, such as Lymphocyte-activation gene 3 (LAG-3), T cell immunoglobulin mucin-3 (TIM-3) and T cell immunoreceptor with immunoglobulin and ITIM domains (TIGIT).45–47 Both LAG-3, TIM-3 and TIGIT contribute to resistance to ICIs monotherapy as they are upregulated under PD-1/PD-L1 blockade, similar to what happens with CTLA-4.48–50 This provides a strong rationale for combining anti-PD-1/PD-L1 antibodies with agents targeting these new inhibitory immune checkpoint molecules. An increased expression of TIM-3 on tumor-infiltrating lymphocytes and tumor-associated macrophages has been correlated with poor prognosis and a higher risk of recurrence in HCC patients, and several clinical trials are evaluating the role of anti-TIM-3 agents in advanced solid tumors (eg, NCT02817633, NCT03708328, NCT03680508).51–55 Of note, NCT03680508 is a phase II trial investigating the association of cobolimab (an anti-TIM-3 antibody) with dostarlimab (an anti-PD-1 antibody) in advanced HCC patients as first-line treatment, and the results of this study are expected in 2024.54 IL-27 plays a central role in inducing the expression of TIM-3 on immune cells, thus representing a potential therapeutic target.56 SRF388 (an anti-IL-27 antibody) in association with pembrolizumab will be tested in a phase I study in advanced HCC and renal cell carcinoma.57 The association of relatlimab (an anti-LAG-3 antibody) plus nivolumab has been recently approved by FDA for the treatment of advanced melanoma, given the results of the large phase III RELATIVITY-047 trial.58,59 Currently, relatlimab is under evaluation in a phase II trial in association with nivolumab in immunotherapy naïve advanced HCC patients (NCT04567615).60 Clinical trials investigating dual immune checkpoint blockade in advanced HCC are summarized in Table 2.
 |
Table 2 Clinical Trials of Dual Immune Checkpoint Blockade for Advanced Hepatocellular Carcinoma |
Other Immune-Based Strategies
Triplets
Given the modest results shown by large phase III studies of ICIs in association with TKIs, such as LEAP 002 and COSMIC-312 trials, the addition of a second ICI to combination therapies with anti-PD-1/PD-L1 plus TKIs or anti VEGF agents has received growing attention22 [LBA34 ESMO 2022]. Triplets under investigation for treatment of advanced HCC could be divided into two groups: (i) anti-PD-1/PD-L1 plus anti-CTLA-4 with anti-angiogenics (TKIs or anti-VEGF); (ii) anti-PD-1/PD-L1 plus anti-VEGF with agents targeting alternative immune pathways.
Early clinical data of the association of dual immune checkpoint blockade with TKIs were provided by an arm of the Checkmate 040 trial in which 71 advanced HCC patients were randomized to receive nivolumab 240 mg every two weeks plus cabozantinib 40 mg daily or nivolumab 3 mg/kg every two weeks + ipilimumab 1mg/kg every six weeks + cabozantinib 40 mg daily. Although the small sample size did not allow drawing definitive conclusions, the triplet arm showed an improved ORR and mPFS with 26% vs 17% and 6.8 vs 5.5 months respectively.61 The rationale for considering the association of cabozantinib with dual immune checkpoint blockade is provided by the evidence that cabozantinib may exert an immunomodulatory effect not only via the inhibition of VEGF, but also through the inhibition of other targets such as MET and TAM family of receptor kinases. In particular, the inhibition of TAM family of kinases determines an increase in circulating and tumor-infiltrating cytotoxic T lymphocytes, while MET inhibition impaired the recruitment of immunosuppressive neutrophils into tumor bed in response to immunotherapy.62,63 Moreover, the addition of cabozantinib to dual immune checkpoint blockade seems able to overcome primary resistance to anti-PD-1 plus anti-CTLA-4 by targeting myeloid-derived suppressor cells in castration-resistant prostate cancer mice models.64 Cabozantinib is under evaluation in association with nivolumab plus ipilimumab and transarterial chemoembolization (TACE) in a phase II study (NCT04472767), while the CAMILLA study (NCT03539822) is evaluating the role of cabozantinib in association with durvalumab plus tremelimumab in advanced HCC.65,66
It has been demonstrated in HCC mice models that lenvatinib reduced regulatory T lymphocytes differentiation and the number of tumor-associated macrophages (TAMs), while it increased the percentage of activated CD8+ T cells producing interferon-γ.67,68 Interestingly, also tremelimumab treatment is associated with an increased median proliferating CD8+ T-cell counts, compared to durvalumab treatment, with a tremelimumab dose-dependent increase in T-cell clonal expansion which is associated with improved ORR and OS.69,70 These data provide a rationale for combining lenvatinib with anti-PD-1 plus anti-CTLA-4 and by hypothesizing a possible synergistic effect. Lenvatinib plus durvalumab and tremelimumab is now under investigation in the phase III EMERALD-3 trial in association with TACE, while MK-1308A (a coformulation of pembrolizumab and the anti-CTLA-4 antibody quavonlimab) is under evaluation in association with lenvatinib as first-line treatment in advanced HCC.71,72
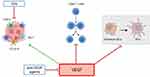
It has been demonstrated that VEGF promotes tumor growth not only via the stimulation of tumor vascularization, but also through an immunosuppressive effect.17 In particular, preclinical data on mice models showed that VEGF induces the expression of immune checkpoint molecules, including PD-1, CTLA-4 and LAG-3, resulting in a reduced activity of anti-PD-1 treatment.16 In addition, VEGF reduces the number of infiltrating CD8+ effector T lymphocytes by increasing the expression of FasL on tumor endothelial cells, making them capable of killing CD8+ T lymphocytes.73 In melanoma patients the addition of bevacizumab to ipilimumab determined an increase in infiltrating CD8+ T cells compared to ipilimumab alone, suggesting a possible synergistic effect with anti-CTLA-4 antibodies.74 Immunosuppressive effects of VEGF are summarized in Figure 1. Combination treatment with atezolizumab plus bevacizumab has already proven its synergistic effect in HCC, thus adding a second ICI could represent a promising therapeutic strategy. Clinical trials investigating the association of anti-VEGF agents plus anti-PD-1/PD-L1 antibody and agents targeting CTLA-4, LAG-3, TIGIT and IL-27 in advanced HCC are summarized in Table 3.
 |
Table 3 Clinical Trials Investigating Triplet Systemic Strategy for Advanced Hepatocellular Carcinoma |
Beyond ICIs
Chimeric Antigen Receptor T Cells (CAR-T) therapy consist of T lymphocytes which are engineered to express a chimeric antigen receptor that specifically recognizes tumor associated-antigens. CAR-T has recently emerged as a promising strategy in the treatment of hematological malignancies and several studies are evaluating its possible application also in the treatment of solid tumors. Glypican-3 (GPC3), NK group 2 member D (NKG2D) and CD147 represent potential targets for CAR-T in HCC of which GPC3 is probably the most studied.75–77 GPC3 is a heparan sulfate proteoglycan playing an important role in cell proliferation and metastasis which is expressed in approximately 75% of HCC cells, but not in healthy liver tissue.78 It has been demonstrated in HCC mice models that GPC3-targeted CAR-T cells induced perforin- and granzyme-mediated apoptosis in GPC3-positive HCC cells with also a reduction of Wnt signaling in cancer cells.79 Despite preclinical studies showing a certain activity of CAR-T therapy alone, its efficacy against solid tumors, including HCC, is still limited due to several obstacles such as the immunosuppressive tumor microenvironment in which PD-1 plays a relevant role.75 This is particularly true in HCC where the GPC3-targeted CAR-T cells have been suggested to present a reduced cytotoxic effect in PD-L1 positive HCC mice models compared to PD-L1 negative mice, while GPC3-CAR-T cells carrying PD-1 blockade agents showed a significantly increased tumor suppression capacity compared to “classic” GPC3-CAR-T cells.80,81 These data provide a rationale for combining CAR-T therapy with ICIs.
The rationale for using vaccines in cancer treatment is based on their ability of inducing a tumor-specific immune response by generating new antigen-specific T cell responses and enhancing existing responses. Various peptide vaccines based on defined antigens have been studied in HCC including vaccines targeting alphafetoprotein, multidrug resistance-associated protein 3 and GPC3.82–84 Particularly, a GPC3-derived peptide vaccine was tested in a phase I trial on 33 HCC patients determining only 1 partial response, even though this treatment induced a relevant GPC3-specific immune response.83 It has become clear that vaccines alone are not able to exert a satisfactory anticancer response and their association with other agents, such as ICIs, represents a promising strategy.85 One of the causes of primary resistance to ICIs is the absence of tumor antigens able to effectively prime and activate T cells resulting in a “cold” TME. This is particularly relevant in HCC if we consider high rates of primary progression reported in phase III trials of ICIs monotherapy (nivolumab 37%, pembrolizumab 33%, durvalumab 45.2% and tislelizumab 49.4%)9–11 [ESMO Congress 2022, LBA36]. Vaccines can produce many neoepitope-specific T cells on which ICIs could exert their stimulatory effect.86 In addition, several studies showed that vaccines up-regulate the expression of molecules targeted by anti-PD-1/PD-L1 and in some reports were the neoantigen specific T cells, induced by vaccine, which expressed PD-1, thus hypothesizing a reduced vaccine efficacy related to PD-1 up-regulation.87–89 Cold-Inducible RNA Binding Protein (CIRP) is a toll-like-receptor-4 ligand released under stress conditions which induces the production of inflammatory cytokines. Silva et al developed a CIRP-based vaccine containing GPC3 (CIRP- GPC3 vaccine) and tested it alone and in association with anti-PD-1 and anti-CTLA-4 agents in HCC mice models. The authors found that the association of CIRP- GPC3 vaccine with anti-PD-1 and anti-CTLA-4 antibodies determined an increased immune response, mainly directed against the 522–530 epitope of HLA-A2*01, compared to vaccine alone, without significantly increased hepatic toxicity in mice.90 Combination of vaccines plus ICIs has already showed promising results in a small series of advanced melanoma patients, and this strategy is receiving growing attention also in HCC.89,91 Clinical trials investigating the association of CAR-T cell therapy and vaccines plus ICIs in advanced HCC are summarized in Table 4.
 |
Table 4 Clinical Trial Investigating the Association of CAR-T Cell Therapy and Vaccines Plus ICIs in Advanced HCC |
Immune-Driven Mechanisms of Epatocarcinogenesis and Tumor Progression
Several factors such as immunity suppression, chronic inflammation, and the decreased recognition of cancer cells have been suggested to play a role in promoting tumor antigen tolerance and epatocarcinogenesis.92,93 In particular, a number of recent trials have highlighted that the onset of HCC may be favored by alterations in cytokine levels as well as in immune cells’ function and number.94,95 Of note, changes in the expression of immune components cause some shifts in terms of immune response, leading to tumor tolerance and tumor progression. Interestingly, several tumor-related cells, including CD4+ T cells, myeloid-derived suppressor cells (MDSCs), natural killer (NK) cells, regulatory T cells (Tregs), and cytotoxic T cells, are involved in HCC development and tumor progression.96,97 Disease progression from liver cirrhosis to HCC sees some changes in terms of immune cells’ function and regulation; among these, the Tregs’ recruitment and development is promoted by the differentiation of macrophages into other phenotypes, something that results in a Th2-type immune response.98 Several studies have suggested a correlation between Tregs and disease progression in HCC patients, and Tregs have been reported to exert a negative effect on other immune-related cells, such as dendritic cells (DCs), NK cells, and T cells, promoting the differentiation of Th17 cells by cytokines with immunosuppressive properties.99,100 In fact, there is a reduced secretion of Th1 cytokines caused by the loss of the antigen presentation capabilities of DCs as well as a lower cytolytic activity by NK cells. In addition, β-catenin pathway also impairs DCs recruitment and induces resistance to anti-PD-1 agents, thus promoting immune escape.101
Based on these premises, it is readily apparent that HCC tumor microenvironment (TME) presents several types of immune cells harboring distinct features, including myeloid cells, NK cells and T cells.102,103 This “ecosystem” is modified by a number of interactions between tumor and immune cells, with these processes resulting in the exhaustion of pro-inflammatory immune cells and the parallel impairment of anti-tumor response.104 Interestingly, several recent studies have suggested the presence of some “immune clusters” playing a prognostic role, with some of these clusters being associated with better outcomes.105,106 In particular, improved survival was reported in HCC patients with low levels of macrophages and high levels of CD8+ T cells; conversely, a more aggressive clinical course was observed in HCC TME with high levels of Tregs, tumor-associated macrophages, and dysfunctional NK cells.107 Characterization of the tumor immune microenvironment could also represent a promising strategy for the identification of predictors of response to ICIs. Zhu et al analyzed tumor biopsies from 358 patients treated with atezolizumab/bevacizumab, atezolizumab alone or sorafenib in two different clinical trials. They found that an increased Treg to effector T cell ratio was associated with a reduced benefit of atezolizumab/bevacizumab, while an increased expression of CD274, T-effector signature and intratumoral CD8+ T cell density were associated with an improved clinical outcome with atezolizumab/bevacizumab.108 In a retrospective analysis conducted on tumor samples from the CheckMate 040 trial, PD-L1 ≥1% was associated with improved mOS in HCC patients treated with nivolumab. Despite this, the relatively small sample size does not allow drawing definitive conclusions.109
Future Perspectives
Atezolizumab/bevacizumab has revolutionized the treatment of advanced HCC, but it also raised several issues. First, in real-life setting only approximately one third of advanced HCC patients are eligible for atezolizumab/bevacizumab treatment if we consider inclusion criteria of IMbrave 150 trial.110 In particular, Child-Pugh B patients were excluded and only few data from retrospective studies are available.111 In this setting, ICIs monotherapy may maintain a role as it has comparable efficacy versus TKIs with a more favorable tolerability profile compared to both TKIs and atezolizumab/bevacizumab.10 In the fifth cohort of CheckMate 040 trial, nivolumab was tested in Child-Pugh B patients showing good tolerability and these results are also supported by small retrospective studies.112,113 Currently, NCCN guidelines consider nivolumab as a therapeutic option for advanced HCC in Child-Pugh B patients; despite this, further studies are needed in order to better understand the real role of nivolumab in this setting and also whether atezolizumab/bevacizumab could be considered in selected Child-Pugh B patients.114 In addition, orthotopic liver transplant still represents an absolute contraindication to treatment with ICIs, thus excluding patients with recurrent disease, from first-line atezolizumab/bevacizumab. Second, there are no data from prospective studies of subsequent systemic treatment after atezolizumab/bevacizumab. Several small retrospective studies suggest a benefit of treatment with TKIs, and ESMO guidelines recommend TKIs as potential options for second line treatment.115,116 Currently, several clinical trials are investigating the role of various systemic treatments in this setting (eg, NCT04770896 and NCT05134532). Given the different mechanism of action, treatment with sorafenib or lenvatinib represents, in our opinion, a reasonable option after progression to first-line atezolizymab/bevacizumab.
ICIs-based combinations will probably dominate treatment scenario of advanced HCC in coming years with dual-immune checkpoint blockade being one of the most promising strategies since this therapeutic strategy seems to maintain a survival benefit over TKIs also in nonviral HCC. Despite this, the modest results shown by LEAP 002 and COSMIC-321 trials underline the absence of reliable predictors of response to ICIs treatment and the lack of data on systemic treatment based on HCC etiology as a fundamental unmet need in this setting.117–119
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Cancer today; 2022. Available from: http://gco.iarc.fr/today/home.
2. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(Suppl 1):4–13. doi:10.1002/hep.31288
3. Younossi Z, Stepanova M, Ong JP, et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. 2019;17(4):748–755.e3. doi:10.1016/j.cgh.2018.05.057
4. She WH, Chok KS. Strategies to increase the resectability of hepatocellular carcinoma. World J Hepatol. 2015;7(18):2147–2154. doi:10.4254/wjh.v7.i18.2147
5. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
6. Rizzo A, Nannini M, Novelli M, Dalia Ricci A, Scioscio VD, Pantaleo MA. Dose reduction and discontinuation of standard-dose regorafenib associated with adverse drug events in cancer patients: a systematic review and meta-analysis. Ther Adv Med Oncol. 2020;12:1758835920936932. doi:10.1177/1758835920936932
7. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. doi:10.1056/NEJMoa1717002
8. Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–296. doi:10.1016/S1470-2045(18)30937-9
9. Yau T, Park JW, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23(1):77–90. doi:10.1016/S1470-2045(21)00604-5
10. Abou-Alfa GK, Lau G, Kudo M, et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evidence. 2022;1(8):EVIDoa2100070. doi:10.1056/EVIDoa2100070
11. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193–202. doi:10.1200/JCO.19.01307
12. Rs F, Q S, I M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:20.
13. C B, Y Q, W W. Intimate communications within the tumor microenvironment: stromal factors function as an orchestra. J Biomed Sci. 2023;30:1.
14. Xie Q, Zhang P, Wang Y, Mei W, Zeng C. Overcoming resistance to immune checkpoint inhibitors in hepatocellular carcinoma: challenges and opportunities. Front Oncol. 2022;12:958720. doi:10.3389/fonc.2022.958720
15. Rizzo A, Ricci AD, Di Federico A, et al. Predictive biomarkers for checkpoint inhibitor-based immunotherapy in hepatocellular carcinoma: where do we stand? Front Oncol. 2021;11:803133. doi:10.3389/fonc.2021.803133
16. Voron T, Colussi O, Marcheteau E, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212(2):139–148. doi:10.1084/jem.20140559
17. Ntellas P, Mavroeidis L, Gkoura S, et al. Old player-new tricks: non angiogenic effects of the VEGF/VEGFR pathway in cancer. Cancers. 2020;12(11):3145. doi:10.3390/cancers12113145
18. Jiang X, Wang J, Deng X, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 2019;18(1):10. doi:10.1186/s12943-018-0928-4
19. Sagmeister P, Daza J, Ofner A, et al. Comparative response of HCC Cells to TKIs: modified in vitro testing and descriptive expression analysis. J Hepatocell Carcinoma. 2022;9:595–607. doi:10.2147/JHC.S356333
20. Finn RS, Qin S, Ikeda M, et al. IMbrave150: updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J Clin Oncol. 2023;39:267. doi:10.1200/JCO.2021.39.3_suppl.267
21. Xu J, Shen J, Gu S, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a nonrandomized, open-label, phase II trial. Clin Cancer Res. 2021;27(4):1003–1011. doi:10.1158/1078-0432.CCR-20-2571
22. Kelley RK, Rimassa L, Cheng AL, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(8):995–1008. doi:10.1016/S1470-2045(22)00326-6
23. Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, Phase 2-3 study. Lancet Oncol. 2021;22(7):977–990. doi:10.1016/S1470-2045(21)00252-7
24. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974–1982. doi:10.1200/JCO.2014.59.4358
25. Brahmer JR, Lee JS, Ciuleanu TE, et al. Five-year survival outcomes with nivolumab plus ipilimumab versus chemotherapy as first-line treatment for metastatic non-small cell lung cancer in checkMate 227. J Clin Oncol. 2022;2022:101200J.
26. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290. doi:10.1056/NEJMoa1712126
27. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. New England J Med. 2019;381(16):1535–1546. doi:10.1056/NEJMoa1910836
28. Yau T, Kang YK, Kim TY, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the checkmate 040 randomized clinical trial. PubMed. 2022;6(11):e204564.
29. El-Khoueiry AB, Yau T, Kang YK, et al. Nivolumab (NIVO) plus ipilimumab (IPI) combination therapy in patients (Pts) with advanced hepatocellular carcinoma (aHCC): long-term results from CheckMate 040. JCO. 2021;39(3_suppl):269. doi:10.1200/JCO.2021.39.3_suppl.269
30. Sangro B, Yau T, El-Khoueiry AB, et al. Exposure-response analysis for nivolumab + ipilimumab combination therapy in patients with advanced hepatocellular carcinoma (CheckMate 040). Clin Transl Sci. 2023;2023:1.
31. Squibb B-M. A randomized, multi-center, phase 3 study of nivolumab in combination with ipilimumab compared to sorafenib or lenvatinib as first-line treatment in participants with advanced hepatocellular carcinoma. Clin Trial Regist. 2022;2022:1.
32. Boutros C, Tarhini A, Routier E, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13(8):473–486. doi:10.1038/nrclinonc.2016.58
33. Fan J, Qin S, Sun HC. An open-label, multi-center phase iiib study of durvalumab and tremelimumab as first-line treatment in patients with unresectable hepatocellular carcinoma. clinicaltrials.gov; 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT05557838.
34. Kelley RK, Sangro B, Harris W, et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: randomized expansion of a phase I/II study. J Clin Oncol. 2021;39(27):2991–3001. doi:10.1200/JCO.20.03555
35. Haber PK, Puigvehí M, Castet F, et al. Evidence-based management of hepatocellular carcinoma: systematic review and meta-analysis of randomized controlled trials (2002–2020). Gastroenterology. 2021;161(3):879–898. doi:10.1053/j.gastro.2021.06.008
36. Pfister D, Núñez NG, Pinyol R, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592(7854):450–456. doi:10.1038/s41586-021-03362-0
37. Rimini M, Rimassa L, Ueshima K, et al. Atezolizumab plus bevacizumab versus lenvatinib or sorafenib in non-viral unresectable hepatocellular carcinoma: an international propensity score matching analysis. ESMO Open. 2022;7(6):100591.
38. Zhou J, Shi YH, Liu B, et al. A phase Ib, multicenter, open-label study to assess the safety, tolerability, and preliminary efficacy of sintilimab plus IBI310 (anti-CTLA4 mAb) in patients with advanced hepatocellular carcinoma. JCO. 2022;40(4_suppl):421. doi:10.1200/JCO.2022.40.4_suppl.421
39. Innovent Biologics (Suzhou) Co. Ltd. A randomized, open-label, controlled, multicenter phase III clinical study to compare the effectiveness and safety of ibi310 combined with sintilimab versus sorafenib in the first-line treatment of advanced hepatocellular carcinoma. clinicaltrials.gov; 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT04720716.
40. Zimmer L, Apuri S, Eroglu Z, et al. Ipilimumab alone or in combination with nivolumab after progression on anti-PD-1 therapy in advanced melanoma. Eur J Cancer. 2017;75:47–55. doi:10.1016/j.ejca.2017.01.009
41. Viscardi G, Tralongo AC, Massari F, et al. Comparative assessment of early mortality risk upon immune checkpoint inhibitors alone or in combination with other agents across solid malignancies: a systematic review and meta-analysis. Eur J Cancer. 2022;177:175–185. doi:10.1016/j.ejca.2022.09.031
42. Mollica V, Santoni M, Matrana MR, et al. Concomitant proton pump inhibitors and outcome of patients treated with nivolumab alone or plus ipilimumab for advanced renal cell carcinoma. Target Oncol. 2022;17(1):61–68. doi:10.1007/s11523-021-00861-y
43. Wong JSL, Kwok GGW, Tang V, et al. Ipilimumab and nivolumab/pembrolizumab in advanced hepatocellular carcinoma refractory to prior immune checkpoint inhibitors. J Immunother Cancer. 2021;9(2):e001945. doi:10.1136/jitc-2020-001945
44. Academic and Community Cancer Research United. A phase II study of nivolumab + ipilimumab in advanced HCC patients who have progressed on first line atezolizumab + bevacizumab. clinicaltrials.gov; 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT05199285.
45. Duan X, Liu J, Cui J, et al. Expression of TIGIT/CD155 and correlations with clinical pathological features in human hepatocellular carcinoma. Mol Med Rep. 2019;20(4):3773–3781. doi:10.3892/mmr.2019.10641
46. Guo M, Yuan F, Qi F, et al. Expression and clinical significance of LAG-3, FGL1, PD-L1 and CD8+T cells in hepatocellular carcinoma using multiplex quantitative analysis. J Transl Med. 2020;18(1):306. doi:10.1186/s12967-020-02469-8
47. Li H, Wu K, Tao K, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56(4):1342–1351. doi:10.1002/hep.25777
48. Stecher C, Battin C, Leitner J, et al. PD-1 blockade promotes emerging checkpoint inhibitors in enhancing T cell responses to allogeneic dendritic cells. Front Immunol. 8;2017.
49. Shayan G, Srivastava R, Li J, Schmitt N, Kane LP, Ferris RL. Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K-Akt pathway in head and neck cancer. Oncoimmunology. 2017;6(1):e1261779. doi:10.1080/2162402X.2016.1261779
50. Chiu DKC, Yuen VWH, Cheu JWS, et al. Hepatocellular Carcinoma Cells Up-regulate PVRL1, Stabilizing PVR and Inhibiting the Cytotoxic T-cell response via TIGIT to mediate tumor resistance to PD1 inhibitors in mice. Gastroenterology. 2020;159(2):609–623. doi:10.1053/j.gastro.2020.03.074
51. Yan W, Liu X, Ma H, et al. Tim-3 fosters HCC development by enhancing TGF-β-mediated alternative activation of macrophages. Gut. 2015;64(10):1593–1604. doi:10.1136/gutjnl-2014-307671
52. Zhou G, Sprengers D, Boor PPC, et al. Antibodies against immune checkpoint molecules restore functions of tumor-infiltrating T cells in hepatocellular carcinomas. Gastroenterology. 2017;153(4):1107–1119.e10. doi:10.1053/j.gastro.2017.06.017
53. Tesaro, Inc. A Phase 1 dose escalation and cohort expansion study of TSR-022, an Anti-TIM-3 monoclonal antibody, in patients with advanced solid tumors (AMBER). clinicaltrials.gov; 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT02817633.
54. University of Hawaii. Phase II Study of TSR-022 (Cobolimab) in Combination With TSR-042 (Dostarlimab) for the treatment of advanced hepatocellular carcinoma. clinicaltrials.gov; 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT03680508.
55. Hoffmann-La Roche. An open label, multicenter, dose escalation and expansion, phase 1 study to evaluate safety, pharmacokinetics, and preliminary anti-tumor activity of RO7121661, a PD-1/TIM-3 bispecific antibody, in patients with advanced and/or metastatic solid tumors. clinicaltrials.gov; 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT03708328.
56. Aghayev T, Mazitova AM, Fang JR, et al. IL27 signaling serves as an immunologic checkpoint for innate cytotoxic cells to promote hepatocellular carcinoma. Cancer Discov. 2022;12(8):1960–1983. doi:10.1158/2159-8290.CD-20-1628
57. Naing A, Mantia C, Morgensztern D, et al. First-in-human study of SRF388, a first-in-class IL-27 targeting antibody, as monotherapy and in combination with pembrolizumab in patients with advanced solid tumors. JCO. 2022;40(16_suppl):2501. doi:10.1200/JCO.2022.40.16_suppl.2501
58. Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386(1):24–34. doi:10.1056/NEJMoa2109970
59. Paik J. Nivolumab plus relatlimab: first approval. Drugs. 2022;82(8):925–931. doi:10.1007/s40265-022-01723-1
60. Bristol-Myers Squibb. A phase 2, randomized, open-label study of relatlimab in combination with nivolumab in participants with advanced hepatocellular carcinoma who are naive to IO therapy but progressed on tyrosine kinase inhibitors (RELATIVITY-073). clinicaltrials.gov; 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT04567615.
61. Yau T, Zagonel V, Santoro A, et al. Nivolumab (NIVO) + ipilimumab (IPI) + cabozantinib (CABO) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): results from CheckMate 040. JCO. 2020;38(4_suppl):478. doi:10.1200/JCO.2020.38.4_suppl.478
62. Akalu YT, Rothlin CV, Ghosh S. TAM receptor tyrosine kinases as emerging targets of innate immune checkpoint blockade for cancer therapy. Immunol Rev. 2017;276(1):165–177. doi:10.1111/imr.12522
63. Glodde N, Bald T, Van den boorn-konijnenberg D, et al. Reactive neutrophil responses dependent on the receptor tyrosine kinase c-MET limit cancer immunotherapy. Immunity. 2017;47(4):789–802.e9. doi:10.1016/j.immuni.2017.09.012
64. Lu X, Horner JW, Paul E, et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature. 2017;543(7647):728–732. doi:10.1038/nature21676
65. Saeed A. A phase I/II trial of cabozantinib in combination with durvalumab (MEDI4736) with or without tremelimumab in patients with advanced gastroesophageal cancer and other gastrointestinal (GI) malignancies (CAMILLA). clinicaltrials.gov; 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT03539822.
66. Dayyani F. Phase 2 study of cabozantinib combined with ipilimumab/nivolumab and transarterial chemoembolization (TACE) in patients with hepatocellular carcinoma (HCC) who are not candidates for curative intent treatment. clinicaltrials.gov; 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT04472767.
67. Kato Y, Tabata K, Kimura T, et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One. 2019;14(2):e0212513. doi:10.1371/journal.pone.0212513
68. Yi C, Chen L, Lin Z, et al. Lenvatinib Targets FGF Receptor 4 to enhance antitumor immune response of anti-programmed cell death-1 in HCC. Hepatology. 2021;74(5):2544–2560. doi:10.1002/hep.31921
69. McCoon P, Lee YS, Kelley RK, et al. T-cell receptor pharmacodynamics associated with survival and response to tremelimumab (T) in combination with durvalumab (D) in patients (pts) with unresectable hepatocellular carcinoma (uHCC). JCO. 2021;39(15_suppl):4087. doi:10.1200/JCO.2021.39.15_suppl.4087
70. Kelley RK, Sangro B, Harris WP, et al. Efficacy, tolerability, and biologic activity of a novel regimen of tremelimumab (T) in combination with durvalumab (D) for patients (pts) with advanced hepatocellular carcinoma (aHCC). JCO. 2020;38(15_suppl):4508.
71. AstraZeneca. A Phase III, randomized, open-label, sponsor-blinded, multicenter study of durvalumab in combination with tremelimumab ± lenvatinib given concurrently with TACE Compared to TACE alone in patients with locoregional hepatocellular carcinoma (EMERALD-3). clinicaltrials.gov; 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT05301842.
72. Perets R, Bar J, Rasco DW, et al. Safety and efficacy of quavonlimab, a novel anti-CTLA-4 antibody (MK-1308), in combination with pembrolizumab in first-line advanced non-small-cell lung cancer. Annal Oncol. 2021;32(3):395–403. doi:10.1016/j.annonc.2020.11.020
73. Motz GT, Santoro SP, Wang LP, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. 2014;20(6):607–615. doi:10.1038/nm.3541
74. Hodi FS, Lawrence D, Lezcano C, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res. 2014;2(7):632–642. doi:10.1158/2326-6066.CIR-14-0053
75. Shen J, Yang D, Ding Y. Advances in promoting the efficacy of chimeric antigen receptor T cells in the treatment of hepatocellular carcinoma. Cancers. 2022;14(20):5018. doi:10.3390/cancers14205018
76. Sun B, Yang D, Dai H, et al. Eradication of hepatocellular carcinoma by NKG2D-Based CAR-T Cells. Cancer Immunol Res. 2019;7(11):1813–1823. doi:10.1158/2326-6066.CIR-19-0026
77. Zhang RY, Wei D, Liu ZK, et al. Doxycycline inducible chimeric antigen receptor T cells targeting CD147 for hepatocellular carcinoma therapy. Front Cell Dev Biol. 2019;7:233. doi:10.3389/fcell.2019.00233
78. Dargel C, Bassani-Sternberg M, Hasreiter J, et al. T cells engineered to express a T-cell receptor specific for glypican-3 to recognize and kill hepatoma cells in vitro and in mice. Gastroenterology. 2015;149(4):1042–1052. doi:10.1053/j.gastro.2015.05.055
79. Li D, Li N, Zhang YF, et al. Persistent polyfunctional chimeric antigen receptor T cells that target glypican 3 eliminate orthotopic hepatocellular carcinomas in mice. Gastroenterology. 2020;158(8):2250–2265.e20. doi:10.1053/j.gastro.2020.02.011
80. Jiang Z, Jiang X, Chen S, et al. Anti-GPC3-CAR T cells suppress the growth of tumor cells in patient-derived xenografts of hepatocellular carcinoma. Front Immunol. 2016;7:690. doi:10.3389/fimmu.2016.00690
81. Pan Z, Di S, Shi B, et al. Increased antitumor activities of glypican-3-specific chimeric antigen receptor-modified T cells by coexpression of a soluble PD1-CH3 fusion protein. Cancer Immunol Immunother. 2018;67(10):1621–1634. doi:10.1007/s00262-018-2221-1
82. Mizukoshi E, Nakagawa H, Kitahara M, et al. Phase I trial of multidrug resistance-associated protein 3-derived peptide in patients with hepatocellular carcinoma. Cancer Lett. 2015;369(1):242–249. doi:10.1016/j.canlet.2015.08.020
83. Sawada Y, Yoshikawa T, Nobuoka D, et al. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res. 2012;18(13):3686–3696. doi:10.1158/1078-0432.CCR-11-3044
84. Butterfield LH, Economou JS, Gamblin TC, Geller DA. Alpha fetoprotein DNA prime and adenovirus boost immunization of two hepatocellular cancer patients. J Transl Med. 2014;12:86. doi:10.1186/1479-5876-12-86
85. Sangro B, Sarobe P, Hervás-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(8):525–543. doi:10.1038/s41575-021-00438-0
86. Liao JY, Zhang S. Safety and efficacy of personalized cancer vaccines in combination with immune checkpoint inhibitors in cancer treatment. Front Oncol. 2021;11. doi:10.3389/fonc.2021.663264
87. Fu J, Malm IJ, Kadayakkara DK, Levitsky H, Pardoll D, Kim YJ. Preclinical evidence that PD1 blockade cooperates with cancer vaccine TEGVAX to elicit regression of established tumors. Cancer Res. 2014;74(15):4042–4052. doi:10.1158/0008-5472.CAN-13-2685
88. Hui E, Cheung J, Zhu J, et al. T cell costimulatory receptor CD28 is a primary target for PD-1–mediated inhibition. Science. 2017;355(6332):1428–1433. doi:10.1126/science.aaf1292
89. Sahin U, Derhovanessian E, Miller M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547(7662):222–226. doi:10.1038/nature23003
90. Silva L, Egea J, Villanueva L, et al. Cold-Inducible RNA binding protein as a vaccination platform to enhance immunotherapeutic responses against hepatocellular carcinoma. Cancers. 2020;12(11):3397. doi:10.3390/cancers12113397
91. Ott PA, Hu Z, Keskin DB, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547(7662):217–221. doi:10.1038/nature22991
92. Zheng C, Zheng L, Yoo JK, et al. Landscape of infiltrating T cells in liver cancer revealed by single-cell sequencing. Cell. 2017;169(7):1342–1356.e16. PMID: 28622514. doi:10.1016/j.cell.2017.05.035
93. Zhang Q, He Y, Luo N, et al. Landscape and dynamics of single immune cells in hepatocellular carcinoma. Cell. 2019;179(4):829–845.e20. PMID: 31675496. doi:10.1016/j.cell.2019.10.003
94. Sun Y, Wu L, Zhong Y, et al. Single-cell landscape of the ecosystem in early-relapse hepatocellular carcinoma. Cell. 2021;184(2):404–421.e16. PMID: 33357445. doi:10.1016/j.cell.2020.11.041
95. Dong LQ, Peng LH, Ma LJ, et al. Heterogeneous immunogenomic features and distinct escape mechanisms in multifocal hepatocellular carcinoma. J Hepatol. 2020;72(5):896–908. PMID: 31887370. doi:10.1016/j.jhep.2019.12.014
96. Kang HJ, Oh JH, Chun SM, et al. Immunogenomic landscape of hepatocellular carcinoma with immune cell stroma and EBV-positive tumor-infiltrating lymphocytes. J Hepatol. 2019;71(1):91–103. PMID: 30930222. doi:10.1016/j.jhep.2019.03.018
97. Shirabe K, Motomura T, Muto J, et al. Tumor-infiltrating lymphocytes and hepatocellular carcinoma: pathology and clinical management. Int J Clin Oncol. 2010;15(6):552–558. PMID: 20963618. doi:10.1007/s10147-010-0131-0
98. Sachdeva M, Chawla YK, Arora SK. Immunology of hepatocellular carcinoma. World J Hepatol. 2015;7(17):2080–2090. PMID: 26301050; PMCID: PMC4539401. doi:10.4254/wjh.v7.i17.2080
99. Fathi F, Saidi RF, Banafshe HR, Arbabi M, Lotfinia M, Motedayyen H. Changes in immune profile affect disease progression in hepatocellular carcinoma. Int J Immunopathol Pharmacol. 2022;36:3946320221078476. PMID: 35226515; PMCID: PMC8891922. doi:10.1177/03946320221078476
100. Romualdo GR, Leroy K, Costa CJS, et al. In vivo and in vitro models of hepatocellular carcinoma: current strategies for translational modeling. Cancers. 2021;13(21):5583. PMID: 34771745; PMCID: PMC8582701. doi:10.3390/cancers13215583
101. de Galarreta M R, Bresnahan E, Molina-Sánchez P, et al. β-catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 2019;9(8):1124–1141. doi:10.1158/2159-8290.CD-19-0074
102. Oura K, Morishita A, Tani J, Masaki T. Tumor immune microenvironment and immunosuppressive therapy in hepatocellular carcinoma: a review. Int J Mol Sci. 2021;22(11):5801. PMID: 34071550; PMCID: PMC8198390. doi:10.3390/ijms22115801
103. Hou J, Zhang H, Sun B, Karin M. The immunobiology of hepatocellular carcinoma in humans and mice: basic concepts and therapeutic implications. J Hepatol. 2020;72(1):167–182. PMID: 31449859. doi:10.1016/j.jhep.2019.08.014
104. Fu Y, Liu S, Zeng S, Shen H. From bench to bed: the tumor immune microenvironment and current immunotherapeutic strategies for hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38(1):396. PMID: 31500650; PMCID: PMC6734524. doi:10.1186/s13046-019-1396-4
105. Xiang S, Li J, Shen J, et al. Identification of prognostic genes in the tumor microenvironment of hepatocellular carcinoma. Front Immunol. 2021;12:653836. PMID: 33897701; PMCID: PMC8059369. doi:10.3389/fimmu.2021.653836
106. Ng HH, Lee RY, Goh S, et al. Immunohistochemical scoring of CD38 in the tumor microenvironment predicts responsiveness to anti-PD-1/PD-L1 immunotherapy in hepatocellular carcinoma. J Immunother Cancer. 2020;8(2):e000987. PMID: 32847986; PMCID: PMC7451957. doi:10.1136/jitc-2020-000987
107. Affo S, Yu LX, Schwabe RF. The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu Rev Pathol. 2017;12:153–186. PMID: 27959632; PMCID: PMC5720358. doi:10.1146/annurev-pathol-052016-100322
108. Zhu AX, Abbas AR, de Galarreta MR, et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat Med. 2022;28(8):1599–1611. doi:10.1038/s41591-022-01868-2
109. Sangro B, Melero I, Wadhawan S, et al. Association of inflammatory biomarkers with clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma. J Hepatol. 2020;73(6):1460–1469. doi:10.1016/j.jhep.2020.07.026
110. Stefanini B, Bucci L, Santi V, et al. Potential feasibility of atezolizumab-bevacizumab therapy in patients with hepatocellular carcinoma treated with tyrosine-kinase inhibitors. Dig Liver Dis. 2022;54(11):1563–1572. doi:10.1016/j.dld.2022.07.003
111. D’Alessio A, Fulgenzi CAM, Nishida N, et al. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-Pugh A and B cirrhosis: a real-world study. Hepatology. 2022;76(4):1000–1012. doi:10.1002/hep.32468
112. Kudo M, Matilla A, Santoro A, et al. CheckMate 040 cohort 5: a phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis. J Hepatol. 2021;75(3).
113. Kambhampati S, Bauer KE, Bracci PM, et al. Nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh class B cirrhosis: safety and clinical outcomes in a retrospective case series. Cancer. 2019;125(18):3234–3241. doi:10.1002/cncr.32206
114. NCCN. NCCN guidelines® updates: hepatobiliary cancers. J Natl Compr Canc Netw. 2021;19(5):xix–xx.
115. Chen CT, Feng YH, Yen CJ, et al. Prognosis and treatment pattern of advanced hepatocellular carcinoma after failure of first-line atezolizumab and bevacizumab treatment. Hepatol Int. 2022;16(5):1199–1207. doi:10.1007/s12072-022-10392-x
116. eUpdate. Hepatocellular carcinoma algorithm; 2022. Available from: https://www.esmo.org/guidelines/guidelines-by-topic/gastrointestinal-cancers/hepatocellular-carcinoma/eupdate-hepatocellular-carcinoma-algorithm.
117. Rizzo A, Cusmai A, Gadaleta-Caldarola G, Palmiotti G. Which role for predictors of response to immune checkpoint inhibitors in hepatocellular carcinoma? Expert Rev Gastroenterol Hepatol. 2022;16(4):333–339. doi:10.1080/17474124.2022.2064273
118. Di Federico A, Rizzo A, Carloni R, et al. Atezolizumab-bevacizumab plus Y-90 TARE for the treatment of hepatocellular carcinoma: preclinical rationale and ongoing clinical trials. Expert Opin Investig Drugs. 2022;31(4):361–369. doi:10.1080/13543784.2022.2009455
119. Rizzo A, Ricci AD, Gadaleta-Caldarola G, Brandi G. First-line immune checkpoint inhibitor-based combinations in unresectable hepatocellular carcinoma: current management and future challenges. Expert Rev Gastroenterol Hepatol. 2021;15(11):1245–1251. doi:10.1080/17474124.2021.1973431
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.